Effects of Proton Pump Inhibitor Administration and Intake of a Combination of Yogurt and Galactooligosaccharides on Bone and Mineral Metabolism in Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Diets
2.2. Animals
2.3. BMD by X-ray CT Analysis
2.4. Dynamic Bone Histomorphometry
2.5. Calcium and Phosphorus Absorption
2.6. Biochemical Analysis
2.7. Statistics
3. Results
3.1. Body Weight and Whole Caecal Weight
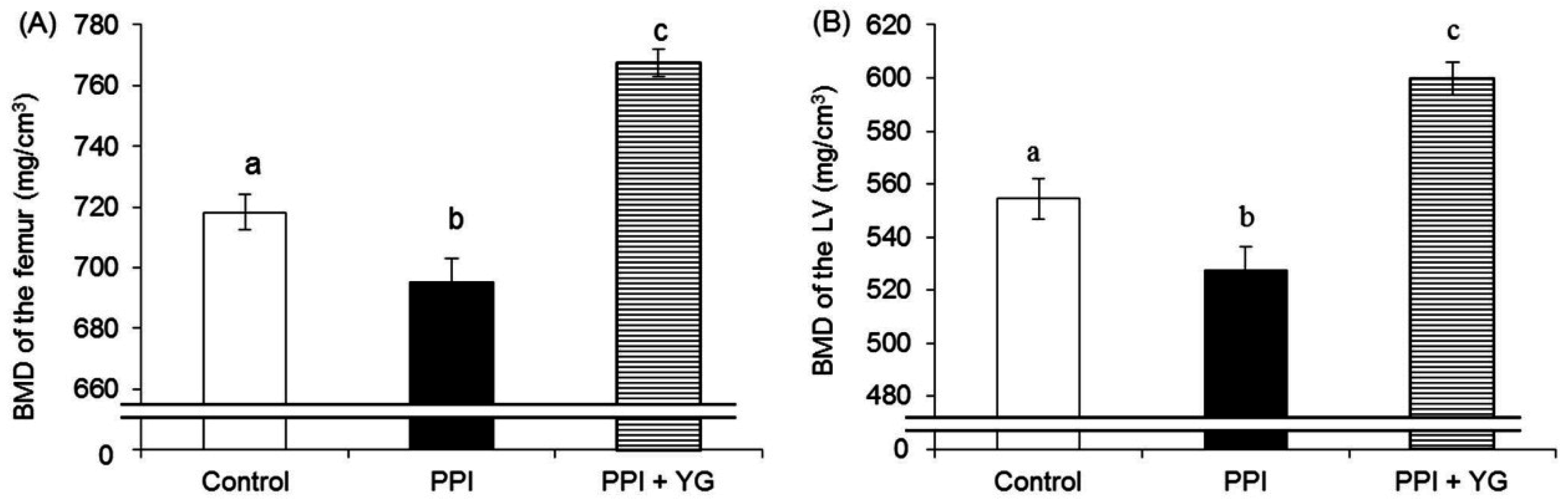
3.2. BMD of the Femur and the LV
3.3. Dynamic Bone Histomorphometry
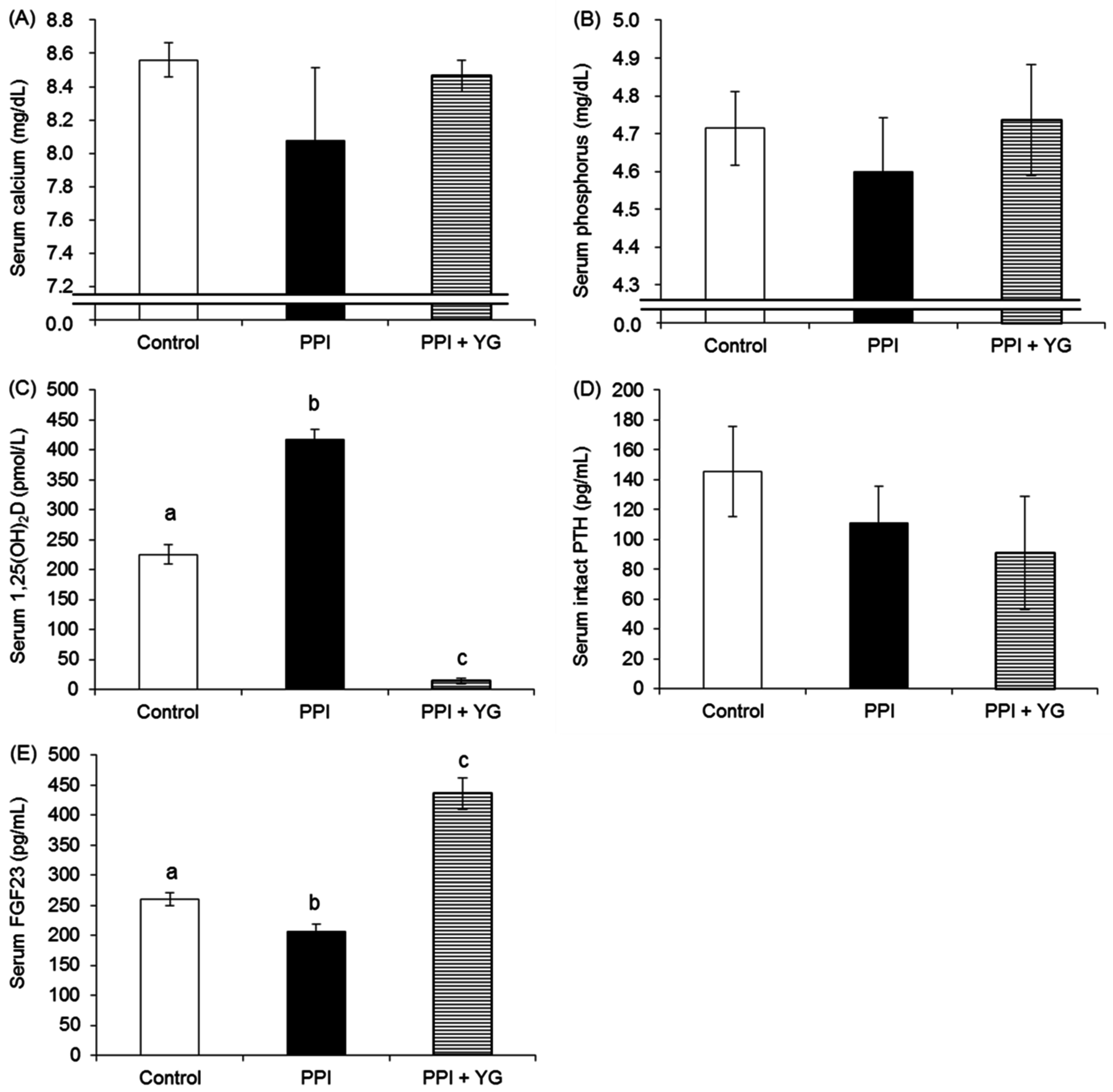
3.4. Biochemical Analysis
3.5. Calcium and Phosphorus Absorption
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomson, A.B.; Sauve, M.D.; Kassam, N.; Kamitakahara, H. Safety of the long-term use of proton pump inhibitors. World J. Gastroenterol. 2010, 16, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif. Tissue Int. 2006, 79, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.A.; Leslie, W.D.; Targownik, L.E.; Papaioannou, A.; Adachi, J.D.; CaMos Research Group. The effect of proton pump inhibitors on fracture risk: Report from the Canadian Multicenter Osteoporosis Study. Osteoporos. Int. 2013, 24, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Heller, D.A.; Ahern, F.M.; Brown, T.V. The relationship between proton pump inhibitor adherence and fracture risk in the elderly. Calcif. Tissue Int. 2014, 94, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Jensen, R.T. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr. Gastroenterol. Rep. 2010, 12, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, S.; Ashida, K.; Maruyama, S.; Komaba, Y.; Kaneko, T.; Yamaji, T. A dairy product fermented by lactobacilli cancels the adverse effects of hypochlorhydria induced by a proton pump inhibitor on bone metabolism in growing rats. Br. J. Nutr. 2011, 106, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, S.; Ashida, K.; Maruyama, S.; Matsukiyo, Y.; Kaneko, T.; Yamaji, T. A combination of a dairy product fermented by lactobacilli and galactooligosaccharides shows additive effects on mineral balances in growing rats with hypochlorhydria induced by a proton pump inhibitor. Biol. Trace Elem. Res. 2013, 153, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Opotowsky, A.R.; Bilezikian, J.P. Racial differences in the effect of early milk consumption on peak and postmenopausal bone mineral density. J. Bone Miner. Res. 2003, 18, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, D.; Lyle, R.M.; Proulx, W.R.; Johnston, C.C.; Weaver, C.M. Previous milk consumption is associated with greater bone density in young women. Am. J. Clin. Nutr. 1999, 69, 1014–1017. [Google Scholar] [PubMed]
- McCabe, L.D.; Martin, B.R.; McCabe, G.P.; Johnston, C.C.; Weaver, C.M.; Peacock, M. Dairy intakes affect bone density in the elderly. Am. J. Clin. Nutr. 2004, 80, 1066–1074. [Google Scholar] [PubMed]
- Chonan, O.; Watanuki, M. Effect of galactooligosaccharides on calcium absorption in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 1995, 41, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Chonan, O.; Watanuki, M. The effect of 6′-galactooligosaccharides on bone mineralization of rats adapted to different levels of dietary calcium. Int. J. Vitam. Nutr. Res. 1996, 66, 244–249. [Google Scholar] [PubMed]
- Van den Heuvel, E.G.; Schoterman, M.H.; Muijs, T. Transgalactooligosaccharides stimulate calcium absorption in postmenopausal women. J. Nutr. 2000, 130, 2938–2942. [Google Scholar] [PubMed]
- Weaver, C.M.; Martin, B.R.; Nakatsu, C.H.; Armstrong, A.P.; Clavijo, A.; McCabe, L.D.; McCabe, G.P.; Duignan, S.; Schoterman, M.H.; van den Heuvel, E.G. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011, 59, 6501–6510. [Google Scholar] [CrossRef] [PubMed]
- Chonan, O.; Matsumoto, K.; Watanuki, M. Effect of galactooligosaccharides on calcium absorption and preventing bone loss in ovariectomized rats. Biosci. Biotechnol. Biochem. 1995, 59, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Porta, A.; Mady, L.J.; Seth, T. Vitamin D and intestinal calcium absorption. Mol. Cell Endocrinol. 2011, 347, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Fleisch, H.; Bonjour, J.P. Role of 1,25-dihydroxyvitamin D3 on intestinal phosphate absorption in rats with a normal vitamin D supply. J. Clin. Investig. 1977, 60, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Schoch, R.D. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit. Rev. Clin. Lab. Sci. 2010, 47, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Toverud, S.U.; Dostal, L.A. Calcium absorption during development: Experimental studies of the rat small intestine. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, S.; Shioyama, M.; Kitade, M.; Nagata, M.; Yamaji, T.; Meiji Co., Ltd., Odawara, Japan. Unpublished data.
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.R.; Dempster, D.W.; Zhou, H.; Shane, E.; Nickolas, T.; Sliney, J., Jr.; Silverberg, S.J.; Bilezikian, J.P. Dynamic and structural properties of the skeleton in hypoparathyroidism. J. Bone Miner. Res. 2008, 23, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Lauretani, F.; Ceda, G.P.; de Vita, F.; Bondi, G.; Corsonello, A.; Cattabiani, C.; Lattanzio, F.; Ruggiero, C.; Nouvenne, A.; et al. Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone 2013, 57, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kirkpantur, A.; Altun, B.; Arici, M.; Turgan, C. Proton pump inhibitor omeprazole use is associated with low bone mineral density in maintenance haemodialysis patients. Int. J. Clin. Pract. 2009, 63, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, S.; Akutsu, M.; Nagata, M. Oral phosphorus supplementation secondarily increases circulating fibroblast growth factor 23 levels at least partially via stimulation of parathyroid hormone secretion. J. Nutr. Sci. Vitaminol. (Tokyo) 2014, 60, 140–144. [Google Scholar] [CrossRef] [PubMed]
- López, I.; Rodríguez-Ortiz, M.E.; Almadén, Y.; Guerrero, F.; de Oca, A.M.; Pineda, C.; Shalhoub, V.; Rodríguez, M.; Aguilera-Tejero, E. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011, 80, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Maeda, A.; Ohtomo, S.; Hirata, M.; Kusano, K.; Kato, S.; Ogata, E.; Segawa, H.; Miyamoto, K.; Fukushima, N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J. Biol. Chem. 2005, 280, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ortiz, M.E.; Lopez, I.; Muñoz-Castañeda, J.R.; Martinez-Moreno, J.M.; Ramírez, A.P.; Pineda, C.; Canalejo, A.; Jaeger, P.; Aguilera-Tejero, E.; Rodriguez, M.; et al. Calcium deficiency reduces circulating levels of FGF23. J. Am. Soc. Nephrol. 2012, 23, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Coudray, C.; Feillet-Coudray, C.; Tressol, J.C.; Gueux, E.; Thien, S.; Jaffrelo, L.; Mazur, A.; Rayssiguier, Y. Stimulatory effect of inulin on intestinal absorption of calcium and magnesium in rats is modulated by dietary calcium intakes short- and long-term balance studies. Eur. J. Nutr. 2005, 44, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, S.; Nakagawa, C.; Kishimoto, Y.; Tagami, H.; Hara, H. Promotive effects of resistant maltodextrin on apparent absorption of calcium, magnesium, iron and zinc in rats. Eur. J. Nutr. 2010, 49, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, M.; Tressol, J.C.; Arnaud, J.; Bornet, F.R.; Bouteloup-Demange, C.; Feillet-Coudray, C.; Brandolini, M.; Ducros, V.; Pépin, D.; Brouns, F.; et al. Effect of short-chain fructooligosaccharides on intestinal calcium absorption and calcium status in postmenopausal women: A stable-isotope study. Am. J. Clin. Nutr. 2003, 77, 449–457. [Google Scholar] [PubMed]
- Demigné, C.; Levrat, M.A.; Younes, H.; Rémésy, C. Interactions between large intestine fermentation and dietary calcium. Eur. J. Clin. Nutr. 1995, 49, S235–S238. [Google Scholar] [PubMed]
- Rémésy, C.; Behr, S.R.; Levrat, M.A.; Demigné, C. Fiber fermentation in the cecum and its physiological consequences. Nutr. Res. 1992, 12, 1235–1244. [Google Scholar] [CrossRef]
- Trinidad, T.P.; Wolever, T.M.; Thompson, L.U. Effects of calcium concentration, acetate, and propionate on calcium absorption in the human distal colon. Nutrition 1999, 15, 529–533. [Google Scholar] [CrossRef]
- Tahiri, M.; Tressol, J.C.; Arnaud, J.; Bornet, F.; Bouteloup-Demange, C.; Feillet-Coudray, C.; Ducros, V.; Pépin, D.; Brouns, F.; Rayssiguier, A.M.; et al. Five-week intake of short-chain fructo-oligosaccharides increases intestinal absorption and status of magnesium in postmenopausal women. J. Bone Miner. Res. 2001, 16, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.S.; Maguire, J.A.; Emmett, M.; Santa Ana, C.A.; Nicar, M.J.; Schiller, L.R.; Fordtran, J.S. Reduction of dietary phosphorus absorption by phosphorus binders. A theoretical, in vitro, and in vivo study. J. Clin. Investig. 1989, 83, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Nordin, B.E. Calcium effects on phosphorus absorption: Implications for the prevention and co-therapy of osteoporosis. J. Am. Coll. Nutr. 2002, 21, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Mineo, H.; Hara, H.; Shigematsu, N.; Okuhara, Y.; Tomita, F. Melibiose, difructose anhydride III and difructose anhydride IV enhance net calcium absorption in rat small and large intestinal epithelium by increasing the passage of tight junctions in vitro. J. Nutr. 2002, 132, 3394–3399. [Google Scholar] [PubMed]
- Koshihara, M.; Katsumata, S.; Uehara, M.; Suzuki, K. Effects of dietary phosphorus intake on bone mineralization and calcium absorption in adult female rats. Biosci. Biotechnol. Biochem. 2005, 69, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Masuyama, R.; Uehara, M.; Suzuki, K. High-phosphorus diet stimulates receptor activator of nuclear factor-kappaB ligand mRNA expression by increasing parathyroid hormone secretion in rats. Br. J. Nutr. 2005, 94, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Masuyama, R.; Koshihara, M.; Matsuzaki, H.; Uehara, M.; Suzuki, K. High phosphorus diet changes phosphorus metabolism regardless of PTH action in rats. Biosci. Biotechnol. Biochem. 2004, 68, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, M.M.; Tillman, I.; Viljakainen, H.T.; Tuukkanen, J.; Peng, Z.; Pekkinen, M.; Lamberg-Allardt, C.J. High dietary phosphate intake reduces bone strength in the growing rat skeleton. J. Bone Miner. Res. 2007, 22, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, R.; Haraikawa, M.; Sogabe, N.; Sugimoto, A.; Kawamura, Y.; Takasugi, S.; Nagata, M.; Nakane, A.; Yamaguchi, A.; Iimura, T.; et al. Retention of bone strength by feeding of milk and dairy products in ovariectomized rats: Involvement of changes in serum levels of 1alpha, 25(OH)2D3 and FGF23. J. Nutr. Biochem. 2013, 24, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Flourié, B. Tolerance to low-digestible carbohydrates: Symptomatology and methods. Br. J. Nutr. 2001, 85, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C. The clinical importance of drug interactions with antiulcer therapy. J. Clin. Gastroenterol. 1990, 12, S54–S63. [Google Scholar] [CrossRef] [PubMed]



| Control (AIN-93M) | Yogurt + GOS | |
|---|---|---|
| Ingredients (g/kg diet) | ||
| Corn starch | 447.10 | 333.03 |
| α-corn starch | 155.00 | 155.00 |
| Casein | 140.00 | 46.67 |
| Lyophilized yogurt | 0.00 | 220.50 |
| Sucrose | 100.00 | 32.25 |
| Soybean oil | 40.00 | 40.00 |
| Cellulose powder | 50.00 | 50.00 |
| AIN-93M mineral premix without calcium and phosphorus | 35.00 | 35.00 |
| Calcium carbonate | 12.41 | 5.54 |
| Potassium phosphate | 8.69 | 2.46 |
| AIN-93 vitamin premix including choline bitartrate | 10.00 | 10.00 |
| l-Cystine | 1.80 | 1.80 |
| GOS ingredient | 0.00 | 67.75 |
| Calculated value (g/kg diet) | ||
| Crude protein | 118.70 | 118.70 |
| Calcium | 5.00 | 5.00 |
| Phosphorus | 3.00 | 3.00 |
| GOS | 0.00 | 50.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takasugi, S.; Shioyama, M.; Kitade, M.; Nagata, M.; Yamaji, T. Effects of Proton Pump Inhibitor Administration and Intake of a Combination of Yogurt and Galactooligosaccharides on Bone and Mineral Metabolism in Rats. Nutrients 2016, 8, 653. https://doi.org/10.3390/nu8100653
Takasugi S, Shioyama M, Kitade M, Nagata M, Yamaji T. Effects of Proton Pump Inhibitor Administration and Intake of a Combination of Yogurt and Galactooligosaccharides on Bone and Mineral Metabolism in Rats. Nutrients. 2016; 8(10):653. https://doi.org/10.3390/nu8100653
Chicago/Turabian StyleTakasugi, Satoshi, Miho Shioyama, Masami Kitade, Masashi Nagata, and Taketo Yamaji. 2016. "Effects of Proton Pump Inhibitor Administration and Intake of a Combination of Yogurt and Galactooligosaccharides on Bone and Mineral Metabolism in Rats" Nutrients 8, no. 10: 653. https://doi.org/10.3390/nu8100653




