Cross-Sectional Associations between Empirically-Derived Dietary Patterns and Indicators of Disease Risk among University Students
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Study Design
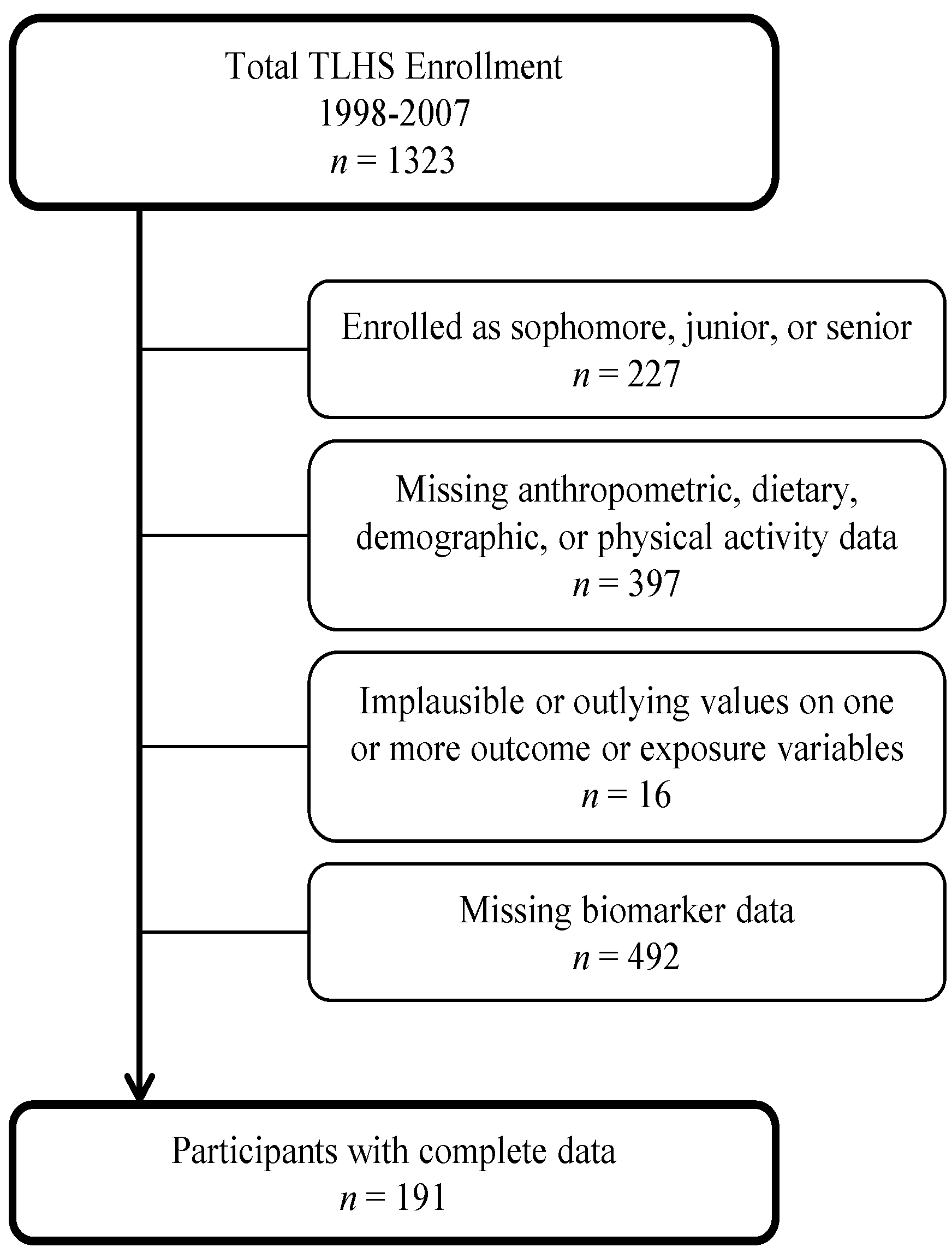
2.2. Assessments
2.2.1. Outcome Variables: Health Indices
Anthropometric Measurements
FFMFemales = _11.03 + 0.70 stature2/resistance + 0.17* weight + 0.02* resistance
Lipid Biomarkers
2.2.2. Exposure/Predictor Variables: Dietary Intake and Dietary Patterns
2.2.3. Confounding and Effect Modifying Variables
Physical Activity
Total Energy
Demographic Variables
2.3. Statistical Analysis
Regression Models
3. Results
3.1. Sample Demographics
| Characteristic | |
|---|---|
| Male (%) | 31.9 |
| Age years | 18.5 ± 0.6 |
| Race/Ethnicity | |
| Caucasian (%) | 76.1 |
| African American (%) | 3.4 |
| Hispanic (%) | 3.7 |
| Other (%) | 16.8 |
| Current smokers (%) | 4.1 |
| Freshman (%) | 100.0 |
| Intention to lose weight (%) | 52.3 |
| Intention to gain weight (%) | 11.2 |
| MET minutes PA per week | 2324.4 ± 2276.6 |
| Total Daily Energy Intake (kJ) | 7824.5 ± 3172.3 |
| Percent Body Fat (%) | 24.3 ± 6.9 |
| BMI (kg/m2) | 22.8 ± 3.0 |
| Lipid Profile (n = 191) | |
| LDL (mg/dL) | 93.8 ± 27.9 |
| HDL (mg/dL) | 54.0 ± 12.0 |
| Total cholesterol (mg/dL) | 167.0 ± 32.6 |
| Triglycerides (mg/dL) | 96.3 ± 42.1 |
3.2. Dietary Pattern Characterization
3.3. Dietary Patterns and Indicators of Disease Risk
| Dietary Pattern | |||||
|---|---|---|---|---|---|
| Western | Prudent | Alcohol | |||
| Foods or Food Groups | Factor Loading | Foods or Food Groups | Factor Loading | Foods or Food Groups | Factor Loading |
| Red meat | 0.66 | Fruit | 0.74 | Liquor | 0.55 |
| French fries | 0.59 | Dark yellow-orange vegetables | 0.60 | Beer | 0.48 |
| Refined grains | 0.58 | Other vegetables | 0.57 | Wine | 0.46 |
| Processed meats | 0.56 | Whole grains | 0.55 | Coffee | 0.38 |
| Snacks | 0.51 | Cruciferous vegetables | 0.52 | Low-energy drinks | 0.30 |
| Potatoes | 0.49 | Green leafy vegetables | 0.51 | Legumes | −0.38 |
| Pizza | 0.48 | Legumes | 0.51 | Other vegetables | −0.40 |
| Butter | 0.45 | Non-cream soups | 0.47 | ||
| High energy drinks | 0.45 | Tomatoes | 0.44 | ||
| Pasta | 0.45 | Yogurt | 0.43 | ||
| Creamy dressings | 0.42 | Nuts | 0.38 | ||
| High fat dairy products | 0.42 | Breakfast cereal | 0.35 | ||
| Ice cream | 0.42 | Fish and seafood | 0.34 | ||
| Poultry | 0.42 | ||||
| Margarine | 0.38 | ||||
| Other fats and oils | 0.38 | ||||
| Fruit juice | 0.37 | ||||
| Sweets and desserts | 0.31 | ||||
| Dietary Pattern | ||||||||
|---|---|---|---|---|---|---|---|---|
| Western | Prudent | Alcohol | ||||||
| Foods/Food Groups | Daily Intake | Foods/Food Groups | Daily Intake (Cups) | Food/Food Groups | Daily Intake (fl oz) | |||
| Q 1 | Q 4 | Q 1 | Q 4 | Q 1 | Q 4 | |||
| Red meat (ounces) | 0.25 | 2.64 | Fruit 3 | 0.25 | 1.31 | Liquor | 0.06 | 0.63 |
| French fries (cups) | 0.07 | 0.68 | Dark yellow-orange vegetables 4 | 0.04 | 0.27 | Beer | 0.00 | 5.4 |
| Refined grains (pieces) 1 | 0.56 | 1.89 | Other vegetables | 0.04 | 0.29 | Wine | 0.05 | 0.40 |
| Processed meats (pieces) 2 | 0.12 | 1.02 | whole grains 5 | 0.11 | 0.52 | Coffee | 0.36 | 4.68 |
| Snacks (cups) | 0.07 | 0.33 | Cruciferous vegetables | 0.03 | 0.25 | Low-energy drinks | 0.72 | 7.44 |
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | se | p | β | se | p | β | se | p | |
| Outcomes | Western | ||||||||
| Body fat (%) | (p-interaction = 0.0426) | −0.08 | 0.37 | 0.818 | 0.31 | 0.36 | 0.381 | ||
| BMI (kg/m2) | (p-interaction = 0.0247) | −0.10 | 0.21 | 0.640 | 0.21 | 0.20 | 0.305 | ||
| HDL (mg/dL) | (p-interaction = 0.0128) | −1.89 | 1.58 | 0.234 | −1.55 | 1.60 | 0.336 | ||
| LDL(mg/dL) | (p-interaction = 0.0460) | (p-interaction = 0.0134) | (p-interaction = 0.0148) | ||||||
| Triglycerides(mg/dL) | (p-interaction = 0.0210) | (p-interaction = 0.0190) | (p-interaction = 0.0139) | ||||||
| Total Cholesterol(mg/dL) | 1.40 | 2.46 | 0.569 | (p-interaction = 0.0271) | (p-interaction = 0.0303) | ||||
| Prudent | |||||||||
| Body fat (%) | 0.36 | 0.26 | 0.176 | −0.26 | 0.22 | 0.236 | −0.42 | 0.21 | 0.046 * |
| BMI (kg/m2) | −0.18 | 0.11 | 0.114 | −0.15 | 0.12 | 0.217 | −0.29 | 0.12 | 0.017 * |
| HDL (mg/dL) | 2.17 | 0.88 | 0.014 * | 1.29 | 0.92 | 0.165 | 1.19 | 0.96 | 0.216 |
| LDL(mg/dL) | 1.48 | 2.07 | 0.476 | 1.03 | 2.34 | 0.660 | 0.79 | 2.47 | 0.748 |
| Triglycerides(mg/dL) | 0.18 | 3.13 | 0.954 | −1.88 | 3.48 | 0.590 | −1.96 | 3.55 | 0.581 |
| Total Cholesterol(mg/dL) | 3.70 | 2.41 | 0.126 | 1.95 | 2.68 | 0.467 | 1.60 | 2.82 | 0.572 |
| Alcohol | |||||||||
| Body fat (%) | 0.46 | 0.27 | 0.083 + | 0.33 | 0.22 | 0.130 | 0.15 | 0.21 | 0.482 |
| BMI (kg/m2) | 0.19 | 0.12 | 0.105 | 0.24 | 0.12 | 0.052 + | 0.13 | 0.12 | 0.280 |
| HDL (mg/dL) | 2.49 | 0.92 | 0.007 * | 1.94 | 0.92 | 0.036 * | 2.16 | 0.93 | 0.021 * |
| LDL(mg/dL) | −4.07 | 2.16 | 0.061 + | −5.43 | 2.31 | 0.020 * | −5.46 | 2.38 | 0.023 * |
| Triglycerides (mg/dL) | 1.39 | 3.29 | 0.673 | −0.33 | 3.49 | 0.924 | −0.59 | 3.48 | 0.866 |
| Total Cholesterol (mg/dL) | −1.29 | 2.55 | 0.612 | −3.55 | 2.67 | 0.186 | −3.40 | 2.75 | 0.218 |
| % Body Fat | BMI | HDL | LDL | Triglycerides | Total Cholesterol | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Males | 0.58 (0.35), p = 0.1 | 0.21 (0.21), p = 0.30 | −0.72 (1.31), p = 0.58 | 8.34 (3.4), p = 0.02 * | 14.1 (4.54), p = 0.003 * | NA |
| Females | −0.21 (0.30), p = 0.5 | −0.21 (0.17), p = 0.21 | 2.48 (1.37), p = 0.07 + | −2.58 (3.28), p = 0.43 | −2.43 (5.22), p = 0.64 | NA |
| Model 2 | ||||||
| Males | NA | NA | NA | 13.0 (6.55), p = 0.05 + | 24.7 (8.59), p = 0.01 * | 14.5 (6.99), p = 0.04 * |
| Females | NA | NA | NA | −0.84 (5.26), p = 0.87 | −1.83 (8.14), p = 0.82 | −1.08 (6.24), p = 0.86 |
| Model 3 | ||||||
| Males | NA | NA | NA | 12.2 (6.75), p = 0.08 + | 23.7 (8.19), p = 0.005 * | 13.98 (7.22), p = 0.06 + |
| Females | NA | NA | NA | −1.38 (5.53), p = 0.80 | −4.98 (8.28), p = 0.55 | −1.88(6.54), p = 0.77 |
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- VanKim, N.A.; Larson, N.; Laska, M.N. Emerging adulthood: A critical age for preventing excess weight gain? Adolesc. Med. State Art Rev. 2012, 23, 571–588. [Google Scholar] [PubMed]
- Nelson, M.C.; Story, M.; Larson, N.I.; Neumark-Sztainer, D.; Lytle, L.A. Emerging adulthood and college-aged youth: An overlooked age for weight-related behavior change. Obesity 2008, 16, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M. Patterns of beverage use across the lifecycle. Physiol. Behav. 2010, 100, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, J.; Gillespie, C.; Seymour, J.; Serdula, M.; Blanck, H.M. Fruit and vegetable intake among adolescents and adults in the United States: Percentage meeting individualized recommendations. Medscape J. Med. 2009, 11, 26. [Google Scholar] [PubMed]
- Lytle, L.A.; Seifert, S.; Greenstein, J.; McGovern, P. How do children’s eating patterns and food choices change over time? Results from a cohort study. Am. J. Health Promot. 2000, 14, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Demory-Luce, D.; Morales, M.; Nicklas, T.; Baranowski, T.; Zakeri, I.; Berenson, G. Changes in food group consumption patterns from childhood to young adulthood: The Bogalusa Heart Study. J. Am. Diet. Assoc. 2004, 104, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.I.; Neumark-Sztainer, D.; Hannan, P.J.; Story, M. Trends in adolescent fruit and vegetable consumption, 1999–2004: Project EAT. Am. J. Prev. Med. 2007, 32, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Niemeier, H.M.; Raynor, H.A.; Lloyd-Richardson, E.E.; Rogers, M.L.; Wing, R.R. Fast food consumption and breakfast skipping: Predictors of weight gain from adolescence to adulthood in a nationally representative sample. J. Adolesc. Health 2006, 39, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, S.; Fryar, C.D.; Ogden, C.L. Caloric Intake from Fast Food among Children and Adolescents in the United States, 2011–2012. NCHS Data Brief 2015, 213, 1–8. [Google Scholar] [PubMed]
- U.S. Department of Education Institute of Education Sciences National Center for Education Statistics. Fast Facts: Back to School Statistics. Available online: http://nces.ed.gov/fastfacts/display.asp?id=372 (accessed on 12 December 2015).
- Vella-Zarb, R.A.; Elgar, F.J. The “freshman 5”: A meta-analysis of weight gain in the freshman year of college. J. Am. Coll. Health 2009, 58, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Das, B.M.; Evans, E.M.; Dishman, R.K. Change in weight and adiposity in college students: A systematic review and meta-analysis. Am. J. Prev. Med. 2014, 47, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Vadeboncoeur, C.; Townsend, N.; Foster, C. A meta-analysis of weight gain in first year university students: Is freshman 15 a myth? BMC Obes. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Bodenlos, J.S.; Gengarelly, K.; Smith, R. Gender differences in freshmen weight gain. Eating Behav. 2015, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kapinos, K.A.; Yakusheva, O.; Eisenberg, D. Obesogenic environmental influences on young adults: Evidence from college dormitory assignments. Econ. Hum. Biol. 2014, 12, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Yakusheva, O.; Kapinos, K.; Weiss, M. Peer effects and the freshman 15: Evidence from a natural experiment. Econ. Hum. Biol. 2011, 9, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Gillen, M.M.; Lefkowitz, E.S. The “freshman 15”: Trends and predictors in a sample of multiethnic men and women. Eating Behav. 2011, 12, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; Cecil, J.; Higgs, S.; Hill, A.; Hetherington, M. Susceptibility to weight gain. Eating behaviour traits and physical activity as predictors of weight gain during the first year of university. Appetite 2012, 58, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.R.; Annunziato, R.A.; Markowitz, J.T.; Didie, E.; Bellace, D.L.; Riddell, L.; Maille, C.; McKinney, S.; Stice, E. Multiple types of dieting prospectively predict weight gain during the freshman year of college. Appetite 2006, 47, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Pliner, P.; Saunders, T. Vulnerability to freshman weight gain as a function of dietary restraint and residence. Physiol. Behav. 2008, 93, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Provencher, V.; Polivy, J.; Wintre, M.G.; Pratt, M.W.; Pancer, S.M.; Birnie-Lefcovitch, S.; Adams, G.R. Who gains or who loses weight? Psychosocial factors among first-year university students. Physiol. Behav. 2009, 96, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.R. Gender, residence and ethnicity affect freshman BMI and dietary habits. Am. J. Health Behav. 2010, 34, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.E.; Laska, M.N. Campus food and beverage purchases are associated with indicators of diet quality in college students living off campus. Am. J. Health Promot. 2013, 28, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.; Chang, K. College students’ attitude toward body weight control, health-related lifestyle and dietary behavior by self-perception on body image and obesity index. J. Korean Soc. Food Sci. Nutr. 2005, 34, 1559–1565. [Google Scholar]
- Querido, J.; Morrell, J. How does inaccurate perception of weight compared to actual BMI status affect the diet score of college students? FASEB J. 2015, 29, LB315. [Google Scholar]
- Brevard, P.B.; Ricketts, C.D. Residence of college students affects dietary intake, physical activity, and serum lipid levels. J. Am. Diet. Assoc. 1996, 96, 35–38. [Google Scholar] [CrossRef]
- Anding, J.D.; Suminski, R.R.; Boss, L. Dietary intake, body mass index, exercise, and alcohol: Are college women following the dietary guidelines for Americans? J. Am. Coll. Health 2001, 49, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Song, W.O.; Schemmel, R.A.; Hoerr, S.M. What do college students eat? Food selection and meal pattern. Nutr. Res. 1994, 14, 1143–1153. [Google Scholar] [CrossRef]
- Dinger, M.K.; Waigandt, A. Dietary intake and physical activity behaviors of male and female college students. Am. J. Health Promot. 1997, 11, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Haberman, S.; Luffey, D. Weighing in college students’ diet and exercise behaviors. J. Am. Coll. Health 1998, 46, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Laska, M.N. Nutrition label use partially mediates the relationship between attitude toward healthy eating and overall dietary quality among college students. J. Acad. Nutr. Dietet. 2012, 112, 414–418. [Google Scholar] [CrossRef]
- Block, J.P.; Gillman, M.W.; Linakis, S.K.; Goldman, R.E. “If It Tastes Good, I’m Drinking It”: Qualitative Study of Beverage Consumption Among College Students. J. Adolesc. Health 2013, 52, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Tully, L.R.; Morrell, J.S.; Mastriano, C.M. Multivitamin/mineral usage, MyPlate adherence, and diet quality among college students. FASEB J. 2013, 27, lb255. [Google Scholar]
- Kelly, N.R.; Mazzeo, S.E.; Bean, M.K. Systematic review of dietary interventions with college students: Directions for future research and practice. J. Nutr. Educ. Behav. 2013, 45, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Muller, D.; Tucker, K.L. Associations of empirically derived eating patterns with plasma lipid biomarkers: A comparison of factor and cluster analysis methods. Am. J. Clin. Nutr. 2004, 80, 759–767. [Google Scholar] [PubMed]
- Economos, C.D.; Hildebrandt, M.L.; Hyatt, R.R. College freshman stress and weight change: Differences by gender. Am. J. Health Behav. 2008, 32, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Chumlea, W.C.; Heymsfield, S.B.; Lukaski, H.C.; Schoeller, D.; Friedl, K.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Hubbard, V.S. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am. J. Clin. Nutr. 2003, 77, 331–340. [Google Scholar] [PubMed]
- Fukuyama, N.; Homma, K.; Wakana, N.; Kudo, K.; Suyama, A.; Ohazama, H.; Tsuji, C.; Ishiwata, K.; Eguchi, Y.; Nakazawa, H.; Tanaka, E. Validation of the friedewald equation for evaluation of plasma LDL-cholesterol. J. Clin. Biochem. Nutr. 2008, 43, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Center, N.C. Minnesota Nutrition Data System (NDS) Software; University of Minnesota: Minneapolis, MN, USA, 1992. [Google Scholar]
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000, 72, 912–921. [Google Scholar] [PubMed]
- Finley, C.E.; LaMonte, M.J.; Waslien, C.I.; Barlow, C.E.; Blair, S.N.; Nichaman, M.Z. Cardiorespiratory fitness, macronutrient intake, and the metabolic syndrome: The Aerobics Center Longitudinal Study. J. Am. Diet. Assoc. 2006, 106, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, J.C.; Wickel, E.E.; Welk, G.J.; Blair, S.N. Relationship between adolescent fitness and fatness and cardiovascular disease risk factors in adulthood: The Aerobics Center Longitudinal Study (ACLS). Am. Heart J. 2005, 149, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Stofan, J.R.; DiPietro, L.; Davis, D.; Kohl, H.W., III; Blair, S.N. Physical activity patterns associated with cardiorespiratory fitness and reduced mortality: The Aerobics Center Longitudinal Study. Am. J. Public Health 1998, 88, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.A.; Collins, J.L.; Warren, C.; Kann, L.; Gold, R.; Clayton, S.; Ross, J.G.; Kolbe, L.J. Results from the 1995 national college health risk behavior survey. J. Am. Coll. Health 1997, 46, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, L.J.; Kann, L.; Collins, J.L. Overview of the youth risk behavior surveillance system. Public Health Rep. 1993, 108 (Suppl. S1), 2–10. [Google Scholar] [PubMed]
- Brener, N.D.; Kann, L.; McManus, T.; Kinchen, S.A.; Sundberg, E.C.; Ross, J.G. Reliability of the 1999 youth risk behavior survey questionnaire. J. Adolesc. Health 2002, 31, 336–342. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 591–611. [Google Scholar] [CrossRef]
- Ning, H.; van Horn, L.; Shay, C.M.; Lloyd-Jones, D.M. Associations of dietary fiber intake with long-term predicted cardiovascular disease risk and C-reactive protein levels (from the National Health and Nutrition Examination Survey Data (2005–2010)). Am. J. Cardiol. 2014, 113, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Fallaize, R.; Forster, H.; Macready, A.L.; Walsh, M.C.; Mathers, J.C.; Brennan, L.; Gibney, E.R.; Gibney, M.J.; Lovegrove, J.A. Online dietary intake estimation: Reproducibility and validity of the Food4Me food frequency questionnaire against a 4-day weighed food record. J. Med. Internet Res. 2014, 16. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Exploratory data analysis. Addison-Wesley: Boston, MA, USA, 1977. [Google Scholar]
- Fung, T.T.; Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Dietary patterns and the risk of coronary heart disease in women. Arch. Intern. Med. 2001, 161, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.; Hu, F.B.; Fuchs, C.; Giovannucci, E.; Hunter, D.J.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Major dietary patterns and the risk of colorectal cancer in women. Arch. Intern. Med. 2003, 163, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Dietary patterns and risk for type 2 diabetes mellitus in US men. Ann. Intern. Med. 2002, 136, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Pasco, J.A.; Mykletun, A.; Williams, L.J.; Hodge, A.M.; O’Reilly, S.L.; Nicholson, G.C.; Kotowicz, M.A.; Berk, M. Association of Western and traditional diets with depression and anxiety in women. Am. J. Psychiatry 2010, 167, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Kerver, J.M.; Yang, E.J.; Bianchi, L.; Song, W.O. Dietary patterns associated with risk factors for cardiovascular disease in healthy US adults. Am. J. Clin. Nutr. 2003, 78, 1103–1110. [Google Scholar] [PubMed]
- Heidemann, C.; Schulze, M.B.; Franco, O.H.; van Dam, R.M.; Mantzoros, C.S.; Hu, F.B. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 2008, 118, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am. J. Clin. Nutr. 2007, 85, 910–918. [Google Scholar] [PubMed]
- Schulze, M.B.; Fung, T.T.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dietary patterns and changes in body weight in women. Obesity 2006, 14, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Manson, J.E.; Willett, W.C.; Meigs, J.B.; Weikert, C.; Heidemann, C.; Colditz, G.A.; Hu, F.B. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am. J. Clin. Nutr. 2005, 82, 675–684. [Google Scholar] [PubMed]
- Brennan, S.F.; Cantwell, M.M.; Cardwell, C.R.; Velentzis, L.S.; Woodside, J.V. Dietary patterns and breast cancer risk: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.L.; Robinson, M.; Smith, G.J.; Ambrosini, G.L.; Piek, J.P.; Oddy, W.H. ADHD is associated with a “Western” dietary pattern in adolescents. J. Atte. Disord. 2011, 15, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; Robinson, M.; Ambrosini, G.L.; Therese, A.; de Klerk, N.H.; Beilin, L.J.; Silburn, S.R.; Zubrick, S.R.; Stanley, F.J. The association between dietary patterns and mental health in early adolescence. Prev. Med. 2009, 49, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.L.; Huang, R.; Mori, T.A.; Hands, B.P.; O’Sullivan, T.A.; de Klerk, N.H.; Beilin, L.J.; Oddy, W.H. Dietary patterns and markers for the metabolic syndrome in Australian adolescents. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.; Hosig, K.; Davy, B.; Serrano, E.; Davis, L. Whole-grain intake is associated with body mass index in college students. J. Nutr. Educ. Behav. 2007, 39, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Zamora, D.; Gordon-Larsen, P.; Jacobs, D.R., Jr.; Popkin, B.M. Diet quality and weight gain among black and white young adults: The Coronary Artery Risk Development in Young Adults (CARDIA) Study (1985–2005). Am. J. Clin. Nutr. 2010, 92, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, L.D.; Spector, P.; Stevens, M.J.; Schmidt, M.M.; Schreiber, G.B.; Striegel-Moore, R.H.; Wang, M.C.; Crawford, P.B. Dietary patterns in adolescence are related to adiposity in young adulthood in black and white females. J. Nutr. 2007, 137, 399–406. [Google Scholar] [PubMed]
- Fung, T.T.; Rimm, E.B.; Spiegelman, D.; Rifai, N.; Tofler, G.H.; Willett, W.C.; Hu, F.B. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Clin. Nutr. 2001, 73, 61–67. [Google Scholar] [PubMed]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Arranz, S.; Lamuela-Raventos, R.M.; Estruch, R. Effects of wine, alcohol and polyphenols on cardiovascular disease risk factors: Evidences from human studies. Alcohol Alcohol. 2013, 48, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. Dietary patterns and their association with food and nutrient intake in the European Prospective Investigation into Cancer and Nutrition (EPIC)—Potsdam study. Br. J. Nutr. 2001, 85, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Engeset, D.; Alsaker, E.; Ciampi, A.; Lund, E. Dietary patterns and lifestyle factors in the Norwegian EPIC cohort: The Norwegian Women and Cancer (NOWAC) study. Eur. J. Clin. Nutr. 2005, 59, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Garcia-Aymerich, J.; Monier, F.; le Moual, N.; de Batlle, J.; Miranda, G.; Pison, C.; Romieu, I.; Kauffmann, F.; Maccario, J. Assessment of dietary patterns in nutritional epidemiology: Principal component analysis compared with confirmatory factor analysis. Am. J. Clin. Nutr. 2012, 96, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, B.; Peleteiro, B.; Lunet, N. Dietary patterns and colorectal cancer: Systematic review and meta-analysis. Eur. J. Cancer Prev. 2012, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Hingson, R. The burden of alcohol use: Excessive alcohol consumption and related consequences among college students. Alcohol Res. Curr. Rev. 2014, 35, 201–218. [Google Scholar]
- Malinauskas, B.M.; Aeby, V.G.; Overton, R.F.; Carpenter-Aeby, T.; Barber-Heidal, K. A survey of energy drink consumption patterns among college students. Nutr. J. 2007, 6, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lenk, K.M.; Erickson, D.J.; Nelson, T.F.; Winters, K.C.; Toomey, T.L. Alcohol policies and practices among four-year colleges in the United States: Prevalence and patterns. J. Stud. Alcohol. Drugs 2012, 73, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.E.; Whiteman, S.; Piazza-Gardner, A.K.; Jensen, A.C. Gender differences in the associations among body mass index, weight loss, exercise, and drinking among college students. J. Am. Coll. Health 2013, 61, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Antin, T.M.; Paschall, M.J. Weight perception, weight change intentions, and alcohol use among young adults. Body Image 2011, 8, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Weeder, S. Binge drinking and disordered eating in college students. J. Am. Acad. Nurse Pract. 2011, 23, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.C.; Lust, K.; Story, M.; Ehlinger, E. Alcohol use, eating patterns, and weight behaviors in a university population. Am. J. Health Behav. 2009, 33, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Sayon-Orea, C.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Alcohol consumption and body weight: A systematic review. Nutr. Rev. 2011, 69, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.A.; Goldstein, R.B.; Saha, T.D.; Grant, B.F. Changes in alcohol consumption: United States, 2001–2002 to 2012–2013. Drug Alcohol Depend. 2015, 148, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Yi, H.; Faden, V.B. Surveillance Report# 101: Trends in Underage Drinking in the United States, 1991–2013; Division of Epidemiology and Prevention Research, Alcohol Epidemiologic Data System; NIAAA: Rockville, MD, USA, 2015. [Google Scholar]
- Bryant, J.B.; Darkes, J.; Rahal, C. College students’ compensatory eating and behaviors in response to alcohol consumption. J. Am. Coll. Health 2012, 60, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ma, D.; Zhang, Y.; Liu, Z.; Wang, P. The effect of coffee consumption on serum lipids: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2012, 66, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Scientific Report of the 2015 Dietary Guidelines for Americans Advisory Committee; US Department of Agriculture; US Department of Health and Human Services: Washington DC, WA, USA, 2015.
- Deshmukh-Taskar, P.; Nicklas, T.; Morales, M.; Yang, S.; Zakeri, I.; Berenson, G. Tracking of overweight status from childhood to young adulthood: The Bogalusa Heart Study. Eur. J. Clin. Nutr. 2006, 60, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, D.A.; Halbmaier, C.A.; Mrdjenovic, G. The freshman weight gain: A model for the study of the epidemic of obesity. Int. J. Obes. 2004, 28, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Brunt, A.R.; Rhee, Y.S. Obesity and lifestyle in US college students related to living arrangemeents. Appetite 2008, 51, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Small, M.; Bailey-Davis, L.; Morgan, N.; Maggs, J. Changes in eating and physical activity behaviors across seven semesters of college: Living on or off campus matters. Health Educ. Behav. 2013, 40, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, J.C.; Welk, G.J.; Wickel, E.E.; Blair, S.N. Stability of variables associated with the metabolic syndrome from adolescence to adulthood: The Aerobics Center Longitudinal Study. Am. J. Hum. Biol. 2004, 16, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Tapsell, L.C. Food synergy:The key to a healthy diet. Proc. Nutr. Soc. 2013, 72, 200–206. [Google Scholar]
- McCann, S.E.; Marshall, J.R.; Brasure, J.R.; Graham, S.; Freudenheim, J.L. Analysis of patterns of food intake in nutritional epidemiology: Food classification in principal components analysis and the subsequent impact on estimates for endometrial cancer. Public Health Nutr. 2001, 4, 989–997. [Google Scholar]
- Hamer, M.; McNaughton, S.; Bates, C.; Mishra, G. Dietary patterns, assessed from a weighed food record, and survival among elderly participants from the United Kingdom. Eur. J. Clin. Nutr. 2010, 64, 853–861. [Google Scholar]
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000, 72, 912–921. [Google Scholar]
- Michels, K.B.; Schulze, M.B. Can dietary patterns help us detect diet-disease associations? Nutr. Res. Rev. 2005, 18, 241–248. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blondin, S.A.; Mueller, M.P.; Bakun, P.J.; Choumenkovitch, S.F.; Tucker, K.L.; Economos, C.D. Cross-Sectional Associations between Empirically-Derived Dietary Patterns and Indicators of Disease Risk among University Students. Nutrients 2016, 8, 3. https://doi.org/10.3390/nu8010003
Blondin SA, Mueller MP, Bakun PJ, Choumenkovitch SF, Tucker KL, Economos CD. Cross-Sectional Associations between Empirically-Derived Dietary Patterns and Indicators of Disease Risk among University Students. Nutrients. 2016; 8(1):3. https://doi.org/10.3390/nu8010003
Chicago/Turabian StyleBlondin, Stacy A., Megan P. Mueller, Peter J. Bakun, Silvina F. Choumenkovitch, Katherine L. Tucker, and Christina D. Economos. 2016. "Cross-Sectional Associations between Empirically-Derived Dietary Patterns and Indicators of Disease Risk among University Students" Nutrients 8, no. 1: 3. https://doi.org/10.3390/nu8010003





