Additional Value of CH4 Measurement in a Combined 13C/H2 Lactose Malabsorption Breath Test: A Retrospective Analysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Methods
2.2.1. 13C/H2 Breath Test
2.2.2. Analytical Methods
2.2.3. Standard Interpretation of Breath Test Results
2.2.4. Additional Breath Test Parameters
2.2.5. Statistics
3. Results
3.1. Patient Characteristics
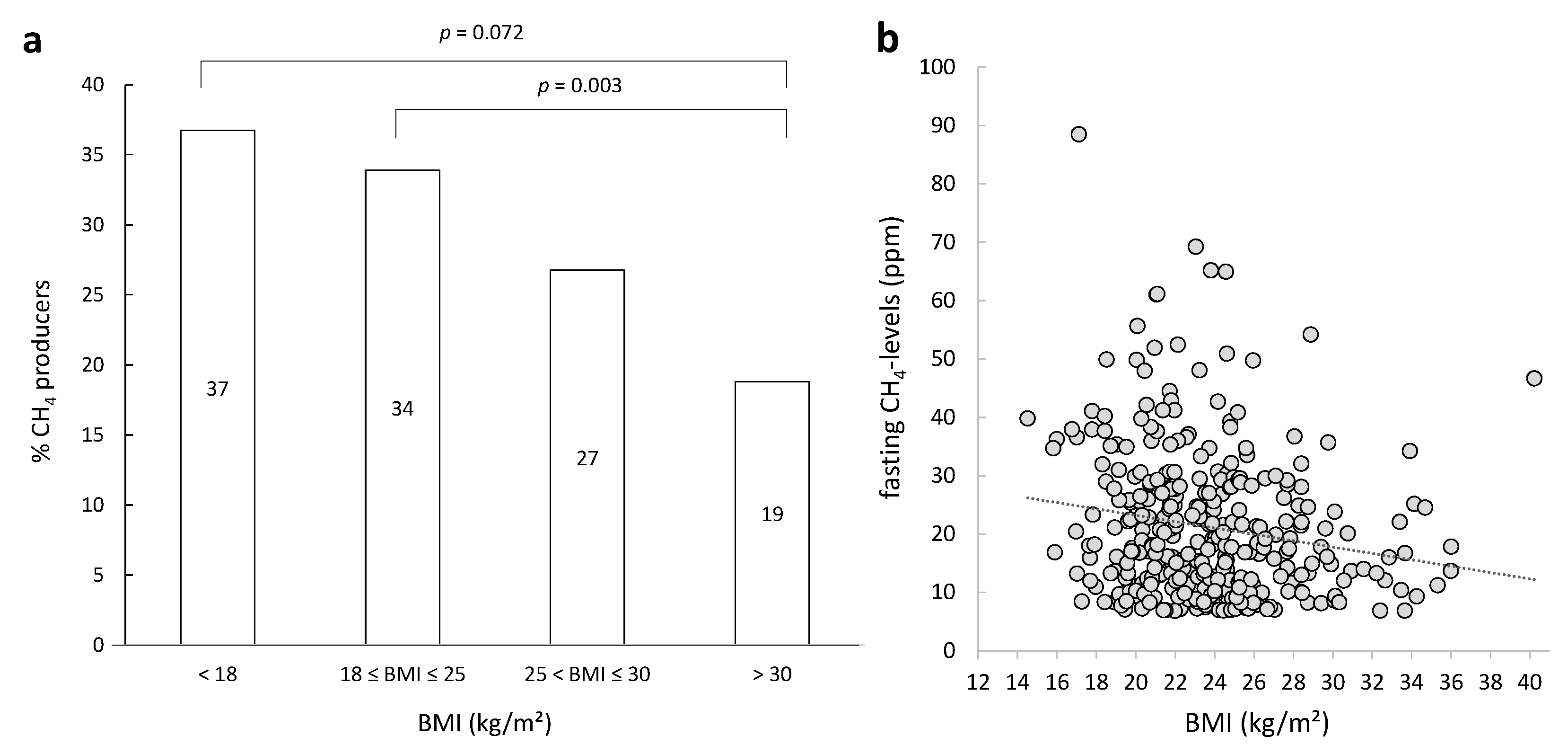
3.2. Diagnosis
3.3. Occurrence of Symptoms on the Day of the Breath Test
| Diagnosis | Number of Subjects | Cumulative 13C-Excretion after 4 h (%) | Maximum H2-Excretion (ppm) | Maximum CH4-Excretion (ppm) |
|---|---|---|---|---|
| Lactase deficiency | All subjects (314) | 11.2 (8.4–13.0) | 21.8 (3.7–96.5) | 0.3 (0.1–1.7) |
| Discomfort + (214) | 10.7 (7.6–12.5) a | 60.9 (6.9–116.0) b | 0.3 (0.1–1.6) | |
| Discomfort − (100) | 12.1 (9.4–13.7) a | 4.4 (1.8–17.6) b | 0.5 (0.1–2.0) | |
| Lactose malabsorption/SIBO | All subjects (138) | 19.0 (16.7–21.8) | 48.1 (31.2–91.3) | 0.2 (0.1–0.7) |
| Discomfort + (97) | 18.7 (16.5–21.4) | 53.0 (34.3–103.0) c | 0.2 (0.1–0.8) | |
| Discomfort − (41) | 20.1 (18.0–22.2) | 37.0 (27.0–57.3) c | 0.2 (0.1–0.5) | |
| Normal test | All subjects (599) | 19.6 (17.4–33.2) | 3.9 (1.5–9.5) | 0.3 (0.1–2.4) |
| Discomfort + (305) | 20.2 (17.4–32.1) | 3.5 (1.5–9.6) | 0.3 (0.1–2.1) | |
| Discomfort − (294) | 19.1 (17.2–33.2) | 4.1 (1.4–9.1) | 0.3 (0.1–2.5) |
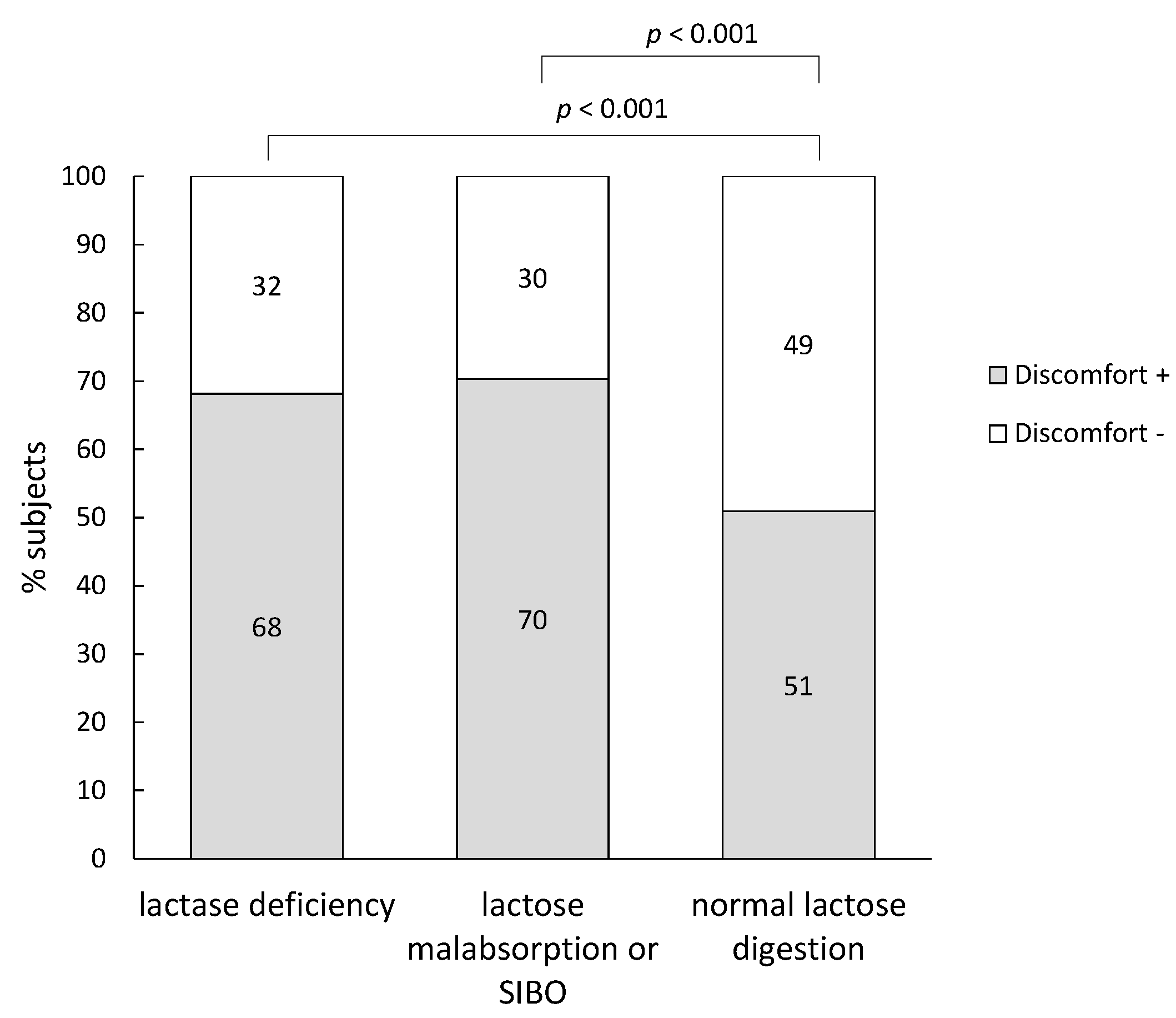
3.4. Subjects Diagnosed with Lactase Deficiency
| Gas production | Number of Subjects | Cumulative 13C-Excretion after 4 h (%) | Maximum H2-Excretion (ppm) | Maximum CH4-Excretion (ppm) |
|---|---|---|---|---|
| H2-producers | All subjects (139) | 9.4 (6.3–11.7) | 95.5 (52.3–144.5) | 0.2 (0.1–0.6) |
| Discomfort + (117) | 9.58 (6.0–12.7) | 101.5 (64.7–148.3) a | 0.2 (0.1–0.5) | |
| Discomfort − (22) | 8.8 (6.0–12.7) | 50.7 (30.6–90.6) a | 0.3 (0.1–0.9) | |
| CH4-producers | All subjects (25) | 11.8 (11.1–13.4) | 3.0 (1.0–9.2) | 11.2 (6.8–15.6) |
| Discomfort + (10) | 11.6 (11.1–12.5) | 3.2 (0.6–8.4) | 11.3 (6.9–14.8) | |
| Discomfort − (15) | 12.7 (10.9–13.5) | 2.5 (1.6–8.2) | 11.2 (7.4–19.8) | |
| H2- and CH4-producers | All subjects (21) | 12.4 (9.4–13.0) | 94.5 (74.5–116.0) | 14.8 (7.7–24.2) |
| Discomfort + (21) | 12.4 (9.4–13.1) | 94.5 (74.5–116.0) | 14.8 (7.7–24.2) | |
| Discomfort − (0) | - | - | - | |
| Non-producers | All subjects (129) | 12.2 (10.1–13.6) | 3.7 (1.5–7.5) | 0.2 (0.1–0.9) |
| Discomfort + (66) | 11.8 (9.7–13.1) | 4.6 (1.5–8.1) | 0.2 (0.1–0.6) | |
| Discomfort − (63) | 12.5 (10.3–13.8) | 3.0 (1.3–7.3) | 0.3 (0.1–1.0) |

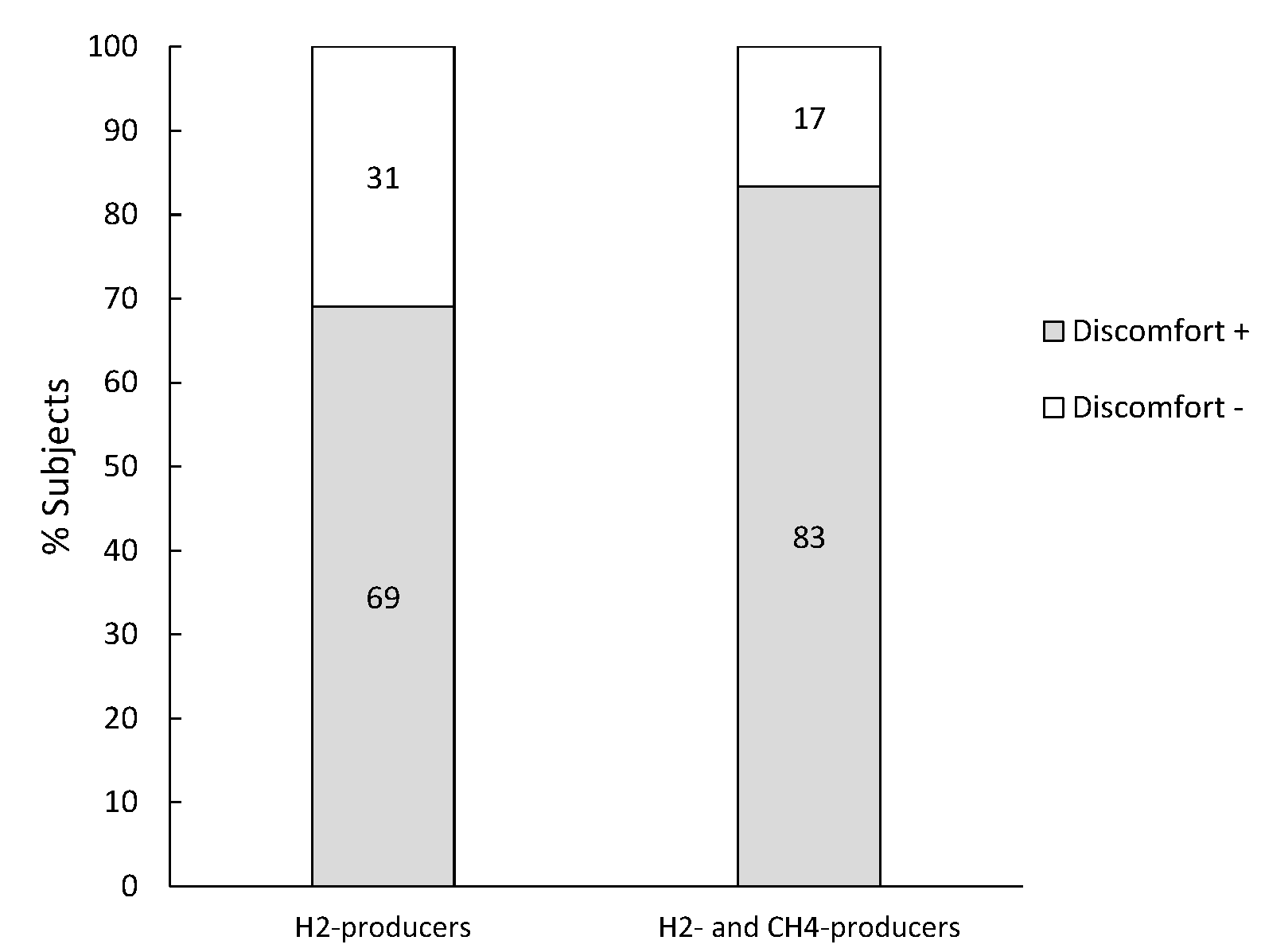
3.5. Subjects Diagnosed with Lactose Malabsorption or Bacterial Overgrowth
| Gas production | Number of Subjects | Cumulative 13C-Excretion after 4 h (%) | Maximum H2-Excretion (ppm) | Maximum CH4-Excretion (ppm) |
|---|---|---|---|---|
| H2-producers | All subjects (126) | 19.0 (16.7–21.6) | 48.5 (31.2–91.3) | 0.2 (0.1–0.4) |
| Discomfort + (87) | 18.3 (16.4–21.0) a | 57.7 (35.2–103.2) b | 0.2 (0.1–0.4) | |
| Discomfort − (39) | 20.1 (18.1–22.1) a | 38.3 (27.5–57.6) b | 0.2 (0.1–0.4) | |
| H2- and CH4-producers | All subjects (12) | 20.8 (18.2–23.8) | 41.0 (31.8–100.0) | 20.9 (11.5–31.1) |
| Discomfort + (10) | 20.8 (19.1–24.0) | 47.0 (35.1–100.0) c | 17.3 (10.5–29.9) | |
| Discomfort − (2) | 19.3 (17.1–21.5) | 25.0 (24.3–25.8) c | 46.8 (34.3–59.3) |
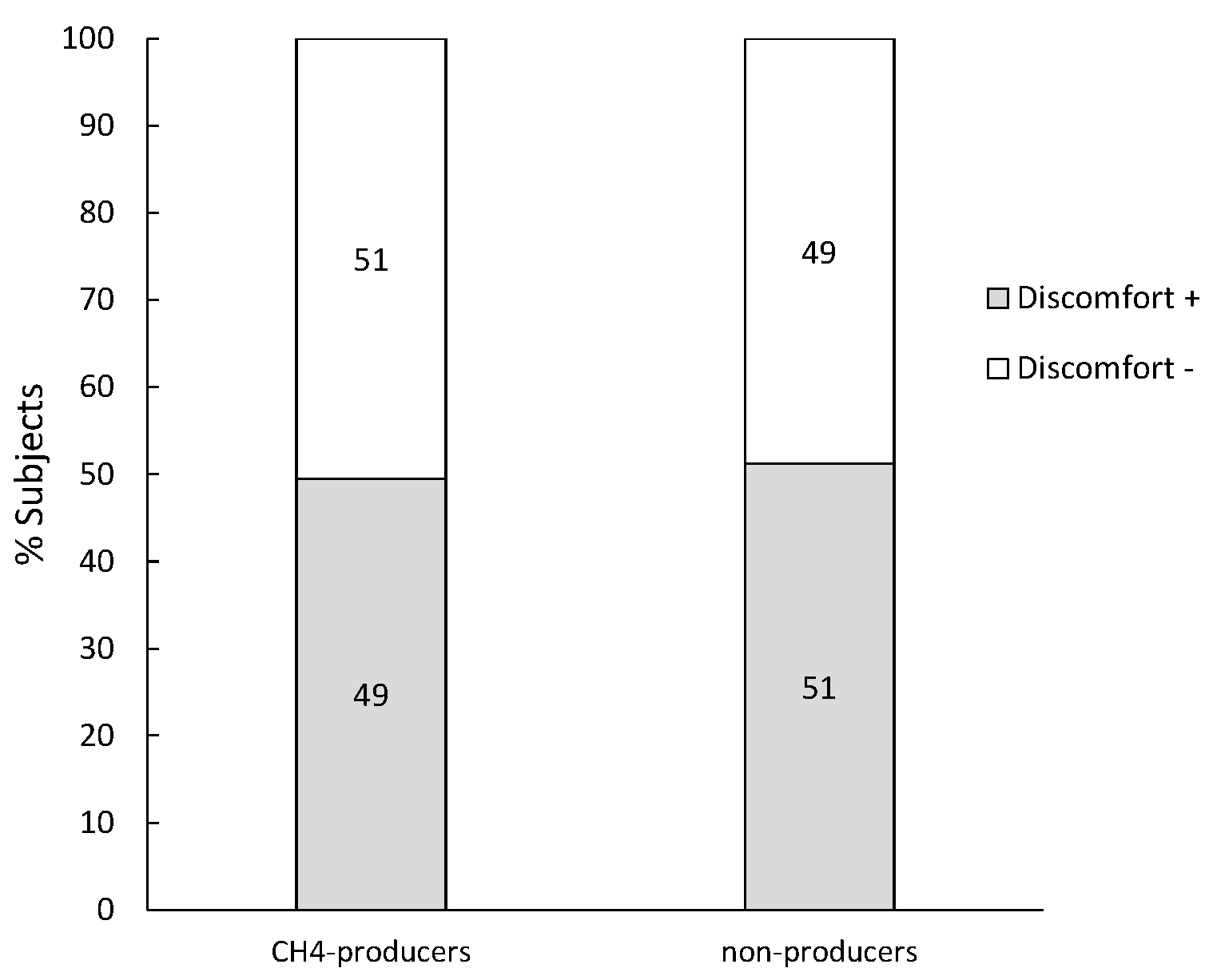
3.6. Subjects Diagnosed with a Normal Lactose Digestion and H2 ≤ 20 ppm
| Gas Production | Number of Subjects | Cumulative 13C-Excretion after 4 h (%) | Maximum H2-Excretion (ppm) | Maximum CH4-Excretion (ppm) |
|---|---|---|---|---|
| CH4-producers | All subjects (97) | 19.2 (17.2–21.6) | 3.5 (2.0–7.6) | 10.2 (7.4–16.9) |
| Discomfort + (48) | 19.6 (17.2–21.7) | 3.4 (2.0–7.7) | 10.2 (7.8–16.6) | |
| Discomfort − (49) | 18.9 (17.2–21.5) | 4.7 (1.8–7.6) | 10.2 (7.1–16.9) | |
| non producers | All subjects (502) | 19. 7 (17.4–22.4) | 4.0 (1.5–9.5) | 0.2 (0.1–0.6) |
| Discomfort + (257) | 20.3 (17.4–22.8) | 4.0 (1.5–9.6) | 0.2 (0.1–0.5) | |
| Discomfort − (245) | 19.2 (17.5–21.9) | 4.1 (1.3–9.2) | 0.2 (0.1–0.8) |
3.7. Increase in Methane versus Hydrogen in Breath
3.8. Additional Value of CH4 Measurements
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Misselwitz, B.; Pohl, D.; Fruhauf, H.; Fried, M.; Vavricka, S.R.; Fox, M. Lactose malabsorption and intolerance: Pathogenesis, diagnosis and treatment. UEG J. 2013, 1, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Enattah, N.S.; Sahi, T.; Savilahti, E.; Terwilliger, J.D.; Peltonen, L.; Jarvela, I. Identification of a variant associated with adult-type hypolactasia. Nat. Genet. 2002, 30, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Waud, J.P.; Matthews, S.B.; Campbell, A.K. Measurement of breath hydrogen and methane, together with lactase genotype, defines the current best practice for investigation of lactose sensitivity. Ann. Clin. Biochem. 2008, 45, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wedlake, L.; Thomas, K.; McGough, C.; Andreyev, H.J. Small bowel bacterial overgrowth and lactose intolerance during radical pelvic radiotherapy: An observational study. Eur. J. Cancer 2008, 44, 2212–2217. [Google Scholar] [CrossRef]
- Matthews, S.B.; Waud, J.P.; Roberts, A.G.; Campbell, A.K. Systemic lactose intolerance: A new perspective on an old problem. Postgrad. Med. J. 2005, 81, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Vesa, T.H.; Marteau, P.; Korpela, R. Lactose intolerance. J. Am. Coll. Nutr. 2000, 19, 165S–175S. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Vapaatalo, H.; Korpela, R.; Teuri, U. Lactose intolerance—A confusing clinical diagnosis. Am. J. Clin. Nutr. 2000, 71, 600–602. [Google Scholar] [PubMed]
- Newcomer, A.D.; McGill, D.B.; Thomas, P.J.; Hofmann, A.F. Prospective comparison of indirect methods for detecting lactase deficiency. N. Engl. J. Med. 1975, 293, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, L.; Raia, V.; Potter, J.; Swallow, D.; Ho, M.W.; Fiocca, R.; Finzi, G.; Cornaggia, M.; Capella, C.; Quaroni, A.; et al. Mosaic pattern of lactase expression by villous enterocytes in human adult-type hypolactasia. Gastroenterology 1991, 100, 359–369. [Google Scholar] [PubMed]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; di Stefano, M.; Basilisco, G.; Parodi, A.; Usai-Satta, P.; Vernia, P.; Anania, C.; et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29, 1–49. [Google Scholar] [PubMed]
- Hiele, M.; Ghoos, Y.; Rutgeerts, P.; Vantrappen, G.; Carchon, H.; Eggermont, E. 13CO2 breath test using naturally 13C-enriched lactose for detection of lactase deficiency in patients with gastrointestinal symptoms. J. Lab. Clin. Med. 1988, 112, 193–200. [Google Scholar] [PubMed]
- Corazza, G.R.; Benati, G.; Strocchi, A.; Malservisi, S.; Gasbarrini, G. The possible role of breath methane measurement in detecting carbohydrate malabsorption. J. Lab. Clin. Med. 1994, 124, 695–700. [Google Scholar] [PubMed]
- Hovde, O.; Farup, P.G. A comparison of diagnostic tests for lactose malabsorption—Which one is the best? BMC Gastroenterol. 2009, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.D.; di Palma, J.A. Carbohydrate challenge tests: Do you need to measure methane? South. Med. J. 2012, 105, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, K.; Chang, C.; Pimentel, M. Methanogens, methane and gastrointestinal motility. J. Neurogastroenterol. Motil. 2014, 20, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Haycock, G.B.; Schwartz, G.J.; Wisotsky, D.H. Geometric method for measuring bodysurface area: A height-weight formula validated in infants, children, and adults. J. Pediatr. 1978, 93, 62–66. [Google Scholar] [CrossRef]
- Altman, P.; Dittmer, D. Metabolic rate of boys and girls according to weight in different age classes. In Biological Data Book; Federation of American Societies for Experimental Biology: Bethesda, MD, USA, 1974; p. 1527. [Google Scholar]
- Geypens, B.; Bennink, R.; Peeters, M.; Evenepoel, P.; Mortelmans, L.; Maes, B.; Ghoos, Y.; Rutgeerts, P. Validation of the lactose-[13 C] ureide breath test for determination of orocecal transit time by scintigraphy. J. Nucl. Med. 1999, 40, 1451–1455. [Google Scholar] [PubMed]
- Nucera, G.; Gabrielli, M.; Lupascu, A.; Lauritano, E.C.; Santoliquido, A.; Cremonini, F.; Cammarota, G.; Tondi, P.; Pola, P.; Gasbarrini, G.; et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 2005, 21, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Parkes, G.C.; Sanderson, J.D. Review article: Lactose intolerance in clinical practice—Myths and realities. Aliment. Pharmacol. Ther. 2008, 27, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Miceli, E.; Mazzocchi, S.; Tana, P.; Moroni, F.; Corazza, G.R. Visceral hypersensitivity and intolerance symptoms in lactose malabsorption. Neurogastroenterol. Motil. 2007, 19, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Tomba, C.; Baldassarri, A.; Coletta, M.; Cesana, B.M.; Basilisco, G. Is the subjective perception of lactose intolerance influenced by the psychological profile? Aliment. Pharmacol. Ther. 2012, 36, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Jellema, P.; Schellevis, F.; van der Windt, D.; Kneepkens, C.M.F.; van der Horst, H.E. Lactose malabsorption and intolerance: A systematic review on the diagnostic value of gastrointestinal symptoms and self-reported milk intolerance. QJM 2010, 103, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.M.; Brummer, R.J.; Ruijgers, A.M.; Stockbrugger, R.W. The relationship between lactose tolerance test results and symptoms of lactose intolerance. Am. J. Gastroenterol. 1997, 92, 981–984. [Google Scholar] [PubMed]
- Zhu, Y.; Zheng, X.; Cong, Y.; Chu, H.; Fried, M.; Dai, N.; Fox, M. Bloating and distention in irritable bowel syndrome: The role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am. J. Gastroenterol. 2013, 108, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Suarez, F.L.; Savaiano, D.A.; Levitt, M.D. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose-intolerance. N. Engl. J. Med. 1995, 333, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Monsbakken, K.W.; Vandvik, P.O. Lactose malabsorption in a population with irritable bowel syndrome: Prevalence and symptoms. A case-control study. Scand. J. Gastroenterol. 2004, 39, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Dainese, R.; Casellas, F.; Marine-Barjoan, E.; Vivinus-Nebot, M.; Schneider, S.M.; Hebuterne, X.; Piche, T. Perception of lactose intolerance in irritable bowel syndrome patients. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Vernia, P.; di Camillo, M.; Foglietta, T.; Avallone, V.E.; de Carolis, A. Diagnosis of lactose intolerance and the “nocebo” effect: The role of negative expectations. Dig. Liver Dis. 2010, 42, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Beyerlein, L.; Pohl, D.; Delco, F.; Stutz, B.; Fried, M.; Tutuian, R. Correlation between symptoms developed after the oral ingestion of 50 g lactose and results of hydrogen breath testing for lactose intolerance. Aliment. Pharmacol. Ther. 2008, 27, 659–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertzler, S.R.; Savaiano, D.A. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am. J. Clin. Nutr. 1996, 64, 232–236. [Google Scholar] [PubMed]
- Hertzler, S.R.; Savaiano, D.A.; Levitt, M.D. Fecal hydrogen production and consumption measurements (response to daily lactose ingestion by lactose maldigesters). Dig. Dis. Sci. 1997, 42, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Briet, F.; Pochart, P.; Marteau, P.; Flourie, B.; Arrigoni, E.; Rambaud, J.C. Improved clinical tolerance to chronic lactose ingestion in subjects with lactose intolerance: A placebo effect? Gut 1997, 41, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A. Adaptation to lactose in lactase non persistent people: Effects on intolerance and the relationship between dairy food consumption and evalution of diseases. Nutrients 2015, 7, 6751–6779. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A.; Cohen, A.; Vinokuroff, C.; Ahmad, D.; Nathwani, U.; Yesovitch, S. Deadaption and readaptation with lactose, but no cross-adaptation to lactulose: A case of occult colonic bacterial adaptation. Can. J. Gastroenterol. 2004, 18, 677–680. [Google Scholar] [PubMed]
- Montes, R.G.; Saavedra, J.M.; Perman, J.A. Relationship between methane production and breath hydrogen excretion in lactose-malabsorbing individuals. Dig. Dis. Sci. 1993, 38, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Cloarec, D.; Bornet, F.; Gouilloud, S.; Barry, J.L.; Salim, B.; Galmiche, J.P. Breath hydrogen response to lactulose in healthy subjects: Relationship to methane producing status. Gut 1990, 31, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Moran, S.; Mina, A.; Duque, X.; Anaya, S.; San-Martin, U.; Yanez, P.; Rodriguez-Leal, G. Prevalence of lactose malabsorption in mexican children: Importance of measuring methane in expired air. Arch. Med. Res. 2013, 44, 291–295. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.F.; Eastwood, M.A.; Brydon, W.G. Methane excretion in man—A study of breath, flatus, and faeces. Gut 1985, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Mengoli, C.; Bergonzi, M.; Klersy, C.; Pagani, E.; Miceli, E.; Corazza, G.R. Breath methane excretion is not an accurate marker of colonic methane production in irritable bowel syndrome. Am. J. Gastroenterol. 2015, 110, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Wang, A.; Su, W.; Rozenbloom, S.R.; Taibi, A.; Comelli, E.M.; Wolever, T.M. Age, dietary fiber, breath methane, and fecal short chain fatty acids are interrelated in archaea-positive humans. J. Nutr. 2013, 143, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Kurbel, S.; Kurbel, B.; Vcev, A. Intestinal gases and flatulence: Possible causes of occurrence. Med. Hypotheses 2006, 67, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Mengoli, C.; Bergonzi, M.; Miceli, E.; Pagani, E.; Corazza, G.R. Hydrogen breath test in patients with severe constipation: The interference of the mixing of intestinal content. Neurogastroenterol. Motil. 2014, 26, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Mayer, A.G.; Park, S.; Chow, E.J.; Hasan, A.; Kong, Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig. Dis. Sci. 2003, 48, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Park, S.; Low, K.; Kong, Y.; Pimentel, M. The degree of breath methane production in ibs correlates with the severity of constipation. Am. J. Gastroenterol. 2007, 102, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lin, H.C.; Enayati, P.; van den Burg, B.; Lee, H.R.; Chen, J.H.; Park, S.; Kong, Y.; Conklin, J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1089–G1095. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Mathur, R.; Chang, C. Gas and the microbiome. Curr. Gastroenterol. Rep. 2013, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Basseri, R.J.; Basseri, B.; Pimentel, M.; Chong, K.; Youdim, A.; Low, K.; Hwang, L.; Soffer, E.; Chang, C.; Mathur, R. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol. Hepatol. 2012, 8, 22–28. [Google Scholar]
- Mathur, R.; Amichai, M.; Chua, K.S.; Mirocha, J.; Barlow, G.M.; Pimentel, M. Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J. Clin. Endocrinol. MeTable 2013, 98, E698–E702. [Google Scholar] [CrossRef] [PubMed]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in lactobacillus in obese patients and methanogens in anorexic patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of lactobacillus reuteri, bifidobacterium animalis, methanobrevibacter smithii and escherichia coli. Int. J. Obes. (Lond.) 2013, 37, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in lactobacillus reuteri and depleted in bifidobacterium animalis and methanobrevibacter smithii. Int. J. Obes. (Lond.) 2012, 36, 817–825. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houben, E.; De Preter, V.; Billen, J.; Van Ranst, M.; Verbeke, K. Additional Value of CH4 Measurement in a Combined 13C/H2 Lactose Malabsorption Breath Test: A Retrospective Analysis. Nutrients 2015, 7, 7469-7485. https://doi.org/10.3390/nu7095348
Houben E, De Preter V, Billen J, Van Ranst M, Verbeke K. Additional Value of CH4 Measurement in a Combined 13C/H2 Lactose Malabsorption Breath Test: A Retrospective Analysis. Nutrients. 2015; 7(9):7469-7485. https://doi.org/10.3390/nu7095348
Chicago/Turabian StyleHouben, Els, Vicky De Preter, Jaak Billen, Marc Van Ranst, and Kristin Verbeke. 2015. "Additional Value of CH4 Measurement in a Combined 13C/H2 Lactose Malabsorption Breath Test: A Retrospective Analysis" Nutrients 7, no. 9: 7469-7485. https://doi.org/10.3390/nu7095348





