One Egg per Day Improves Inflammation when Compared to an Oatmeal-Based Breakfast without Increasing Other Cardiometabolic Risk Factors in Diabetic Patients
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Design
2.2. Diet and Exercise Assessment
2.3. Anthropometrics, Body Fat and Blood Pressure
2.4. Plasma Lipids, Oxidized-LDL and Apolipoprotein B
2.5. Glucose, Insulin and Homeostatic Mode Assessment (HOMA)
2.6. Determination of Size and Concentrations of VLDL, LDL and HDL Subfractions
2.7. Inflammatory Markers, Liver Enzymes and Adiponectin
2.8. Statistical Analysis
3. Results
3.1. Flow Chart of the Study
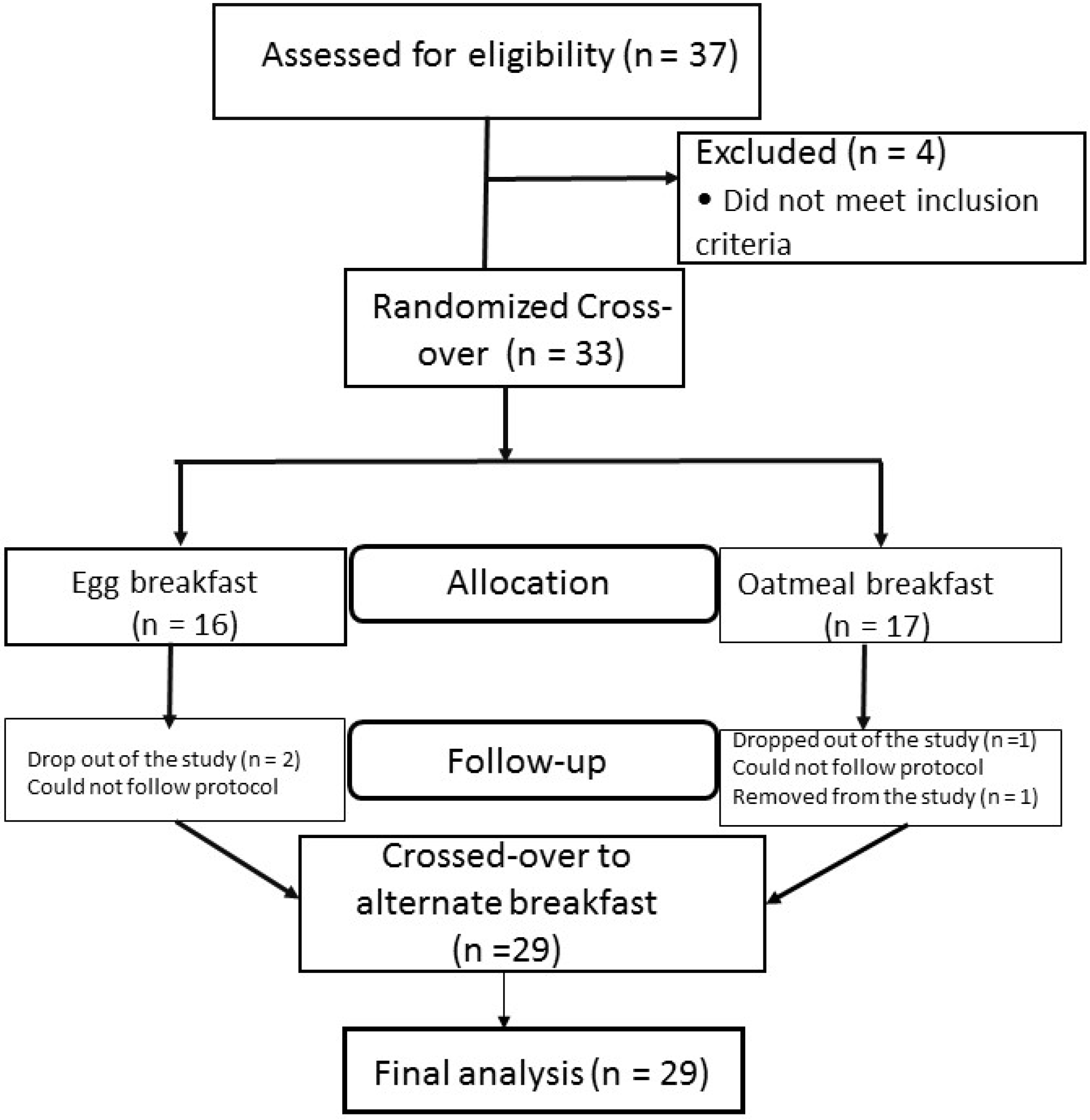
3.2. Baseline Characteristics
| Parameter (n = 29) | Values |
|---|---|
| Age (years) | 53.5 ± 8.3 |
| Gender (n = F/M) | 19/10 |
| Total Cholesterol (mmol/L) | 4.1 ± 0.7 |
| LDL Cholesterol (mmol/L) | 2.3 ± 0.6 |
| Triglycerides (mmol/L) | 2.2 ± 1.0 |
| HDL Cholesterol (mmol/L) | 1.0 ± 0.2 |
| LDL-C/HDL-C | 2.4 ± 1.1 |
| Glucose (mmol/L) | 9.0 ± 3.1 |
| Glycosylated Hemoglobin (%) (mmol/L) | 6.75 ± 0.89 |
| (50 ± 9.7) |
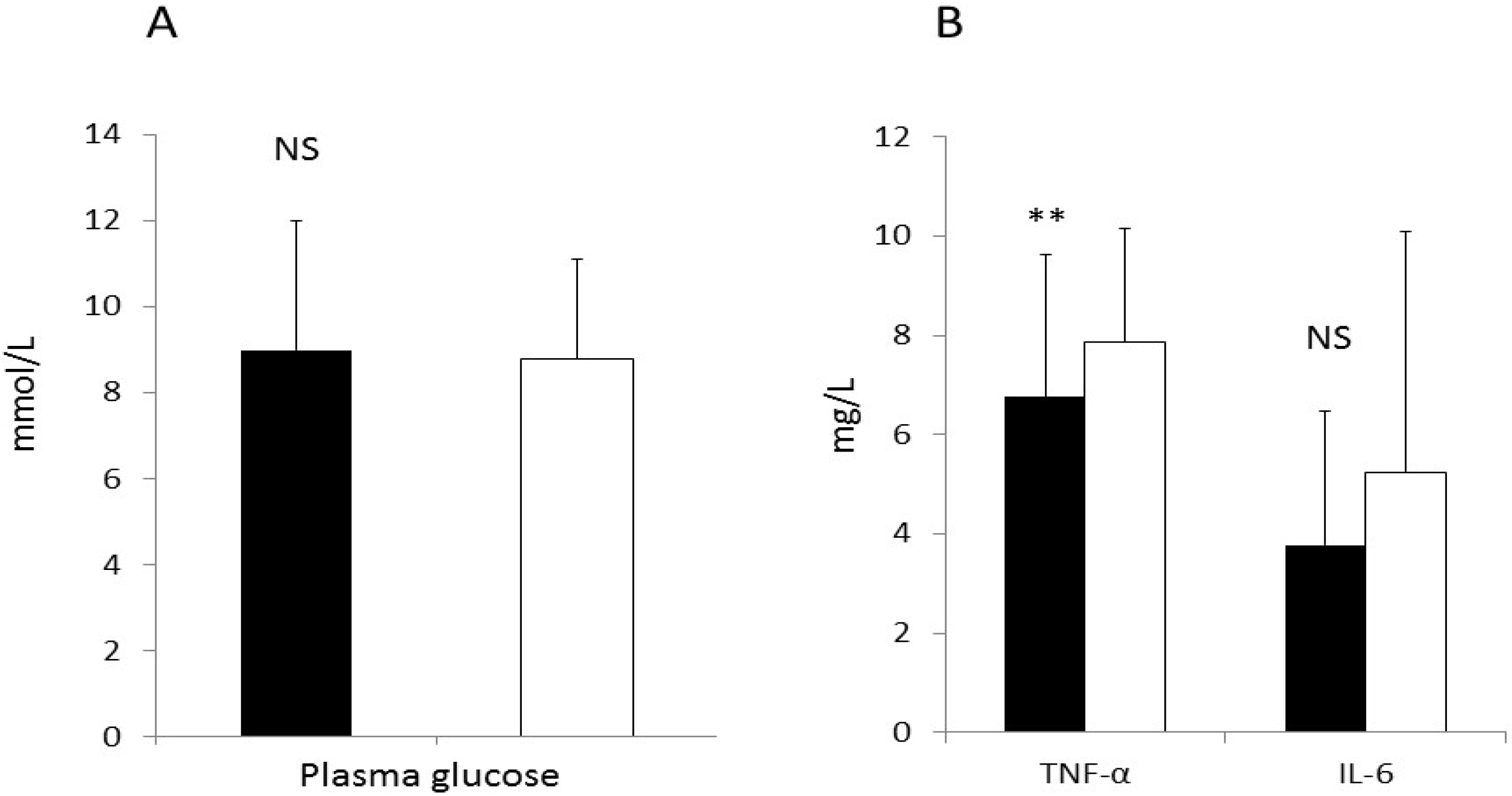
3.3 Primary end Points
3.4. Diet
| Parameter | Egg | Oatmeal | p Value |
|---|---|---|---|
| Energy (Kcal) | 1629 ± 410 | 1686 ± 362 | 0.278 |
| Protein (%) | 18.9 ± 3.5 | 20.0 ± 3.5 | 0.134 |
| Carbohydrate (%) | 50.3 ± 6.5 | 55.1 ± 7.3 | 0.010 |
| Total Fat (%) | 31.6 ± 5.6 | 24.7 ± 6.8 | <0.0001 |
| SFA (g/day) | 21.0 ± 8.9 | 17.3 ± 9.4 | 0.043 |
| MUFA (g/day) | 17.0 ± 5.3 | 12.8 ± 6.6 | 0.006 |
| PUFA (g/day) | 8.1 ± 2.4 | 6.3 ± 2.8 | 0.009 |
| Total Fiber (g/day) | 26.0 ± 10.1 | 27.5 ± 9.2 | 0.345 |
| Soluble Fiber (g/day) | 5.2 ± 3.3 | 6.7 ± 3.1 | 0.008 |
| Insoluble Fiber (g/day) | 13.3 ± 5.6 | 15.2 ± 5.7 | 0.073 |
| Cholesterol (mg/day) | 435.0 ± 119.8 | 149.4 ± 77 | <0.0001 |
| Lutein + Zeaxanthin (μg/day) | 1213 ± 1731 | 1003 ± 1742 | 0.185 |
| Glycemic Index | 49.2 ± 8.7 | 48.5 ± 7.7 | 0.752 |
| Glycemic Load | 17.3 ± 9.4 | 17.1 ± 21.3 | 0.828 |
3.5. Anthropometrics, Blood Pressure, Plasma Lipids, HbA1c and Insulin
3.6. Lipoprotein Number and Subclasses
3.7. CRP and Liver Enzymes
| Parameter (n = 29) | Egg | Oatmeal |
|---|---|---|
| Weight (kg) | 82.1 ±17.0 | 82.1 ± 17.1 |
| BMI (kg/m2) | 30.8 ± 6.4 | 30.8 ± 6.5 |
| Body Fat (%) | 45 ± 9 | 44 ± 8 |
| Systolic BP (mmHg) | 123.5 ± 11.1 | 123.8 ± 11.3 |
| Diastolic BP (mmHg) | 76.1 ± 7.4 | 76.1 ± 8.0 |
| Total cholesterol (mmol/L) | 4.1 ± 1.5 | 4.0 ± 0.8 |
| LDL cholesterol (mmol/L) | 2.5 ± 0.6 | 2.4 ± 0.6 |
| Triglycerides (mmol/L) | 1.48 ± 0.47 | 1.53 ± 0.55 |
| HDL cholesterol (mmol/L) | 1.17 ± 0.23 | 1.14 ± 0.21 |
| LDL-C/HDL-C | 1.99 ± 0.72 | 1.95 ± 0.68 |
| Apolipoprotein B (mg/L) | 90.8 ± 33.9 | 95 ± 38 |
| Oxidized LDL (U/L) | 76.9 ± 25.3 | 80.6 ± 32.7 |
| Glycosylated Hemoglobin (%) | 6.55 ± 0.93 | 6.60 ± 1.04 |
| Insulin (pmol/L) | 101.4 ± 63.2 | 86.8 ± 50.7 |
| HOMA | 3.7 ± 0.7 | 3.1 ± 0.6 |
| Parameter (n = 29) | Egg | Oatmeal |
|---|---|---|
| Total VLDL Particles (mmo/L) | 53.8 ± 27.7 | 53.1 ± 26.4 |
| Large VLDL (mmol/L) | 7.0 ± 4.5 | 7.6 ± 5.1 |
| Medium VLDL (mmol/L) | 21.9 ± 12.3 | 21.6 ± 13.5 |
| Small VLDL(mmol/L) | 24.9 ±17.6 | 23.8 ± 13.1 |
| Total LDL (mmol/L) | 1117 ± 290 | 1064 ± 253 |
| Large LDL(mmol/L) | 189 ± 147 | 215 ± 141 |
| Small LDL(mmol/L) | 775 ± 251 | 715 ± 230 |
| IDL(mmol/L) | 152 ± 89 | 134 ± 103 |
| Total HDL Particles (µmol/L) | 32.7 ± 5.8 | 33.2 ± 6.3 |
| Large HDL(μmol/L) | 3.74 ± 1.88 | 3.64 ± 1.98 |
| Medium HDL(μmol/L) | 8.21 ± 5.33 | 8.34 ± 5.41 |
| Small HDL(μmol/L) | 20.71 ± 3.71 | 21.17 ± 3.94 |

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shin, J.Y.; Xun, P.; Nakamura, Y.; He, K. Egg consumption in relation to risk of cardiovascular disease and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Suri, M.F.K.; Ajmed, S.; Nasar, A.; Divani, A.A.; Kirmani, J.F. Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med. Sci. Monit. 2007, 131, CR1–CR8. [Google Scholar]
- Djousse, L.; Kamineni, A.; Nelson, T.L.; Carnethon, M.; Mozaffarian, D.; Siscovick, D.; Mukamal, K.J. Egg consumption and risk of type 2 diabetes in older adults. Am. J. Clin. Nutr. 2010, 92, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Ebrahim, S. Prevalence and risk factors for self-reported diabetes among adult men and women in India: Findings from a national cross-sectional survey. Pub. Health Nutr. 2011, 15, 1065–1077. [Google Scholar] [CrossRef]
- Radzeviciene, L.; Ostrauskas, R. Egg consumption and the risk of type 2 diabetes mellitus: A case-control study. Public Health Nutr. 2012, 15, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Gaziano, J.M.; Buring, J.E.; Lee, I.M. Egg consumption and risk of type 2 diabetes in men and women. Diabetes Care 2009, 32, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Zazpe, I.; Beunza, J.J.; Bes-Rastrollo, M.; Basterra-Gortari, F.J.; Mari-Sanchis, A.; Martínez-González, M.Á.; SUN Project Investigators. Egg consumption and risk of type 2 diabetes in a Mediterranean cohort: The SUN Project. Nutr. Hosp. 2013, 28, 105–111. [Google Scholar]
- Ng, D.S. Diabetic dyslipidemia: From evolving pathophysiological insight to emerging therapeutic targets. Can. J. Diabetes 2013, 37, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kontoangelos, K.; Papageorgiuo, C.C.; Raptis, A.E.; Tsiotra, P.; Boutati, E.; Lambadiari, V.; Papadimitriou, G.N.; Rabavilas, A.D.; Dimitriadis, G.; Raptis, S.A. Cytokines, diabetes mellitus and psychopathology: A challenging combination. Neuro Endocrinol. Lett. 2014, 35, 159–169. [Google Scholar] [PubMed]
- Thongoun, P.; Pavadhgul, P.; Burmrungpert, A.; Satitvipawee, P.; Harjani, Y.; Kurilich, A. Effect of oat consumption on lipid profiles in hypercholesterolemic adults. J. Med. Assoc. Thai. 2013, 96 (Suppl. 5), S25–S32. [Google Scholar] [PubMed]
- Lammert, A.; Kratzch, J.; Selhorst, J.; Humpert, P.M.; Bierhaus, A.; Birck, R.; Kusterek, K.; Hammes, H.P. Clinical benefit of a short term dietary oatmeal intervention in patients with type 2 diabetes and severe insulin resistance: A pilot study. Exp. Clin. Endocrinol. Diabetes 2008, 116, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, G.; Biiljani, R.L.; Mahapatra, S.C.; Mehta, N.; Lakshmy, R.; Vashist, S.; Manchanda, S.C. The effect of ingestion of egg on serum lipid profile in healthy young free-living subjects. Indian J. Physiol. Pharmacol. 2002, 46, 492–498. [Google Scholar] [PubMed]
- Li, Y.; Zhou, C.; Zhou, X.; Li, L. Egg consumption of cardiovascular diseases and diabetes: A meta-analysis. Atherosclerosis 2013, 229, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Pullinger, C.R.; Goldfind, I.D.; Tanyolac, S.; Movseyan, I.; Faynbaym, M.; Durlach, V.; Chiefan, E.; Foti, D.P.; Malloy, M.J.; Brunette, A.; et al. Evidence that an HMGA1 gene variant associates with type 2 diabetes, body mass index and high density lipoprotein cholesterol in a Hispanic-American population. Metab. Syndr. Rel. Disord. 2014, 12, 25–30. [Google Scholar] [CrossRef]
- Gutiérrez, J.P.; Rivera-Dommarco, J.; Shamah-Levy, T.; Villalpando-Hernandez, S.; Romero-Martinez, M.; Hernandez-Avila, M. Encuesta Nacional de Salud y Nutrición 2012. Resultados Nacionales; Instituto Nacional de Salud Pública (MX): Cuernavaca, México, 2012. [Google Scholar]
- Gonzalez-Villalpando, C.; Davila-Ce rvantes, C.A.; Zamora-Macorra, M.; Trejo-Valdivia, B.; Gonzalez-Villalpando, M.E. Incidence of type II diabetes in Mexico. Results of the Mexico City Diabetes study after 18 years follow-up. Salud Publica Mex. 2014, 56, 11–17. [Google Scholar]
- Albarran, N.B.; Ballesteros, M.N.; Morales, G.G.; Ortega, M.I. Dietary behavior and type 2 diabetes care. Patient Educ. Couns. 2006, 6, 191–199. [Google Scholar] [CrossRef]
- Chung, H.Y.; Rasmussen, H.M.; Johnson, E.J. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J. Nutr. 2004, 134, 1887–1893. [Google Scholar] [PubMed]
- Thurnham, D.I. Macular zeaxanthins and lutein—A review of dietary sources and bioavailability and some relationships with macular pigment optical density and age-related macular disease. Nutr. Res. Rev. 2007, 20, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, J.; Mutungi, G.; Puglisi, M.; Volek, J.S.; Fernandez, M.L. Eggs modulate the inflammatory response to carbohydrate restricted diets in overweight men. Nutr. Metab. (Lond.) 2008, 5, 6. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volk, B.; Volek, J.S.; Fernandez, M.L. Effects of carbohydrate restriction and dietary cholesterol provided by eggs on clinical risk factors of metabolic syndrome. J. Clin. Lipidol. 2013, 7, 463–471. [Google Scholar] [CrossRef]
- Ballesteros, M.N.; Cabrera, R.M.; Saucedo, M.S.; Fernandez, M.L. Intake of two eggs per day increases plasma lutein and zeaxanthin in a pediatric population at high risk for heart disease. Brit. J. Med. Med. Res. 2013, 3, 2203–2213. [Google Scholar] [CrossRef]
- Lorenzo, C.; Hazuda, H.P.; Haffner, S.M. Insulin resistance and excess risk of diabetes in Mexican-Americans: The San Antonio Heart Study. J. Clin. Endocrinol. Met. 2012, 97, 793–799. [Google Scholar] [CrossRef]
- Wood, R.; Volek, J.S.; Liu, Y.; Shachter, N.S.; Contois, J.H.; Fernandez, M.L. Carbohydrate restriction alters lipoprotein metabolism by modifying VLDL, LDL and HDL subfraction distribution and size in overweight men. J. Nutr. 2006, 136, 384–389. [Google Scholar] [PubMed]
- Herron, K.L.; Vega-Lopez, S.; Ramjiganesh, T.; Conde-Knape, K.; Shachter, N.; Fernandez, M.L. Men classified as hypo- or hyper-responders to dietary cholesterol feeding exhibit differences in lipoprotein metabolism. J. Nutr. 2003, 133, 1036–1042. [Google Scholar] [PubMed]
- Greene, C.M.; Zern, T.L.; Wood, R.; Shrestha, S.; Aggarwal, D.; Sharman, M.; Volek, J.S.; Fernandez, M.L. Dietary cholesterol provided by eggs does not result in an increased risk for coronary heart disease in an elderly population. J. Nutr. 2005, 135, 2793–2798. [Google Scholar] [PubMed]
- Pearce, K.L.; Clifton, P.M.; Noakes, M. Egg consumption as part of an energy-restricted high-protein diet improves blood lipid and blood glucose profiles in individuals with type 2 diabetes. Brit. J. Nutr. 2011, 105, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.L.; Romero, J.E.; Laviz, S.; Fernandez, M.L. Cookies enriched with Psyllium and oat bran lower plasma LDL-cholesterol in normal and hypercholesterolemic men from Northern Mexico. J. Am. Coll. Nutr. 1998, 17, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.W. Can dietary oats promote health? Br. J. Biomed. Sci. 1994, 51, 260–270. [Google Scholar] [PubMed]
- Griffin, J.D.; Lichtenstein, A.H. Dietary cholesterol and plasma lipoprotein profiles: Randomized-controlled trials. Clin. Nutr. Rep. 2013, 2, 274–282. [Google Scholar] [CrossRef]
- Ballesteros, M.N.; Cabrera, R.M.; Saucedo, M.S.; Fernandez, M.L. Dietary cholesterol does not increase biomarkers for chronic disease in a pediatric population at risk from Northern Mexico. Am. J. Clin. Nutr. 2004, 80, 855–861. [Google Scholar] [PubMed]
- Andersen, C.J.; Blesso, C.N.; Lee, J.Y.; Sha, D.; Thomas, M.J.; Fernandez, M.L. Egg consumption modulates HDL composition and increases the cholesterol accepting capacity of serum in metabolic syndrome. Lipids 2013, 48, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volek, J.; Fernandez, M.L. Whole egg consumption improves lipoprotein profiles and insulin sensitivity in individuals with metabolic syndrome. Metabolism 2013, 62, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, G.; Waters, D.; Ratliff, J.; Puglisi, M.J.; Clark, R.M.; Volek, J.S.; Fernandez, M.L. Eggs distinctly modulate plasma carotenoid and lipoprotein subclasses in adult men following a carbohydrate restricted diet. J. Nutr. Biochem. 2010, 21, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C.; Pang, M.; Doemler, W.; Krauss, R.M. LDL subclass phenotypes and triglyceride metabolism in non-insulin-dependent diabetes. Arterioscler. Thromb. Vasc. Biol. 1992, 12, 1496–1502. [Google Scholar] [CrossRef]
- Al-Sarraj, T.; Saadi, H.; Volek, J.S.; Fernandez, M.L. Carbohydrate restriction favorably alters lipoprotein metabolism in Emirati subjects classified with the metabolic syndrome. Nutr. Met. Cardiovasc. Dis. 2010, 20, 720–726. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Bolling, B.; Fernandez, M.L. Egg intake improves carotenoid status by increasing HDL cholesterol in adults with metabolic syndrome. Food Funct. 2013, 4, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Na, H.J.; Kim, C.K.; Kim, J.Y.; Ha, K.S.; Lee, H.; Chung, H.T.; Kwon, H.J.; Kwon, Y.G.; Kim, Y.M. The non-provitamin A carotenoid, lutein, inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: Role of H(2)O(2) in NF-kappaB activation. Free Radic. Biol. Med. 2008, 45, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Leite, J.O.; DeOgburn, R.; Smyth, J.A.; Clark, R.M.; Fernandez, M.L. A lutein-enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J. Nutr. 2011, 141, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballesteros, M.N.; Valenzuela, F.; Robles, A.E.; Artalejo, E.; Aguilar, D.; Andersen, C.J.; Valdez, H.; Fernandez, M.L. One Egg per Day Improves Inflammation when Compared to an Oatmeal-Based Breakfast without Increasing Other Cardiometabolic Risk Factors in Diabetic Patients. Nutrients 2015, 7, 3449-3463. https://doi.org/10.3390/nu7053449
Ballesteros MN, Valenzuela F, Robles AE, Artalejo E, Aguilar D, Andersen CJ, Valdez H, Fernandez ML. One Egg per Day Improves Inflammation when Compared to an Oatmeal-Based Breakfast without Increasing Other Cardiometabolic Risk Factors in Diabetic Patients. Nutrients. 2015; 7(5):3449-3463. https://doi.org/10.3390/nu7053449
Chicago/Turabian StyleBallesteros, Martha Nydia, Fabrizio Valenzuela, Alma E. Robles, Elizabeth Artalejo, David Aguilar, Catherine J. Andersen, Herlindo Valdez, and Maria Luz Fernandez. 2015. "One Egg per Day Improves Inflammation when Compared to an Oatmeal-Based Breakfast without Increasing Other Cardiometabolic Risk Factors in Diabetic Patients" Nutrients 7, no. 5: 3449-3463. https://doi.org/10.3390/nu7053449
APA StyleBallesteros, M. N., Valenzuela, F., Robles, A. E., Artalejo, E., Aguilar, D., Andersen, C. J., Valdez, H., & Fernandez, M. L. (2015). One Egg per Day Improves Inflammation when Compared to an Oatmeal-Based Breakfast without Increasing Other Cardiometabolic Risk Factors in Diabetic Patients. Nutrients, 7(5), 3449-3463. https://doi.org/10.3390/nu7053449






