α-Naphthoflavone Increases Lipid Accumulation in Mature Adipocytes and Enhances Adipocyte-Stimulated Endothelial Tube Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Cytotoxicity Analysis
2.4. siRNA Transfection
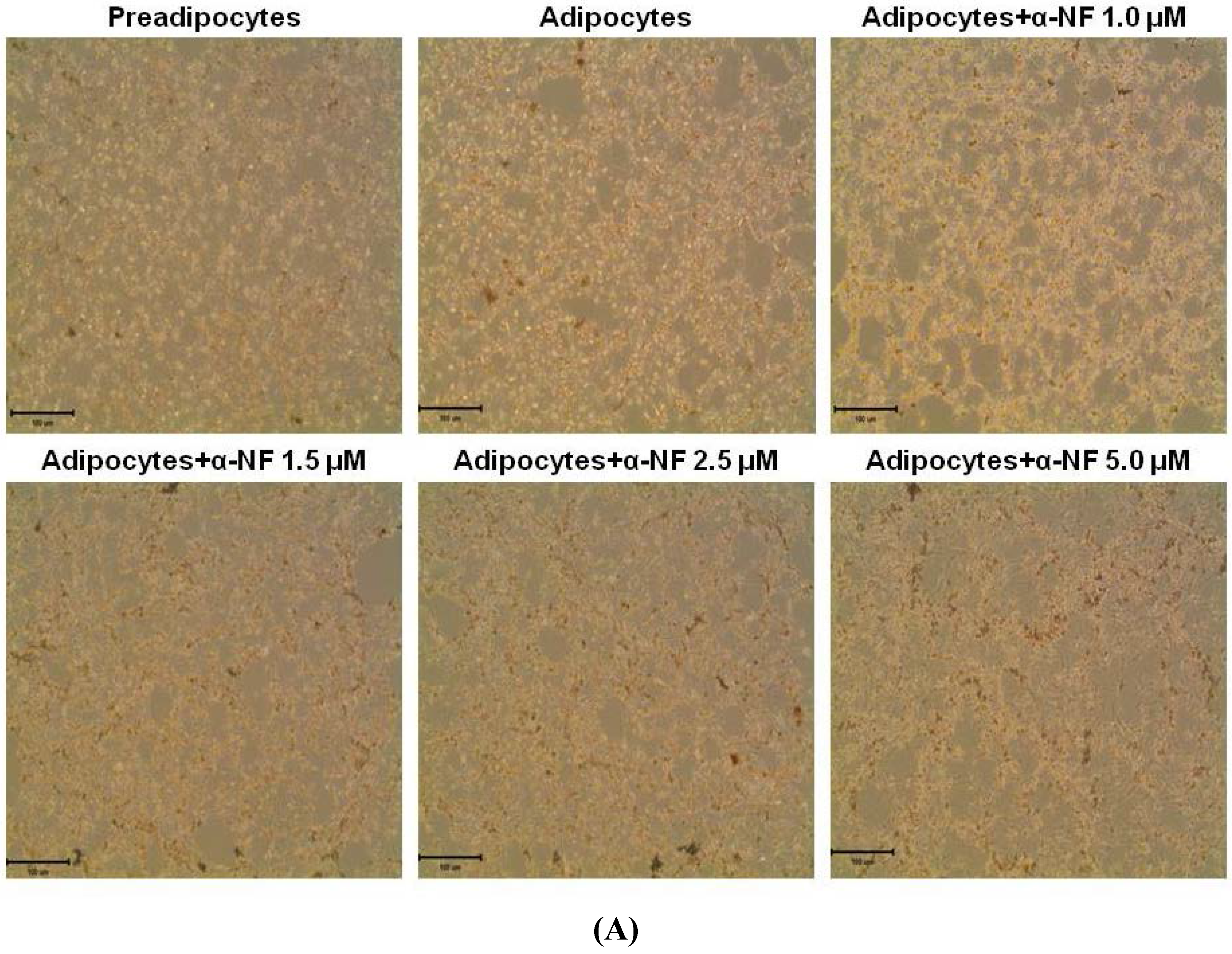
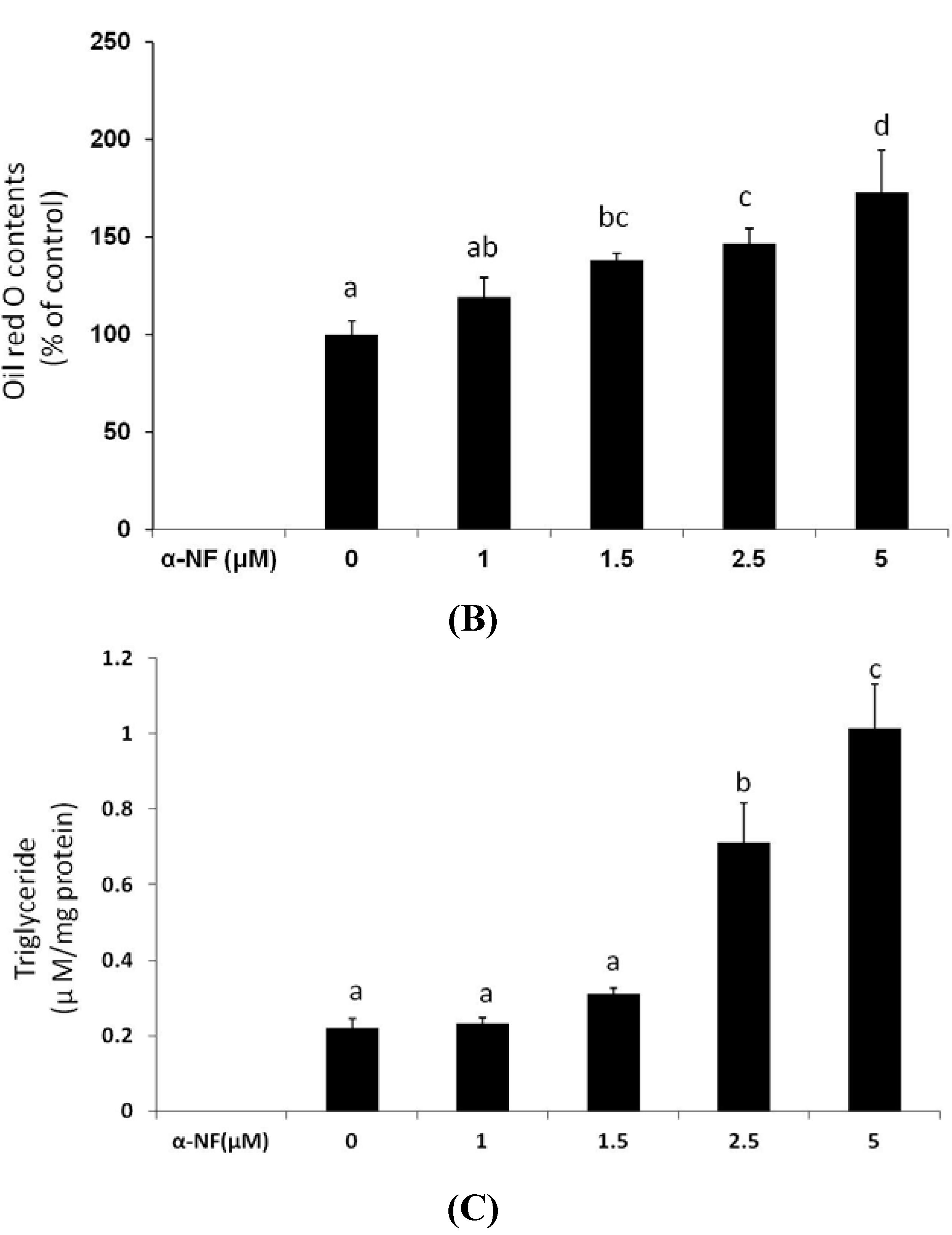
2.5. Lipid Accumulation Determination
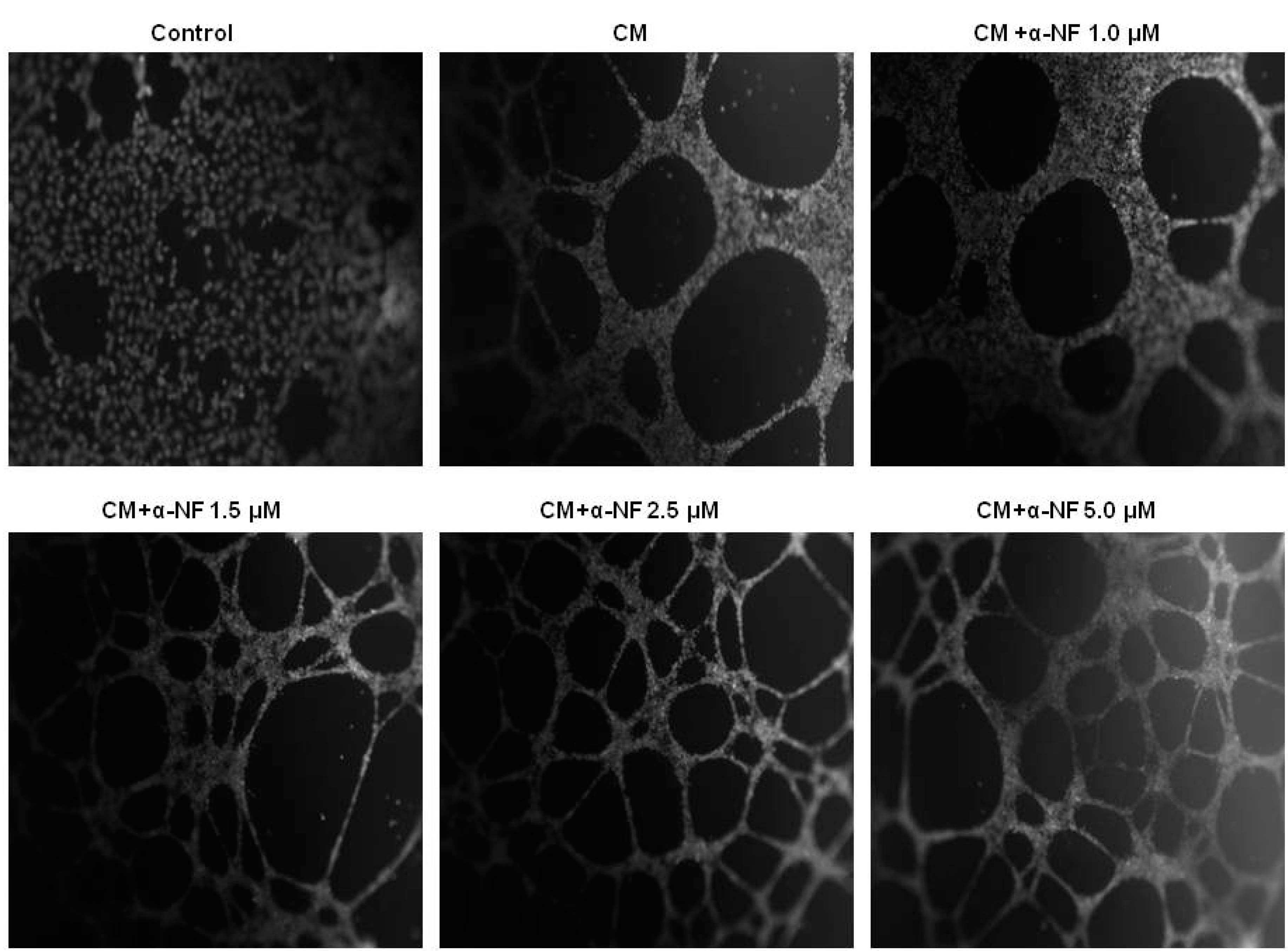
2.6. Tube Formation Assay
2.7. Measurements of VEGF, Insulin-like growth factor 1 (IGF-1), Interleukin 6 (IL-6), and Nitric Oxide (NO)
2.8. Western Blot Analysis
2.9. Statistical analysis
3. Results
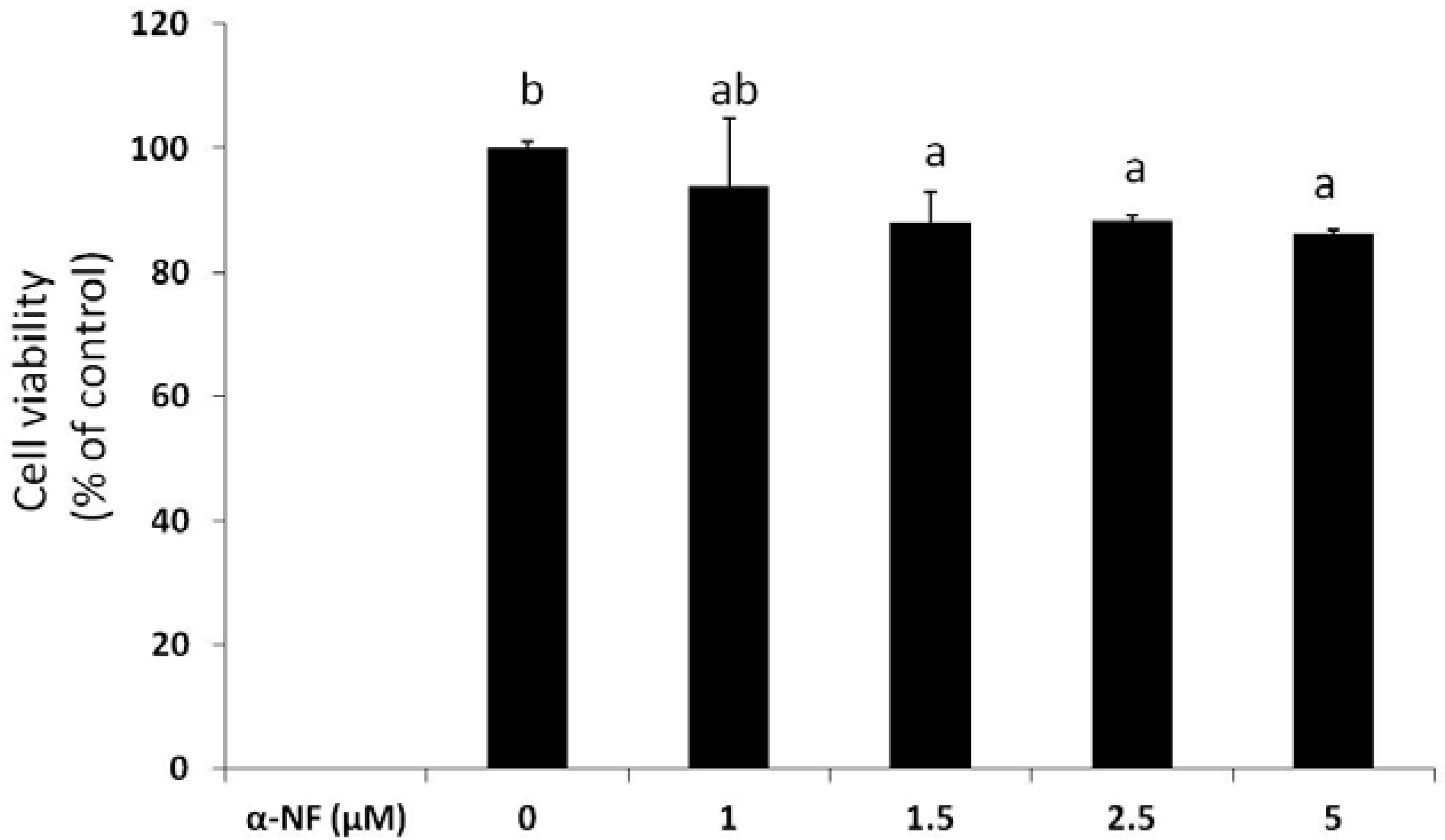
3.1. Effects of α-NF on Cytotoxicity and Lipid Accumulation in Mature Adipocytes
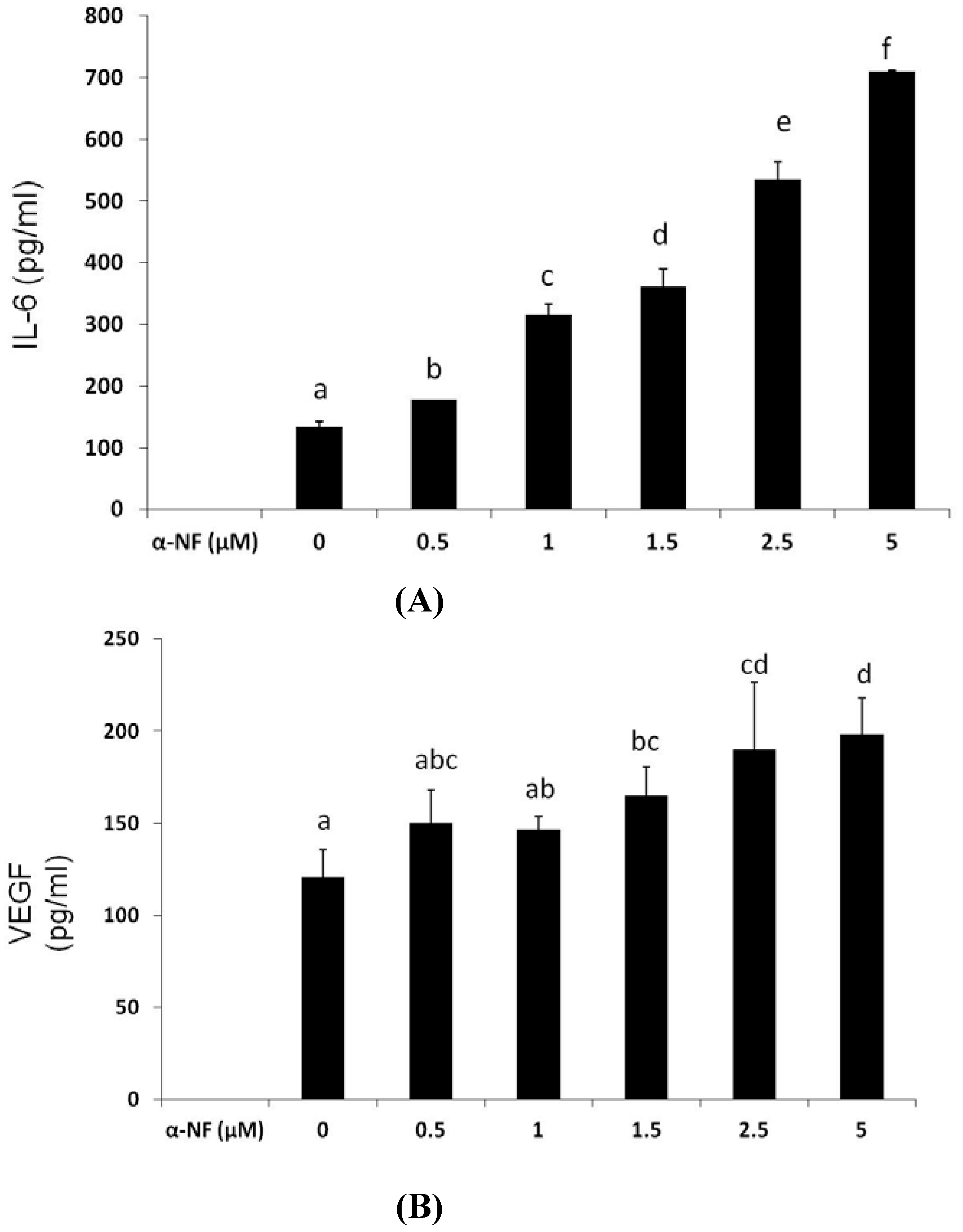
3.2. Effects of α-NF on Adipocyte-Induced Angiogenesis in ECs
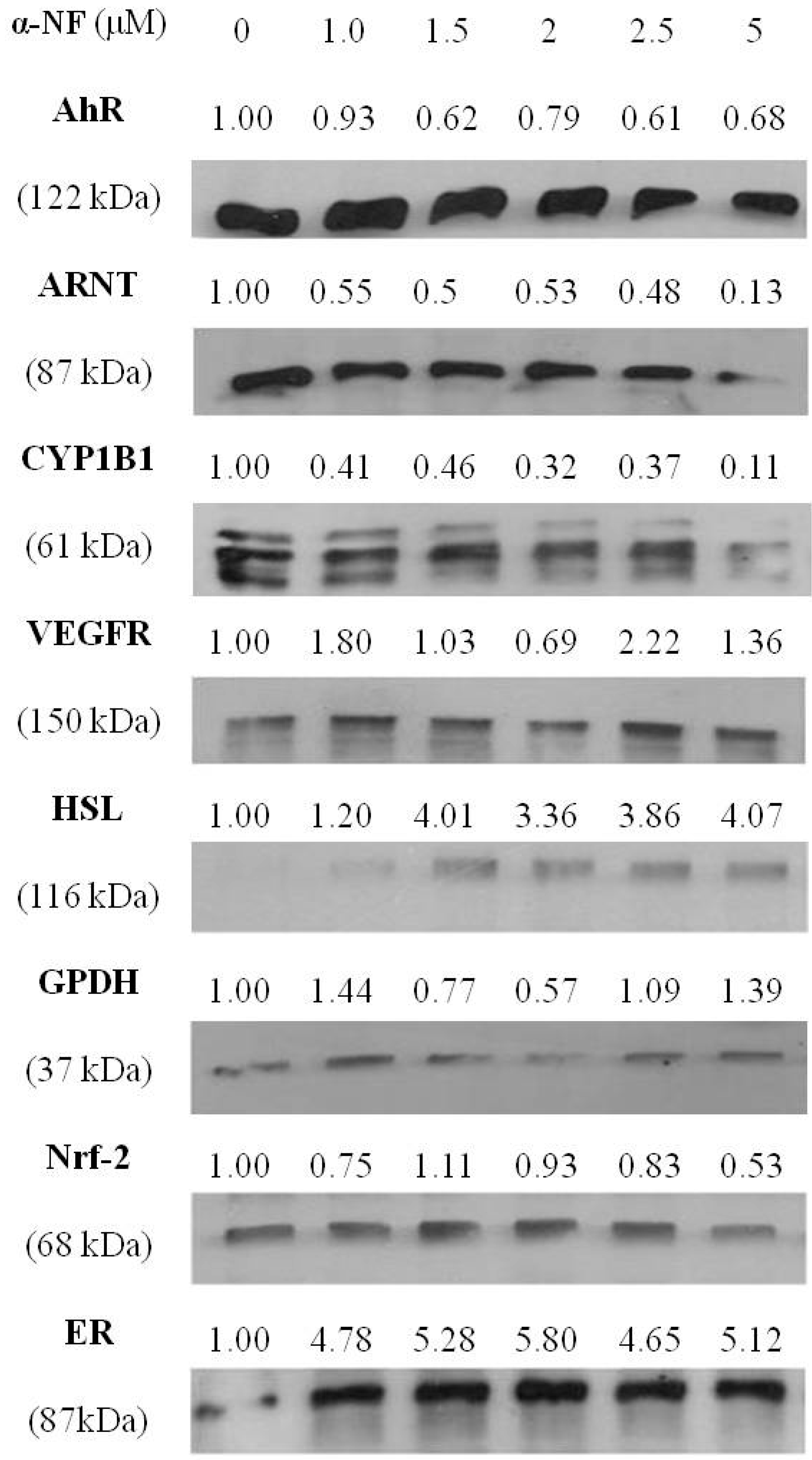
3.3. Effects of α-NF on Expressions of Various Proteins by Mature Adipocytes
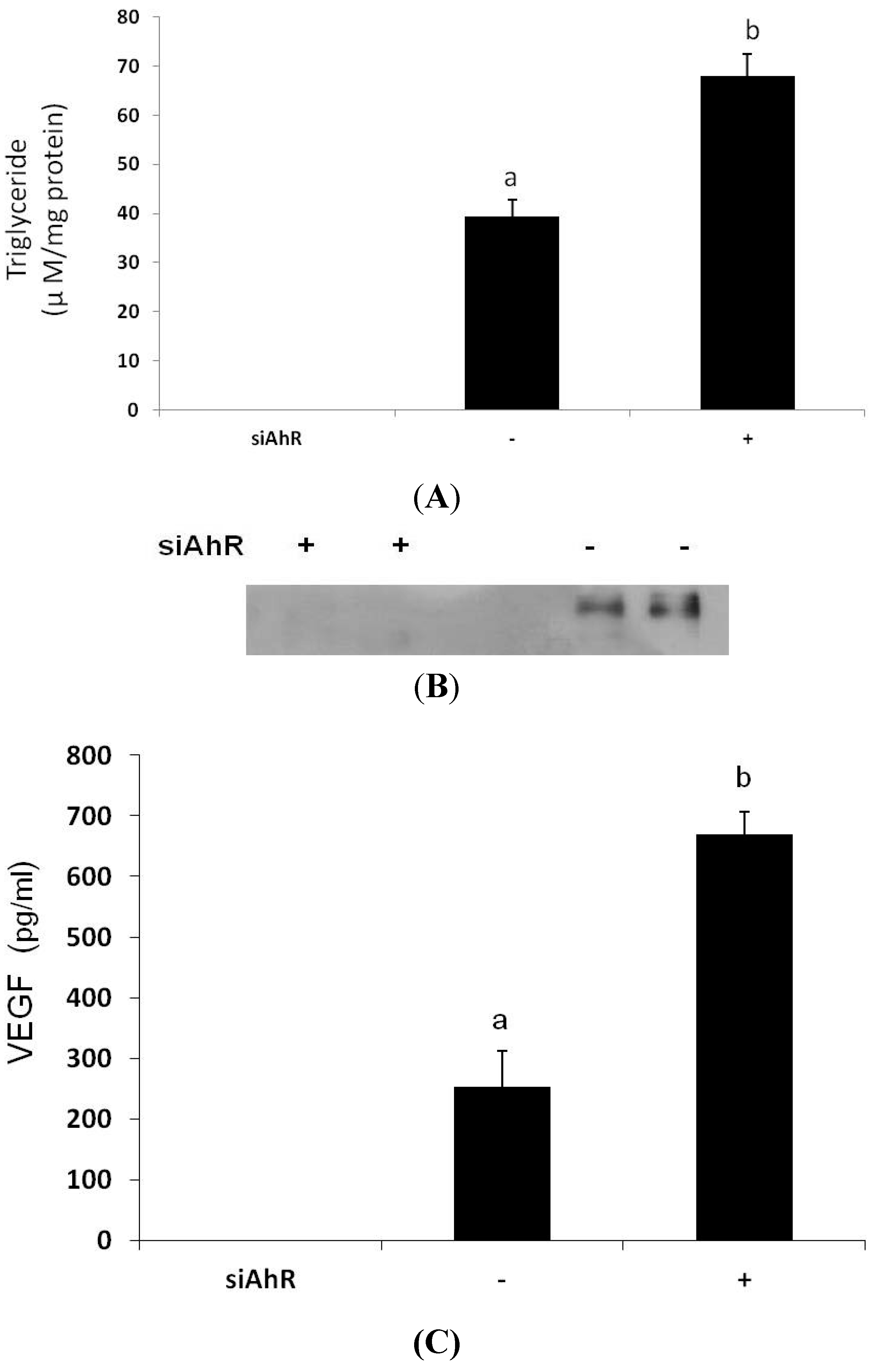
3.4. Effects of AhR Knockout on Lipid Accumulation and VEGF Secretion
4. Discussion

5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desai, M.; Beall, M.; Ross, M.G. Developmental origins of obesity: Programmed adipogenesis. Curr. Diab. Rep. 2013, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, S.M.; Lazar, M.A. Transcriptional control of adipogenesis. Annu. Rev. Nutr. 2000, 20, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R. Angiogenesis and obesity. Cardiovasc. Res. 2008, 78, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ailhaud, G. Autocrine/paracrine effectors of adipogenesis. Ann. Endocrinol. 2002, 63, 83–85. [Google Scholar]
- Rutkowski, J.M.; Davis, K.E.; Scherer, P.E. Mechanisms of obesity and related pathologies: The macro- and microcirculation of adipose tissue. FEBS J. 2009, 276, 5738–5746. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hausman, G.J.; Richardson, R.L. Adipose tissue angiogenesis. J. Anim. Sci. 2004, 82, 925–934. [Google Scholar] [PubMed]
- Safe, S.; Lee, S.O.; Jin, U.H. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol. Sci. 2013, 135, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Gesta, S.; Boucher, J.; Wang, X.L.; Kahn, C.R. The differential role of Hif1beta/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab. 2011, 14, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Kerley-Hamilton, J.S.; Trask, H.W.; Ridley, C.J.; Dufour, E.; Ringelberg, C.S.; Nurinova, N.; Wong, D.; Moodie, K.L.; Shipman, S.L.; Moore, J.H.; Korc, M.; Shworak, N.W.; Tomlinson, C.R. Obesity is mediated by differential aryl hydrocarbon receptor signaling in mice fed a Western diet. Environ. Health Perspect. 2012, 120, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Brodie, A.E.; Azarenko, V.A.; Hu, C.Y. Inhibition of increases of transcription factor mRNAs during differentiation of primary rat adipocytes by in vivo 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) treatment. Toxicol. Lett. 1997, 90, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.C.; Carvajal-Gonzalez, J.M.; Rico-Leo, E.M.; Fernandez-Salguero, P.M. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J. Biol. Chem. 2009, 284, 25135–25148. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Wada, T.; Tezuka, M. Arylhydrocarbon receptor (AhR) is involved in negative regulation of adipose differentiation in 3T3-L1 cells: AhR inhibits adipose differentiation independently of dioxin. J. Cell Sci. 2001, 114, 2809–2817. [Google Scholar] [PubMed]
- Jiang, C.; Qu, A.; Matsubara, T.; Chanturiya, T.; Jou, W.; Gavrilova, O.; Shah, Y.M.; Gonzalez, F.J. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 2011, 60, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Lensu, S.; Tuomisto, J.; Pohjanvirta, R. Dioxins, the aryl hydrocarbon receptor and the central regulation of energy balance. Front. Neuroendocrinol. 2010, 31, 452–478. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Matsumura, F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced down-regulation of glucose transporting activities in mouse 3T3-L1 preadipocyte. J. Environ. Sci. Health B 2002, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Myre, M.; Imbeault, P. Persistent organic pollutants meet adipose tissue hypoxia: Does cross-talk contribute to inflammation during obesity? Obes. Rev. 2014, 15, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Müllerová, D.; Kopecký, J. White adipose tissue: Storage and effector site for environmental pollutants. Physiol. Res. 2007, 56, 375–381. [Google Scholar] [PubMed]
- He, Q.; Huang, C.; Zhao, L.; Feng, J.; Shi, Q.; Wang, D.; Wang, S. Alpha-Naphthoflavone inhibits 3T3-L1 pre-adipocytes differentiation via modulating p38MAPK signaling. Int. J. Clin. Exp. Pathol. 2013, 6, 168–178. [Google Scholar] [PubMed]
- Sun, Y.; Xie, M.; Huang, T.; Zhang, X.; Lei, S.; Shi, Q.; Wang, S.; Fan, C.; Zhang, J. Alpha-Naphthoflavone modulates inflammatory response in adipocytes-macrophages interaction through NFkappaB signaling. Int. J. Clin. Exp. Pathol. 2014, 7, 7768–7774. [Google Scholar] [PubMed]
- Poulos, S.P.; Dodson, M.V.; Hausman, G.J. Cell line models for differentiation: Preadipocytes and adipocytes. Exp. Biol. Med. 2010, 235, 1185–1193. [Google Scholar] [CrossRef]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA. 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Margolis, M.; Schreiner, C.; Edgell, C.J.; Azizkhan, J.; Lazarowski, E.; Juliano, R.L. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: Contributions of induced gene expression, G-proteins, and integrins. J. Cell Physiol. 1992, 153, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Zacarías, J.; Castro-Munozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Gadupudi, G.; Gourronc, F.A.; Ludewig, G.; Robertson, L.W.; Klingelhutz, A.J. PCB126 inhibits adipogenesis of human preadipocytes. Toxicol. In Vitro 2015, 29, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.P.; Wang, M.L.; Chan, M.H.; Chiu, Y.S.; Chen, Y.H. Antiobesity activities of indole-3-carbinol in high-fat-diet-induced obese mice. Nutrition. 2011, 27, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Pelloux, V.; Guyot, E.; Tordjman, J.; Bui, L.C.; Chevallier, A.; Forest, C.; Benelli, C.; Clement, K.; Barouki, R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ. Health Perspect. 2012, 120, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Podechard, N.; Fardel, O.; Corolleur, M.; Bernard, M.; Lecureur, V. Inhibition of human mesenchymal stem cell-derived adipogenesis by the environmental contaminant benzo(a)pyrene. Toxicol. In Vitro 2009, 23, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Arsenescu, V.; Arsenescu, R.I.; King, V.; Swanson, H.; Cassis, L.A. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ. Health Perspect. 2008, 116, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Ellero, S.; Chakhtoura, G.; Barreau, C.; Langouet, S.; Benelli, C.; Penicaud, L.; Beaune, P.; de Waziers, I. Xenobiotic-metabolizing cytochromes p450 in human white adipose tissue: Expression and induction. Drug Metab. Dispos. 2010, 38, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wada, T.; Febbraio, M.; He, J.; Matsubara, T.; Lee, M.J.; Gonzalez, F.J.; Xie, W. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology 2010, 139, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Fukuda, K.; Nagata, Y.; Okada, H.; Haga, A.; Hatakeyama, S.; Yoshida, S.; Okamoto, T.; Hosaka, M.; Sekine, K.; et al. The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio. 2014, 4, 796–803. [Google Scholar]
- Hughes, D.; Guttenplan, J.B.; Marcus, C.B.; Subbaramaiah, K.; Dannenberg, A.J. Heat shock protein 90 inhibitors suppress aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and DNA adduct formation. Cancer Prev. Res. 2008, 1, 485–493. [Google Scholar] [CrossRef]
- Shimba, S.; Todoroki, K.; Aoyagi, T.; Tezuka, M. Depletion of arylhydrocarbon receptor during adipose differentiation in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 1998, 249, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Langin, D.; Manganiello, V.; Belfrage, P.; Degerman, E. Regulation of hormone-sensitive lipase activity in adipose tissue. Methods Enzymol. 1997, 286, 45–67. [Google Scholar] [PubMed]
- Belfrage, P.; Fredrikson, G.; Nilsson, N.O.; Stralfors, P. Regulation of adipose-tissue lipolysis by phosphorylation of hormone-sensitive lipase. Int. J. Obes. 1981, 5, 635–641. [Google Scholar] [PubMed]
- Silha, J.V.; Krsek, M.; Sucharda, P.; Murphy, L.J. Angiogenic factors are elevated in overweight and obese individuals. Int. J. Obes. 2005, 29, 1308–1314. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-alpha and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar] [PubMed]
- Sarkanen, J.R.; Kaila, V.; Mannerstrom, B.; Raty, S.; Kuokkanen, H.; Miettinen, S.; Ylikomi, T. Human adipose tissue extract induces angiogenesis and adipogenesis in vitro. Tissue Eng. Part A 2012, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kawakami, S.; Nakanishi, N. Fat depot-specific differences in angiogenic growth factor gene expression and its relation to adipocyte size in cattle. J. Vet. Med. Sci. 2010, 72, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Rega, G.; Kaun, C.; Demyanets, S.; Pfaffenberger, S.; Rychli, K.; Hohensinner, P.J.; Kastl, S.P.; Speidl, W.S.; Weiss, T.W.; Breuss, J.M.; et al. Vascular endothelial growth factor is induced by the inflammatory cytokines interleukin-6 and oncostatin m in human adipose tissue in vitro and in murine adipose tissue in vivo. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1587–1595. [Google Scholar]
- Anagnostoulis, S.; Karayiannakis, A.J.; Lambropoulou, M.; Efthimiadou, A.; Polychronidis, A.; Simopoulos, C. Human leptin induces angiogenesis in vivo. Cytokine 2008, 42, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Mahadev, K.; Wu, X.; Donnelly, S.; Ouedraogo, R.; Eckhart, A.D.; Goldstein, B.J. Adiponectin inhibits vascular endothelial growth factor-induced migration of human coronary artery endothelial cells. Cardiovasc. Res. 2008, 78, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Lee, C.Y.; Kang, M.S.; Kim, M.H.; Ryu, Y.H.; Bae, K.H.; Shin, S.J.; Lee, S.C.; Ko, Y. Effects of leptin on lipid metabolism and gene expression of differentiation-associated growth factors and transcription factors during differentiation and maturation of 3T3-L1 preadipocytes. Endocr. J. 2008, 55, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, E.; Levy, Y.; Kahana, C.; Shilo, B.Z.; Rubinstein, M.; Cohen, B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998, 17, 5085–5094. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Cheng, Y.W.; Hsu, Y.T.; Hsu, Y.J.; Liao, P.L.; Kang, J.J. Benzo[a]pyrene inhibits angiogenic factors–induced ανβ3 integrin expression, neovasculogenesis, and angiogenesis in human umbilical vein endothelial cells. Toxicol. Sci. 2010, 118, 544–553. [Google Scholar]
- Feng, S.; Cao, Z.; Wang, X. Role of aryl hydrocarbon receptor in cancer. Biochim. Biophys. Acta 2013, 1836, 197–210. [Google Scholar] [PubMed]
- Yamada, K.; Harada, N. Expression of estrogen synthetase (P-450 aromatase) during adipose differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Comm. 1990, 169, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Baglietto, L.; English, D.R.; Hopper, J.L.; MacInnis, R.J.; Morris, H.A.; Tilley, W.D.; Krishnan, K.; Giles, G.G. Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res. Treat. 2009, 115, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Kinneer, K.; Bi, Y.; Chan, J.Y.; Kan, Y.W. Induction of murine NAD(P)H:quinone oxidoreductase by 2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap ‘n’ collar) basic leucine zipper transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2): Cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem. J. 2004, 377, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-L.; Lin, S.-H.; Hou, Y.-Y.; Chen, Y.-H. α-Naphthoflavone Increases Lipid Accumulation in Mature Adipocytes and Enhances Adipocyte-Stimulated Endothelial Tube Formation. Nutrients 2015, 7, 3166-3183. https://doi.org/10.3390/nu7053166
Wang M-L, Lin S-H, Hou Y-Y, Chen Y-H. α-Naphthoflavone Increases Lipid Accumulation in Mature Adipocytes and Enhances Adipocyte-Stimulated Endothelial Tube Formation. Nutrients. 2015; 7(5):3166-3183. https://doi.org/10.3390/nu7053166
Chicago/Turabian StyleWang, Mei-Lin, Shyh-Hsiang Lin, Yuan-Yu Hou, and Yue-Hwa Chen. 2015. "α-Naphthoflavone Increases Lipid Accumulation in Mature Adipocytes and Enhances Adipocyte-Stimulated Endothelial Tube Formation" Nutrients 7, no. 5: 3166-3183. https://doi.org/10.3390/nu7053166



