Resveratrol Protects against Methylglyoxal-Induced Hyperglycemia and Pancreatic Damage In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Diabetes Induction
2.3. Blood Sample Collection
2.4. Oral Glucose Tolerance Test and Insulin Tolerance Test
2.5. Serum Insulin, Hepatic Inflammatory Factors and Tyr-Phosphorylated Insulin Receptor Substrate-1 Protein Expression Assay
2.6. Homeostasis Model Assessment-Insulin Resistance
2.7. Immunohistochemistry Stain
2.8. Statistical Analysis
3. Results
3.1. The Anti-Diabetic Effects of Resveratrol in MG-Treated Mice
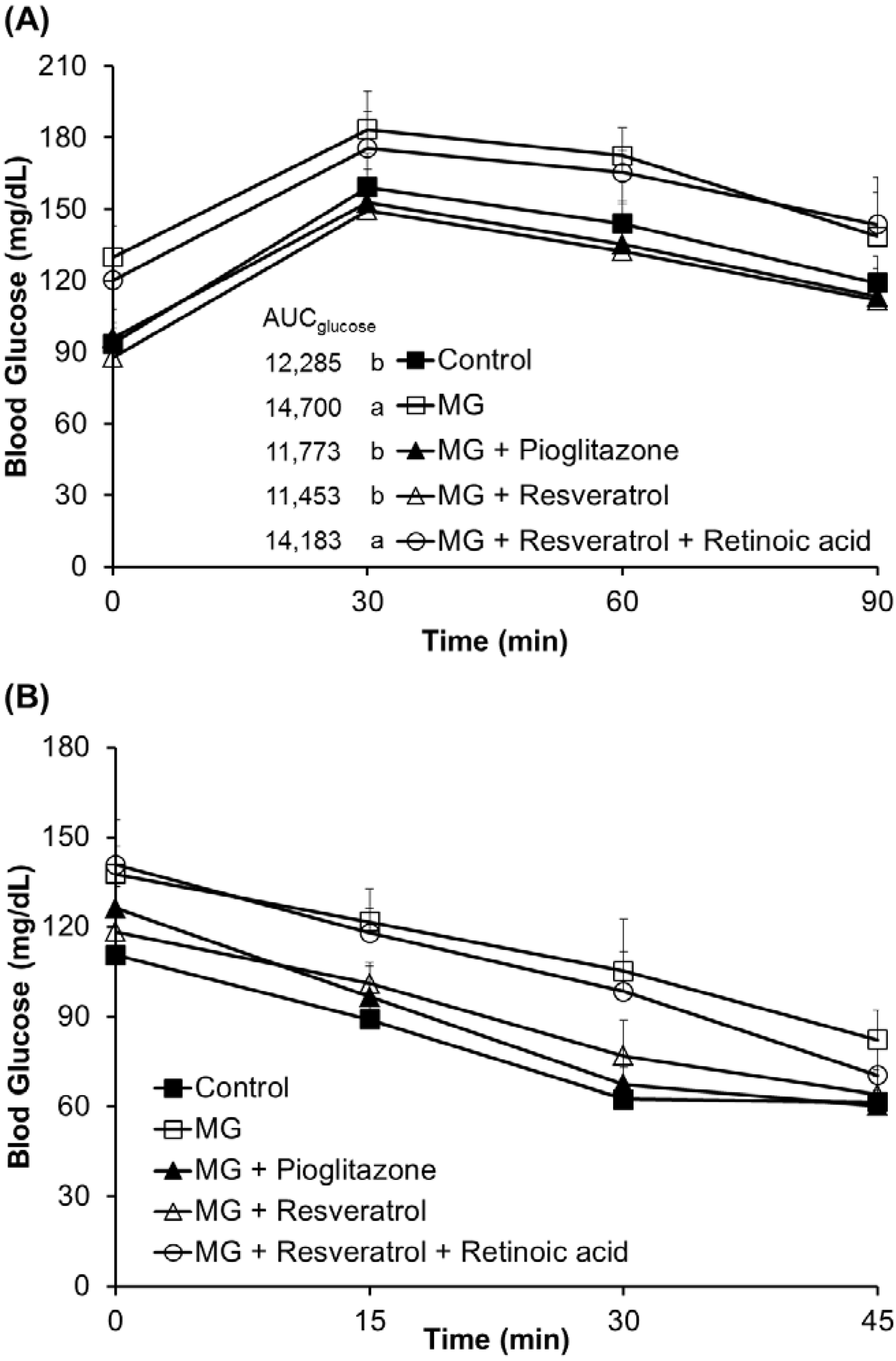
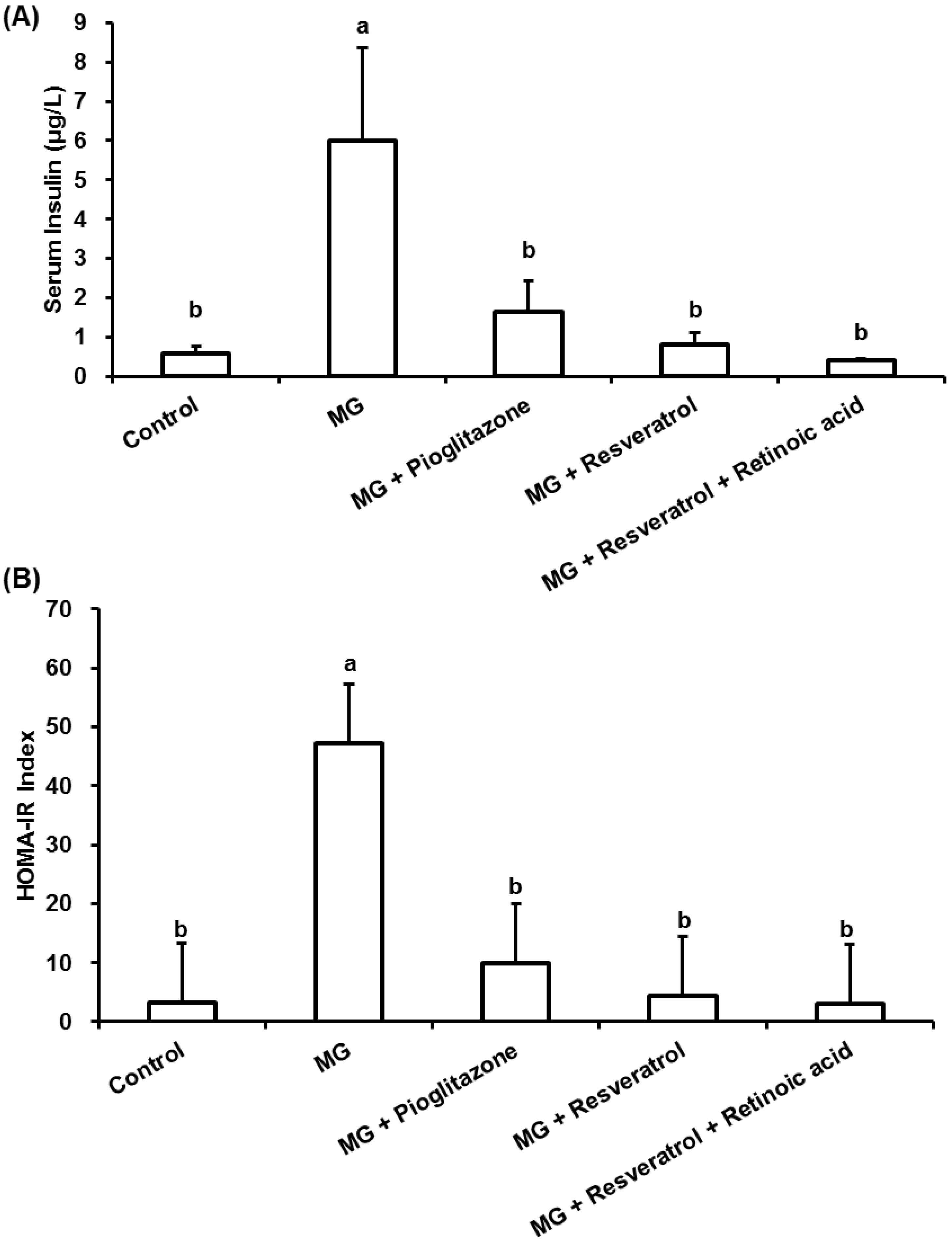
3.2. Anti-Inflammatory Effects of Resveratrol

3.3. Resveratrol-Mediated Elevation of Liver Tyr-Phosphorylated IRS-1 Levels
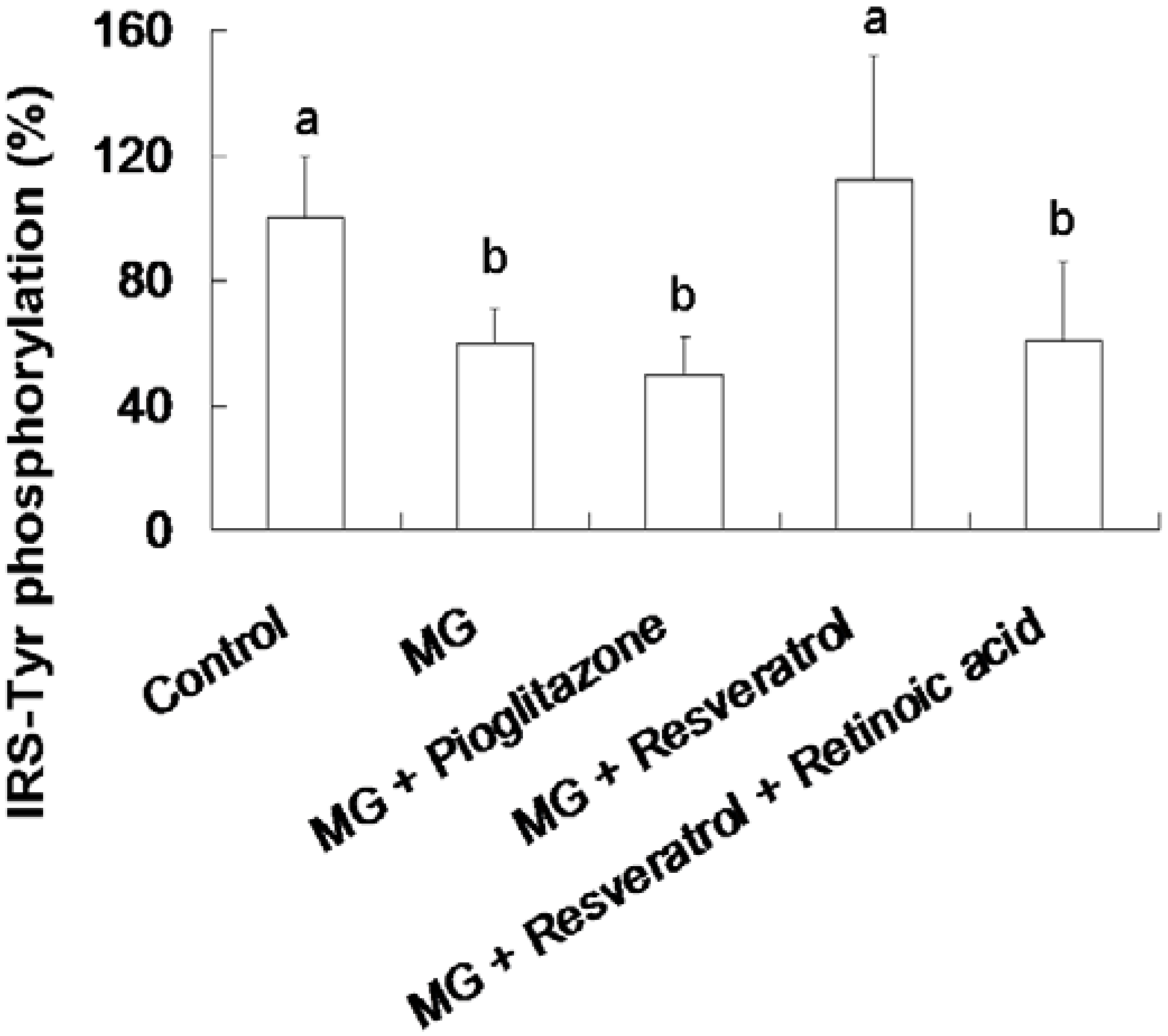
3.4. IHC Stain for Pancreatic Insulin and p-Nrf2

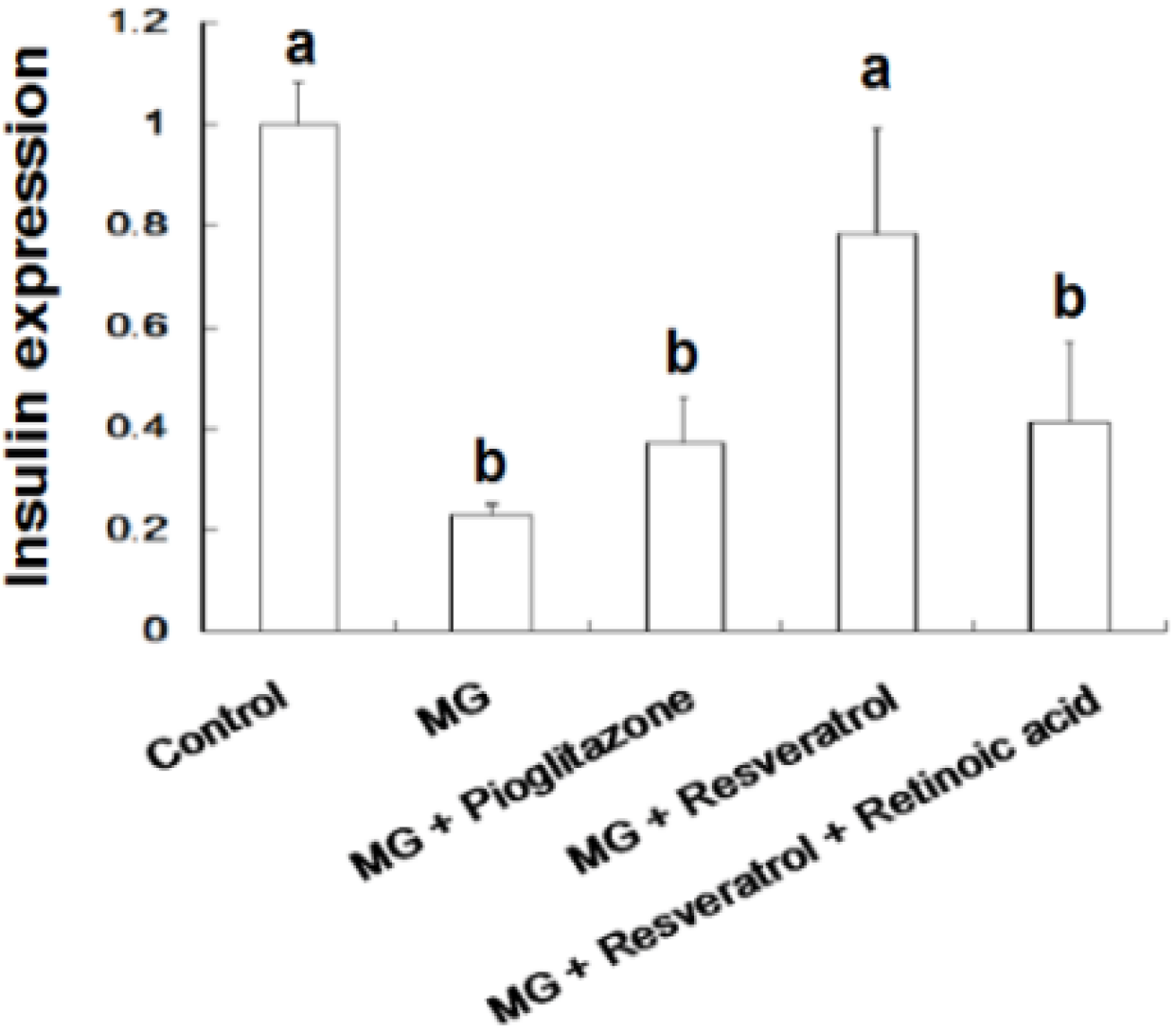
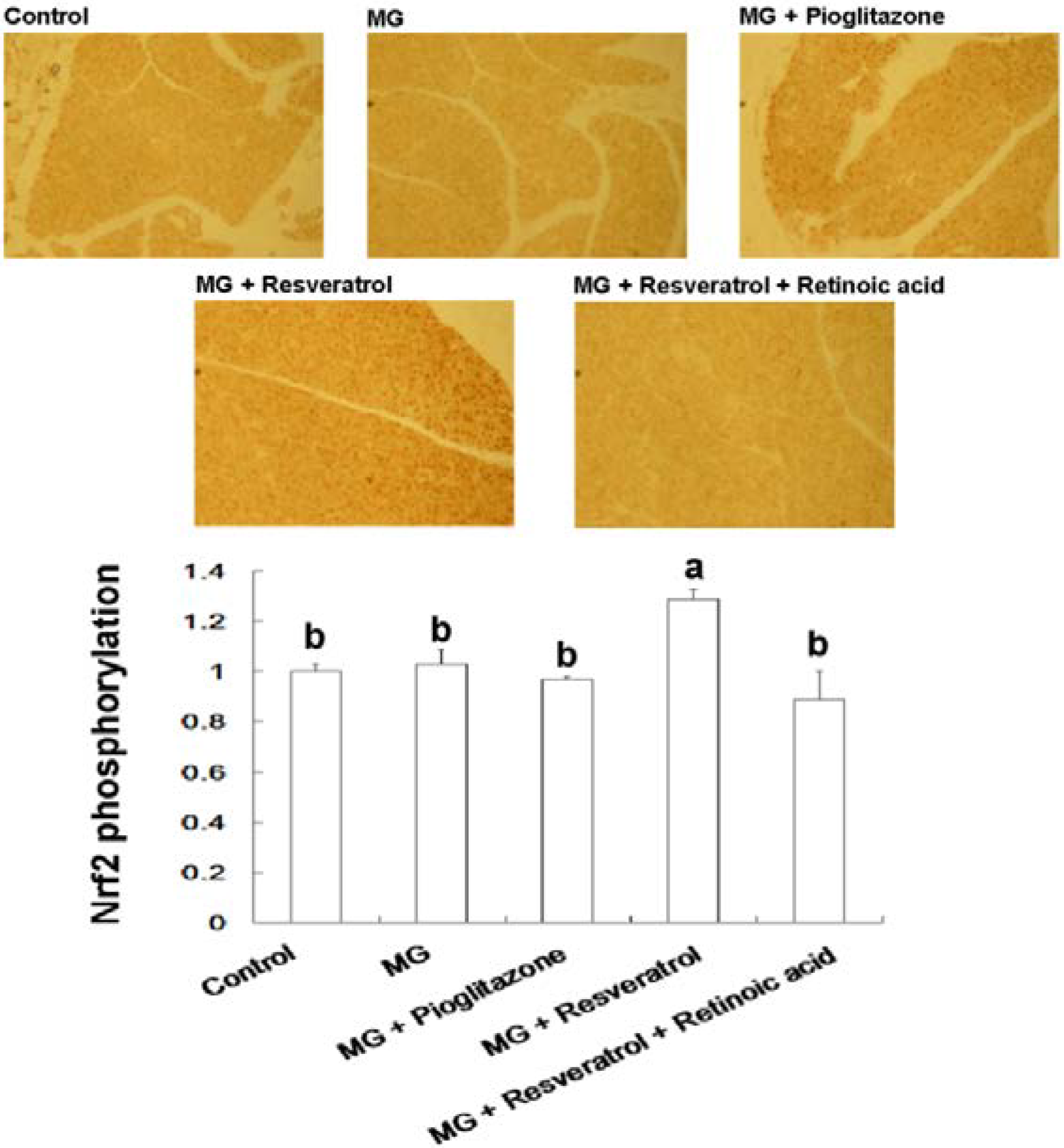
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Negre-Salvayre, A.; Halvayre, R.; Auge, N.; Pamplona, R.; Portero-Otin, M. Hyperglycemia and glycation in diabetic complications. Antioxid. Redox Signal. 2009, 11, 3071–3109. [Google Scholar] [CrossRef] [PubMed]
- Schiekofer, S.; Andrassy, M.; Chen, J.; Rudofsky, G.; Schneider, J.; Wendt, T.; Stefan, N.; Humpert, P.; Fritsche, A.; Stumvoll, M.; et al. Acute hyperglycemia causes intracellular formation of CML and activation of ras p42/44 MAPK and nuclear factor kappaB in PBMCs. Diabetes 2003, 52, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Huang, S.M.; Lin, J.A.; Yen, G.C. Inhibition of advanced glycationend product formation by foodstuffs. Food Funct. 2011, 2, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Desai, K.M.; Wu, L. Alagebrium attenuates acute methylglyoxal induced glucose intolerance in Sprague-Dawley rats. Br. J. Pharmacol. 2010, 159, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Dhar, I.; Jiang, B.; Desai, K.M.; Wu, L. Chronic methylglyoxal injection by minipump causes pancreatic beta-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes 2011, 60, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.S.; Owuor, E.D.; Kim, B.R.; Hu, R.; Kong, A.N. Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethylisothiocyanate (PEITC). Pharm. Res. 2003, 20, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Vander-Jagt, D.; Hunsaker, L. Methylglyoxal metabolism and diabetic complications: Roles of aldose reductase, glyxalase-I, betaine aldehyde dehydrogenase and oxoaldehyde dehydrogenase. Chem. Biol. Int. 2003, 143, 341–351. [Google Scholar] [CrossRef]
- Romero-Sarmiento, Y.; Soto-Rodriguez, I.; Arzaba-Villalba, A.; Garcia, H.S.; Alexander-Aguilera, A. Effects of conjugated linoleic acid on oxidative stress in rats with sucrose-induced non-alcoholic fatty liver disease. J. Funct. Foods 2012, 4, 219–225. [Google Scholar] [CrossRef]
- Khuhawar, M.Y.; Kandhro, A.J.; Khand, F.D. Liquid chromatographic determination of glyoxal and methylglyoxal from serum of diabetic patients using meso-stilbenediamine as derivatizing agent. Anal. Lett. 2006, 39, 2205–2215. [Google Scholar] [CrossRef]
- Wang, J.; Chang, T. Methylglyoxal content in drinking coffee as a cytotoxic factor. J. Food Sci. 2010, 75, H167–H171. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Arrbias-Lorenzo, G.; Morales, F.J. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem. 2010, 58, 2966–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Revel, G.; Bertrand, A. A method for the detection of carbonyl compounds in wine: Glyoxal and methylglyoxal. J. Sci. Food Agric. 1993, 61, 267–272. [Google Scholar] [CrossRef]
- Barros, A.; Rodrigues, J.A.; Almeida, P.J.; Oliva-Teles, M.T. Determination of glyoxal, methylglyoxal and diacetyl in selected beer and wine by HPLC with UV spectrophotometric detection after derivatization with O-phenylendiamine. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 2061–2069. [Google Scholar] [CrossRef]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef] [PubMed]
- Murakamim, A. Modulation of protein quality control systems by food phytochemicals. J. Clin. Biochem. Nutr. 2013, 52, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Xu, Y.; Nie, L.; Yin, Y.G.; Tang, J.L.; Zhou, J.Y.; Li, D.D.; Zhou, S.W. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol. Appl. Pharmacol. 2012, 259, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Fu, Y.C.; Wang, W. Cellular and molecular effects of resveratrol in health and disease. J. Cell Biochem. 2012, 113, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Fiori, J.L.; Shin, Y.K.; Kim, W.; Krzysik-Walker, S.M.; González-Mariscal, I.; Carlson, O.D.; Sanghvi, M.; Moaddel, R.; Farhang, K.; Gadkaree, S.K.; et al. Resveratrol prevents β-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes 2013, 62, 3500–3513. [Google Scholar] [CrossRef] [PubMed]
- Do, G.M.; Jung, U.J.; Park, H.J.; Kwon, E.Y.; Jeon, S.M.; McGregor, R.A.; Choi, M.S. Resveratrol ameliorates diabetesrelated metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 2012, 56, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, L.; Lu, Y.; Zhang, J.; Jian, F.; Liu, Y.; Li, F.; Li, W.; Wang, X.; Li, G. Activation of SIRT1 protects pancreatic β cells against palmitate-induced dysfunction. Biochim. Biophys. Acta 2012, 1822, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.; Zheng, J.; Zeng, T.; Li, H.; Xiao, H.; Deng, X.; Hu, X. The protective effect of resveratrol on islet insulin secretion and morphology in mice on a high-fat diet. Diabetes Res. Clin. Pract. 2012, 97, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.S.; Cheng, Y.H.; Chang, T.L. Resveratrol protects RINm5F pancreatic cells from methylglyoxal-induced apoptosis. J. Funct. Foods 2013, 5, 1774–1783. [Google Scholar] [CrossRef]
- Ku, C.R.; Lee, H.J.; Kim, S.K.; Lee, E.Y.; Lee, M.K.; Lee, E.J. Resveratrol prevents streptozotocin-induced diabetes by inhibiting the apoptosis of pancreatic β-cell and the cleavage of poly (ADP-ribose) polymerase. Endocr. J. 2012, 59, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Rouse, M.; Younès, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic β-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2014. [Google Scholar] [CrossRef]
- Movahed, A.; Nabipour, I.; Louis, X.L.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. eCAM 2013, 2013, 851267–851278. [Google Scholar] [PubMed]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmocokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. J. Pharm. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Blanchard, O. Enhancing the delivery of resveratrol in humans: If low bioavailability is the problem, what is the solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Furimsky, A.; Green. , C.; Sharp, E.; Cats, P.; Adjeu, A.; Parman, T.; Kapetanovic, I.; Weinshilboum, R.; Lyver, L. Effect of resveratrol on 17beta-estradiol sulfation by human hepatic and jejunal S9 and recombinant sulfotransferase 1E1. Drug Metab. Dispos. 2008, 36, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Hayes, J.D.; Henderson, C.J.; Wolf, C.R. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor α. Proc. Natl. Acad. Sci. USA 2007, 104, 19589–19594. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.S.; Cheng, Y.H.; Chiou, C.H.; Chang, T.L. Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells. J. Agric. Food Chem. 2012, 60, 9180–9187. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Lan, K.P.; Liu, H.Y.; Zhang, X.Z.; Lin, Y.F.; Chen, T.Y.; Chiou, H.L. Hepatitis C virus E2 protein involve in insulin resistance through an impairment of Akt/PKB and GSK3ß signaling in hepatocytes. BMC Gastroenterol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Ellmerer, M.; Can Citters, G.W.; Bergman, R.N. Primacy of hepatic insulin resistance in the development of the metabolic syndrome induced by an isocaloric moderate-fat diet in the dog. Diabetes 2003, 52, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, D.L.; Mao, X.Q.; Zou, F.; Jin, H.; Ouyang, I.P. Astragalus polysaccharides decreased the expression of PTP1B through relieving ER stress induced activation of ATF6 in a rat model of type 2 diabetes. Mol. Cell Endocrinol. 2009, 307, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.T.; Wang, J.; Ree, D.; Koll, J.K.; Bryer-Ash, M. Tumor necrosis factor-α induces hepatic insulin resistance in obese zucker (fa/fa) rats via interaction of leukocyte antigen-related tyrosine phosphatase with focal adhesion kinase. Diabetes 2000, 49, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Pan, T.M. Inhibitory effects of dioscorea polysaccharide on TNF-alpha-induced insulin resistance in mouse FL83B cells. J. Agric. Food Chem. 2011, 59, 5279–5285. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Kume, S.; Kanasaki, K.; Takeda-Watanabe, A.; Koya, D. Sirtuins as possible drug targets in type 2 diabetes. Curr. Drug Targets 2013, 14, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Koya, D. SIRT1 in type 2 diabetes: Mechanisms and therapeutic potential. Diabetes Metab. J. 2013, 37, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Hsu, M.C.; Hsieh, C.W.; Lin, J.B.; Lai, P.H.; Wung, B.S. Upregulation of heme oxygenase-1 by epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006, 78, 2889–2897. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Huang, S.M.; Yen, G.C. Silymarin: A novel antioxidant with antiglycation and antiinflammatory properties in vitro and in vivo. Antioxid. Redox Signal. 2011, 14, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, N.A.; Appiah-Opong, R. Toxicity of low levels ofmethylglyoxal: Depletion of blood glutathione and adverse effect onglucose tolerance in mice. Toxicol. Lett. 1999, 109, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Chartoumpekis, D.V.; Ziros, P.G.; Psyrogiannis, A.I.; Papavassiliou, A.G.; Kyriazopoulou, V.E.; Sykiotis, G.P.; Habeos, I.G. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes 2011, 60, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.T.; Whitman, S.A.; Wu, W.; Wondrak, G.T.; Wong, P.K.; Fang, D. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 2011, 60, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Chang, Y.Y.; Hsu, Y.W.; Pan, T.M. A novel natural Nrf2 activator with PPARgamma-agonist (monascin) attenuates the toxicity of methylglyoxal and hyperglycemia. Toxicol. Appl. Pharmacol. 2013, 272, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Li, C.H.; Hsu, Y.W.; Pan, T.M. Monascin and AITC attenuate methylglyoxal-induced PPARgamma phosphorylation and degradation through inhibition of the oxidative stress/PKC pathway depending on Nrf2 activation. J. Agric. Food Chem. 2013, 61, 5996–6006. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Huang, T.; Chang, Y.Y.; Hsu, Y.W.; Pan, T.M. Effects of monascin on anti-inflammation mediated by Nrf2 activation in advanced glycation end product-treated THP-1 monocytes and methylglyoxal-treated Wistar rats. J. Agric. Food Chem. 2013, 61, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Hsu, Y.W.; Pan, T.M. Dimerumic acid attenuates receptor for advanced glycationend products signal to inhibit inflammation and diabetes mediated by Nrf2 activation and promotes methylglyoxal metabolism into d-lactic acid. Free Radic. Biol. Med. 2013, 60, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Huang, Y.C.; Lee, B.H.; Hsu, Y.W.; Pan, T.M. The improvements of ankaflavin isolated from Monascus-fermented products on dyslipidemia in high-fat diet-induced hamster. J. Funct. Foods 2013, 5, 434–443. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, B.H.; Chang, Y.Y.; Hsu, Y.W.; Pan, T.M. Ankaflavin, a novel Nrf2 activator for attenuating allergic airway inflammation. Free Radic. Biol. Med. 2012, 53, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, L.; Szklarz, G.; Bi, Y.; Ma, Q. Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2-related factor 2 pathway. J. Pharmacol. Exp. Ther. 2012, 342, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim. Biophys. Acta 2011, 1812, 719–731. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, A.-S.; Cheng, Y.-H.; Lee, C.-Y.; Chung, C.-Y.; Chang, W.-C. Resveratrol Protects against Methylglyoxal-Induced Hyperglycemia and Pancreatic Damage In Vivo. Nutrients 2015, 7, 2850-2865. https://doi.org/10.3390/nu7042850
Cheng A-S, Cheng Y-H, Lee C-Y, Chung C-Y, Chang W-C. Resveratrol Protects against Methylglyoxal-Induced Hyperglycemia and Pancreatic Damage In Vivo. Nutrients. 2015; 7(4):2850-2865. https://doi.org/10.3390/nu7042850
Chicago/Turabian StyleCheng, An-Sheng, Yu-Hsiang Cheng, Chi-Ying Lee, Chin-Yuan Chung, and Wen-Chang Chang. 2015. "Resveratrol Protects against Methylglyoxal-Induced Hyperglycemia and Pancreatic Damage In Vivo" Nutrients 7, no. 4: 2850-2865. https://doi.org/10.3390/nu7042850



