Protective Effect of Vanillic Acid against Hyperinsulinemia, Hyperglycemia and Hyperlipidemia via Alleviating Hepatic Insulin Resistance and Inflammation in High-Fat Diet (HFD)-Fed Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Tumor Necrosis Factor-Alpha (TNF-α) Induction of Insulin Resistance
2.4. Uptake of Fluorescent 2-(N-(7-Nitrobenz-2-oxa-1,3-Diazol-4-yl)Amino)-2-Deoxyglucose in FL83B Mouse Hepatocytes
2.5. Animals and Diets
2.6. Blood Sample Preparation
2.7. Biochemical Measurements
2.8. Oral Glucose Tolerance Test (OGTT)
2.9. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) Index [14]
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Phenolic Acids on Cell Viability and Glucose Uptake Ability in Insulin-Resistant FL83B Mouse Hepatocytes
| Concentrations | Cell Viability (%) a | Amelioration Rate (%) b (12.5 μM Phenolic Acid) | ||||
|---|---|---|---|---|---|---|
| Phenolic Acids | 12.5 μM | 25 μM | 50 μM | 100 μM | ||
| Caffeic acid | 102.8 ± 15.8 | 99.4 ± 16.8 | 82.0 ± 14.3 | 74.3 ± 8.6 | −6.60 | |
| Cinnamic acid | 86.2 ± 2.1 | 88.7 ± 2.5 | 88.6 ± 6.7 | 89.6 ± 0.1 | −11.30 | |
| Ferulic acid | 92.7 ± 7.3 | 88.9 ± 9.1 | 86.7 ± 6.7 | 77.4 ± 28.2 | −22.01 | |
| Protocatechuic acid | 91.7 ± 10.4 | 91.8 ± 9.0 | 75.4 ± 8.1 | 71.8 ± 9.0 | −12.90 | |
| Rosmarinic acid | 104.3 ± 17.6 | 97.2 ± 13.1 | 102.5 ± 16.5 | 90.7 ± 22.4 | −10.28 | |
| Sinapic acid | 97.2 ± 2.9 | 102.1 ± 10.1 | 102.0 ± 10.5 | 80.0 ± 9.2 | −15.37 | |
| Syringic acid | 90.9 ± 2.2 | 79.8 ± 17.4 | 70.2 ± 13.1 | 64.6 ± 14.5 | 8.36 | |
| Vanillic acid | 114.2 ± 26.3 | 114.8 ± 18.5 | 109.8 ± 25.5 | 116.7 ± 17.6 | 13.66 | |
3.2. Effect of Vanillic Acid on Glucose Tolerance, Serum Insulin and Insulin Resistance Index in High-Fat Diet (HFD)-Fed Rats
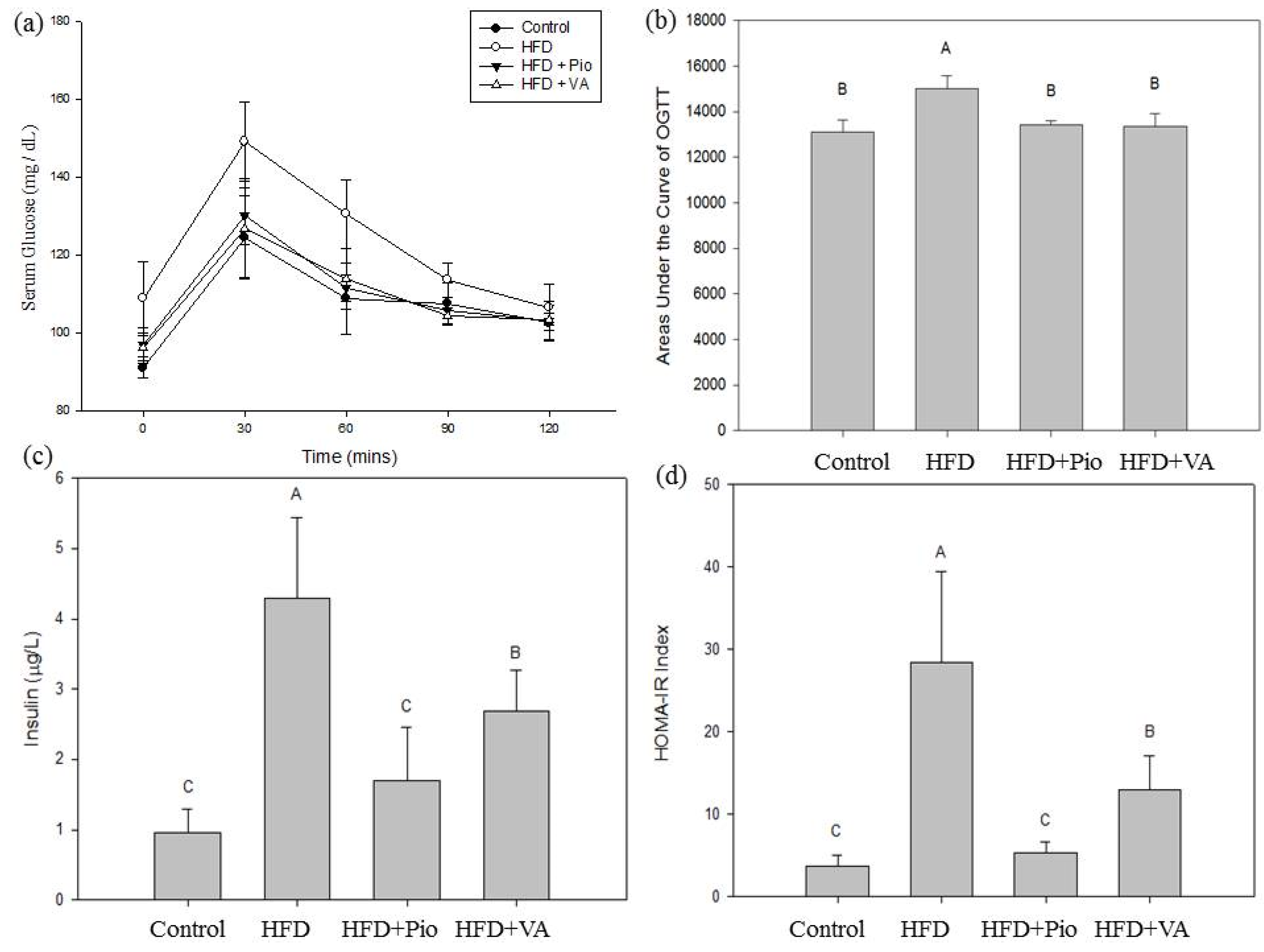
3.3. Effect of Vanillic Acid on Energy Intake, Body Weight, and Selected Organ Weight in HFD-Fed Rats
| Items/Groups | Control | HFD | HFD + Pio | HFD + VA |
|---|---|---|---|---|
| Body weight (g) | 627.1 ± 28.7 C | 760.7 ± 63.1 A | 686.0 ± 30.4 B | 676.9 ± 70.2 B |
| Diet intake (kcal/rat/day) | 104.45 ± 4.43 A | 110.04 ± 11.33 A | 101.63 ± 5.77 A | 104.97 ± 9.91 A |
| Liver weight (g) | 14.6 ± 0.7 B | 18.5 ± 2.2 A | 13.9 ± 1.4 B | 13.9 ± 1.6 B |
| Kidney weight (g) | 1.1 ± 0.4 A | 1.4 ± 0.5 A | 1.7 ± 0.2 A | 0.9 ± 0.6 A |
| Adipose weight (g) | 17.7 ± 4.6 C | 56.1 ± 12.7 A | 36.7 ± 6.4 B | 35.3 ± 12.7 B |
3.4. Effect of Vanillic Acid on Serum Biochemical Parameters in HFD-Fed Rats
| Items/Groups | Control | HFD | HFD + Pio | HFD + VA |
|---|---|---|---|---|
| Glucose (mg/dL) | 90.8 ± 1.7 B | 107.2 ± 5.5 A | 97.0 ± 3.7 B | 94.5 ± 1.9 B |
| Triglyceride (mg/dL) | 74.13 ± 18.20 B | 89.50 ± 11.70 A | 61.50 ± 9.80 B | 71.63 ± 13.42 B |
| Free fatty acid (mmol/L) | 0.92 ± 0.09 B | 1.17 ± 0.26 A | 0.69 ± 0.16 C | 0.76 ± 0.14 B,C |
| Total cholesterol (mg/dL) | 62.38 ± 9.71 A | 51.63 ± 14.35 A | 58.13 ± 9.95 A | 40.00 ± 6.41 B |
| Leptin (ng/mL) | 162.3 ± 32.6 B | 986.3 ± 413.8 A | 429 ± 69.7 B | 303.2 ± 84.2 B |
| Bili-total (mg/dL) | 0.08 ± 0.01 A | 0.07 ± 0.01 A | 0.05 ± 0.01 B | 0.07 ± 0.02 A |
| BUN (mg/dL) | 15.33 ± 1.25 A | 9.55 ± 0.91 C | 12.80 ± 1.05 B | 10.96 ± 2.11 C |
| Creatinine (mg/dL) | 0.35 ± 0.05 AB | 0.38 ± 0.05 A | 0.31 ± 0.04 B | 0.38 ± 0.05 A |
3.5. Effect of Vanillic Acid on Hepatic Insulin Signaling, Inflammation and NEFA Formation in HFD-Fed Rats
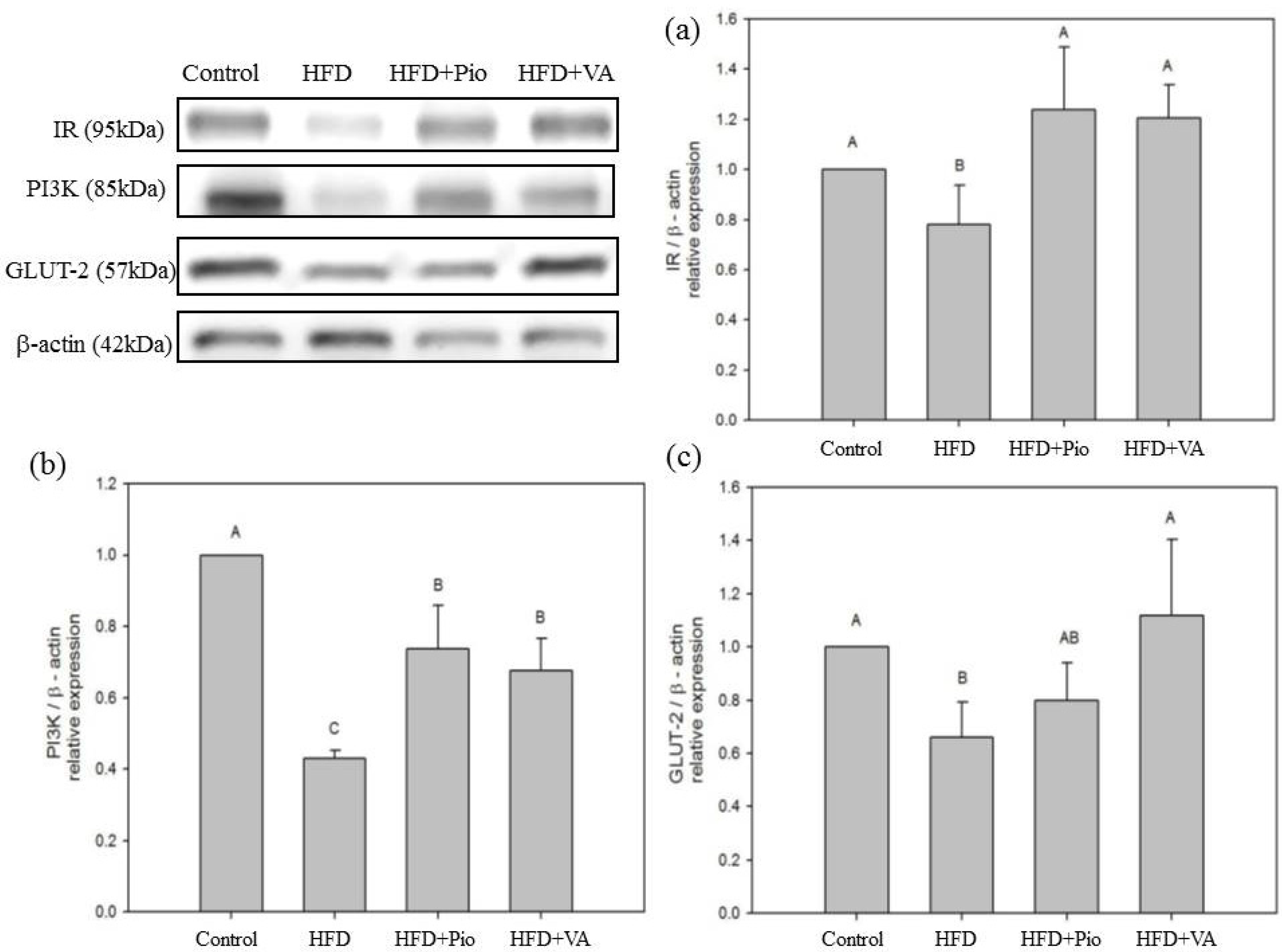

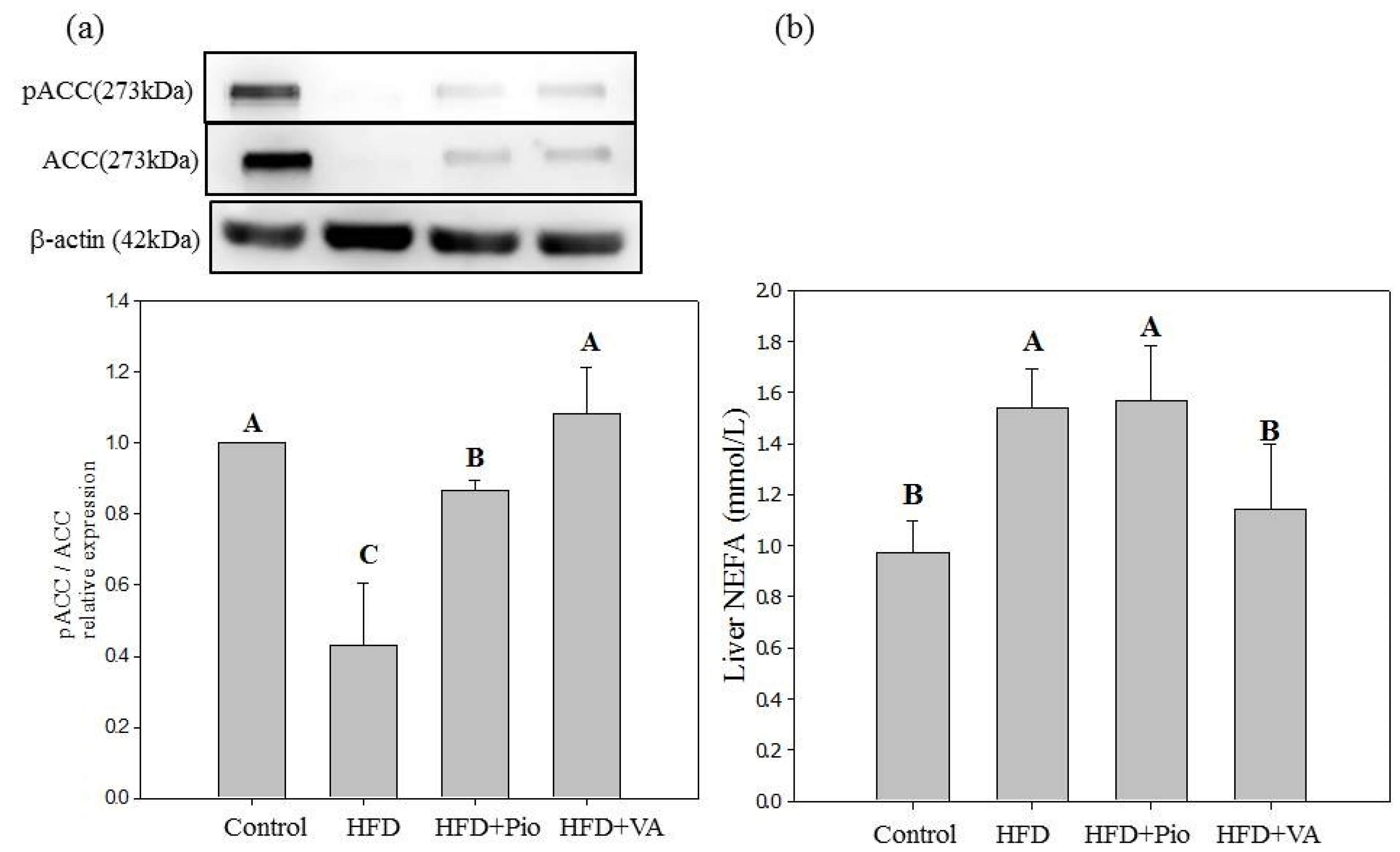
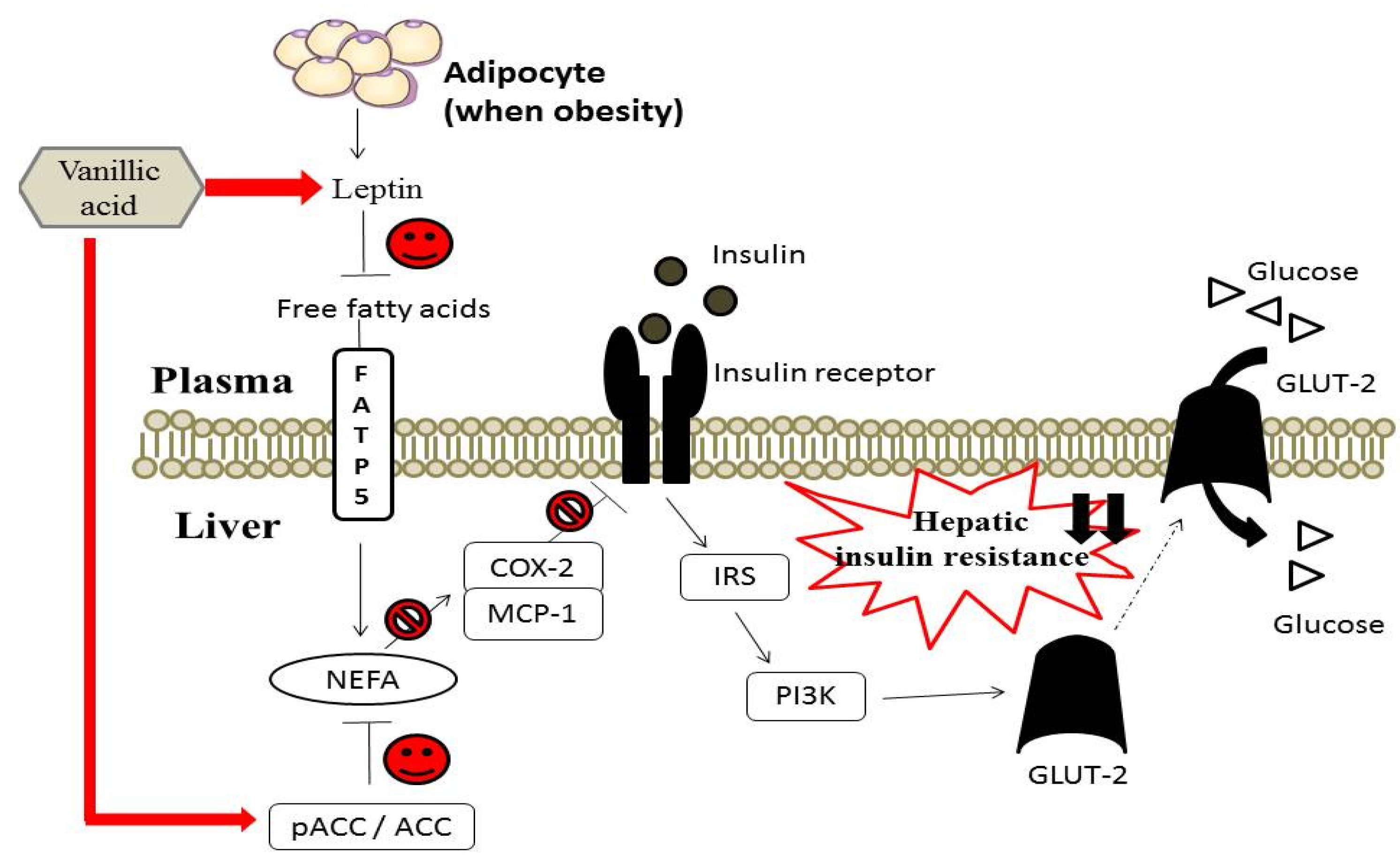
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Preventing Diabetes is Essential for Keeping Health and Well-Being in Tajikistan. 2011. Available online: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/diabetes/news/news/2011/11/preventing-diabetes-is-essential-for-keeping-health-and-well-being-in-tajikistan (accessed on 21 November 2011).
- Buettner, R.; Parhofer, K.G.; Woenckhaus, M.; Wrede, C.E.; Kunz-Schughart, L.A.; Scholmerich, J.; Bollheimer, L.C. Defining high-fat-diet rat models: Metabolic and molecular effect of different fat types. Mol. Endocrinol. 2006, 36, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, Y.; Maddux, B.A.; Mcdonald, A.R.; Logsdon, C.D.; Williams, J.A.; Goldfine, I.D. Mechanisms of insulin-induced insulin-receptor downregulation: Decrease of receptor biosynthesis and mRNA levels. Diabetes 1989, 38, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T. Fructose induced lipogenesis: From sugar to fat to insulin resistance. Trends Endocrinol. Metab. 2011, 22, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKKβ and NF-κB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Qatanani, M.; Lazar, M.A. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Gene. Dev. 2007, 21, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A cyclic voltammetry method suitable for characterizing antioxidant properties of wine and wine phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Roengsamarn, S.; Hsu, W.H.; Yibchok-Anun, S. Mechanisms of antihyperglycemic effect of p-methoxycinnamic acid in normal and streptozotocin-induced diabetic rats. Life Sci. 2005, 78, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Matuo, T.; Tsuno, T.; Hosoda, A.; Normura, E.; Taniguchi, H.; Sasaki, H.; Morishita, H. Antioxidation activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. Biofactors 2004, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Jeon, S.M.; Choi, M.S. Antihyperglycemic and antioxidant properties of caffeic acid I db/db mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Shen, S.C. Effect of water extracts from edible Myrtaceae plants on uptake of 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) in tumor necrosis factor-α-treated FL83B mouse hepatocytes. Phytother. Res. 2013, 27, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diavetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Sargent, J.M. The use of the MTT assay to study drug resistance in fresh tumor samples. Recent Results Cancer Res. 2003, 161, 13–25. [Google Scholar]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O.I. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef]
- Scalbert, A.R.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [PubMed]
- Feinstein, R.; Kanety, H.; Papa, M.Z.; Lunenfeld, B.; Karasik, A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J. Biol. Chem. 1993, 268, 26055–26058. [Google Scholar]
- Peraldi, P.; Hotamisligil, G.S.; Buurman, W.A.; White, M.F.; Spiegelman, B.M. Tumor necrosis factor (TNF-α) inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J. Biol. Chem. 1996, 271, 13018–13022. [Google Scholar]
- Kroder, G.; Bossenmayer, B.; Kellerer, M.; Capp, E.; Stoyanov, B.; Muhlhofer, A.; Berti, L.; Horikoshi, H.; Ullrich, A.; Haring, H. Tumor necrosis factor-alpha and hyperglycemia-induced insulin resistance. J. Clin. Investig. 1996, 97, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Oh, K.B.; Saito, M.; Nemoto, Y.; Matsuoka, H. Evaluation of 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose, a new fluorescent derivative of glucose, for viability assessment of yeast Candida albicans. Appl. Microbiol. Biotechnol. 1996, 46, 400–404. [Google Scholar]
- Louzao, M.C.; Espiña, B.; Vieytes, M.R.; Vega, F.V.; Rubiolo, J.A.; Baba, O.; Terashima, T.; Botana, L.M. “Fluorescent glycogen” formation with sensibility for in vivo and in vitro detection. Glycoconj. J. 2008, 25, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kraegen, E.W.; James, D.E.; Storlien, L.H.; Burleigh, K.M.; Chisholm, D.J. In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: Assessment by euglycaemic clamp plus deoxyglucose administration. Diabetologia 1986, 29, 192–198. [Google Scholar] [CrossRef]
- Pedersen, O.; Kahn, C.R.; Flier, J.S.; Kahn, B.B. High fat feeding causes insulin resistance and a marked decrease in the expression of glucose transporters (Glut 4) in fat cells of rats. Endocrinology 1991, 129, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Ahren, B.; Gudbjartsson, T.; Al-Amin, A.N. Islet perturbations in rats fed a high-fat diet. Pancreas 1999, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kaiyala, K.J.; Prigeon, R.L.; Kahn, S.E.; Woods, S.C.; Porte, D.; Schwartz, M.W. Reduced β-cell function contributes to impaired glucose tolerance in dogs made obese by high-fat feeding. Am. J. Physiol. 1999, 277, E659–E667. [Google Scholar] [PubMed]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, P.S. Metabolic consequences of hyperglycemia and insulin resistance. Clin. Cornerstone 2007, 8, S30–S42. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J. Med. Food 2013, 16, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Hekmatdoost, A.; Mirmiran, P.; Hosseini-Esfahani, F.; Azizi, F. Dietary fatty acid composition and metabolic syndrome in Tehranian adults. Nutrition 2011, 27, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Prada, P.O.; Zecchin, H.G.; Gasparetti, A.L.; Torsoni, M.A.; Ueno, M.; Hirata, A.E.; do Amaral, M.E.; Höer, N.F.; Boschero, A.C.; Saad, M.J. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology 2005, 146, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.W.; Chiang, M.T.; Yao, H.T.; Chiang, W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes. Metab. 2004, 6, 120–126. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Carroll, A.R.; Addepalli, R.; Fechner, G.A.; Avery, V.M.; Quinn, R.J. Vanillic acid derivatives from the green algae Cladophora socialis as potent protein tyrosine phosphatase 1B inhibitors. J. Nat. Prod. 2007, 70, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Fraze, E.; Chiou, M.; Chen, Y.; Reaven, G.M. Age-related changes in postprandial plasma glucose, insulin, and free fatty acid concentrations in nondiabetic individuals. J. Am. Geriatr. Soc. 1987, 35, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Haymond, M.W.; Rizza, R.A.; Cryer, P.E.; Miles, J.M. Influence of body fat distribution on free fatty acid metabolism in obesity. J. Clin. Investig. 1989, 83, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.Y.; Qin, L.Q.; Davaasambuu, G.; Kaneko, T.; Xu, J.; Murata, S.I.; Katoh, R.; Sato, A. The development of diabetes mellitus in wistar rats kept on a high-fat low-carbohydrate diet for long periods. Endocrine 2003, 22, 85–92. [Google Scholar] [CrossRef]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; Mckee, L.J.; Baur, T.L.; et al. Serum immunoreative-leptin concentrations in normal weight and obese human. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Halaas, J.; Ravussin, E.; Pratley, R.E.; Lee, G.H.; Zhang, Y.; Fei, H.; Kim, S.; Lallone, R.; Ranganathan, S.; et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1995, 1, 1155–1161. [Google Scholar] [CrossRef]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Hekmatdoost, A.; Mirmiran, P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int. J. Endocrinol. Metab. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Panzhinskiy, E.; Hua, Y.; Lapchak, P.A.; Topchiy, E.; Lehmann, T.E.; Ren, J.; Nair, S. Novel curcumin derivative CNB-001 mitigates obesity-associated insulin resistance. J. Pharmacol. Exp. Ther. 2014, 349, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Martyn, J.A.; Kaneki, M.; Yasuhara, S. Obesity-induced insulin resistance and hyperglycemia: Etiologic factors and molecular mechanisms. Anesthesiology 2008, 109, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Bose, T.; Alvarenga, J.C.; Tejero, M.E.; Voruganti, V.S.; Proffitt, J.M.; Freeland-Graves, J.H. Association of monocyte chemoattractant protein-1 with adipocyte number, insulin resistance and liver function markers. J. Med. Primatol. 2009, 38, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Investig. 2006, 116, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Iacono, A.; Bianco, G.; Autore, G.; Cuzzocrea, S.; Vajro, P.; Canani, R.B.; Calignano, A.; Raso, G.M.; Meli, R. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J. Nutr. 2009, 139, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Capurso, C.; Capurso, A. From excess adiposity to insulin resistance: The role of free fatty acids. Vascul. Pharmacol. 2012, 57, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.H.; Yao, N.; Wang, Q.Q.; Zhang, J.H.; Sun, Y.; Xiao, N.; Liu, K.; Huang, F.; Fang, S.Z.; Shang, X.L.; et al. Cyclocarya paliurus extract modulates adipokine expression and improves insulin sensitivity by inhibition of inflammation in mice. J. Ethnopharmacol. 2014, 153, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.S.; Jin, J.S.; Chiang, C.F.; Chan, P.C.; Chen, C.H.; Shih, K.C. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity 2009, 17, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, E.E.; Gaubatz, J.W.; Burns, A.R.; Pownall, H.J. Sustained elevations in NEFA induce cyclooxygenase-2 activity and potentiate THP-1 macrophage foam cell formation. Atherosclerosis 2007, 192, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mas, S.; Martínez-Pinna, R.; Martín-Ventura, J.L.; Pérez, R.; Gomez-Garre, D.; Ortiz, A.; Fernandez-Cruz, A.; Vivanco, F.; Egido, J. Local non-esterified fatty acids correlate with inflammation in atheroma plaques of patients with type 2 diabetes. Diabetes 2010, 59, 1292–301. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-C.; Wu, J.S.-B.; Chen, C.-W.; Kuo, P.-L.; Chien, H.-M.; Wang, Y.-T.; Shen, S.-C. Protective Effect of Vanillic Acid against Hyperinsulinemia, Hyperglycemia and Hyperlipidemia via Alleviating Hepatic Insulin Resistance and Inflammation in High-Fat Diet (HFD)-Fed Rats. Nutrients 2015, 7, 9946-9959. https://doi.org/10.3390/nu7125514
Chang W-C, Wu JS-B, Chen C-W, Kuo P-L, Chien H-M, Wang Y-T, Shen S-C. Protective Effect of Vanillic Acid against Hyperinsulinemia, Hyperglycemia and Hyperlipidemia via Alleviating Hepatic Insulin Resistance and Inflammation in High-Fat Diet (HFD)-Fed Rats. Nutrients. 2015; 7(12):9946-9959. https://doi.org/10.3390/nu7125514
Chicago/Turabian StyleChang, Wen-Chang, James Swi-Bea Wu, Chen-Wen Chen, Po-Ling Kuo, Hsu-Min Chien, Yuh-Tai Wang, and Szu-Chuan Shen. 2015. "Protective Effect of Vanillic Acid against Hyperinsulinemia, Hyperglycemia and Hyperlipidemia via Alleviating Hepatic Insulin Resistance and Inflammation in High-Fat Diet (HFD)-Fed Rats" Nutrients 7, no. 12: 9946-9959. https://doi.org/10.3390/nu7125514




