DHA-Containing Oilseed: A Timely Solution for the Sustainability Issues Surrounding Fish Oil Sources of the Health-Benefitting Long-Chain Omega-3 Oils
Abstract
:1. Introduction
2. Consensus for, and Recommendations on, Human Nutrition Needs for Long-Chain Omega-3 Oils
| Group | EPA + DHA, mg/day |
|---|---|
| SACN/COT, UK 2004 [14] | 450 |
| National Heart Foundation (Australia), 2008 [15] | 500 |
| American Dietetic Association and Dieticians of Canada, 2007 [16] | 500 |
| FAO/WHO Expert Consultation, 2008 [17] | 250–2000 * |
| American Heart Association, 2002 [18]: | |
| Coronary heart disease sufferers | 1000 |
| Those seeking to reduce triacylglycerols (blood fats) | 2000–4000 |
| National Health and Medical Research Council (Australia) [19]: | |
| Male adults | 610 |
| Female adults | 430 |
| European Food Safety Authority, 2010 [20] | 250 |
3. The Role of DHA
4. Supply, Demand and Environmental Issues—A Need for Alternative Sources of LC Omega-3 Oils
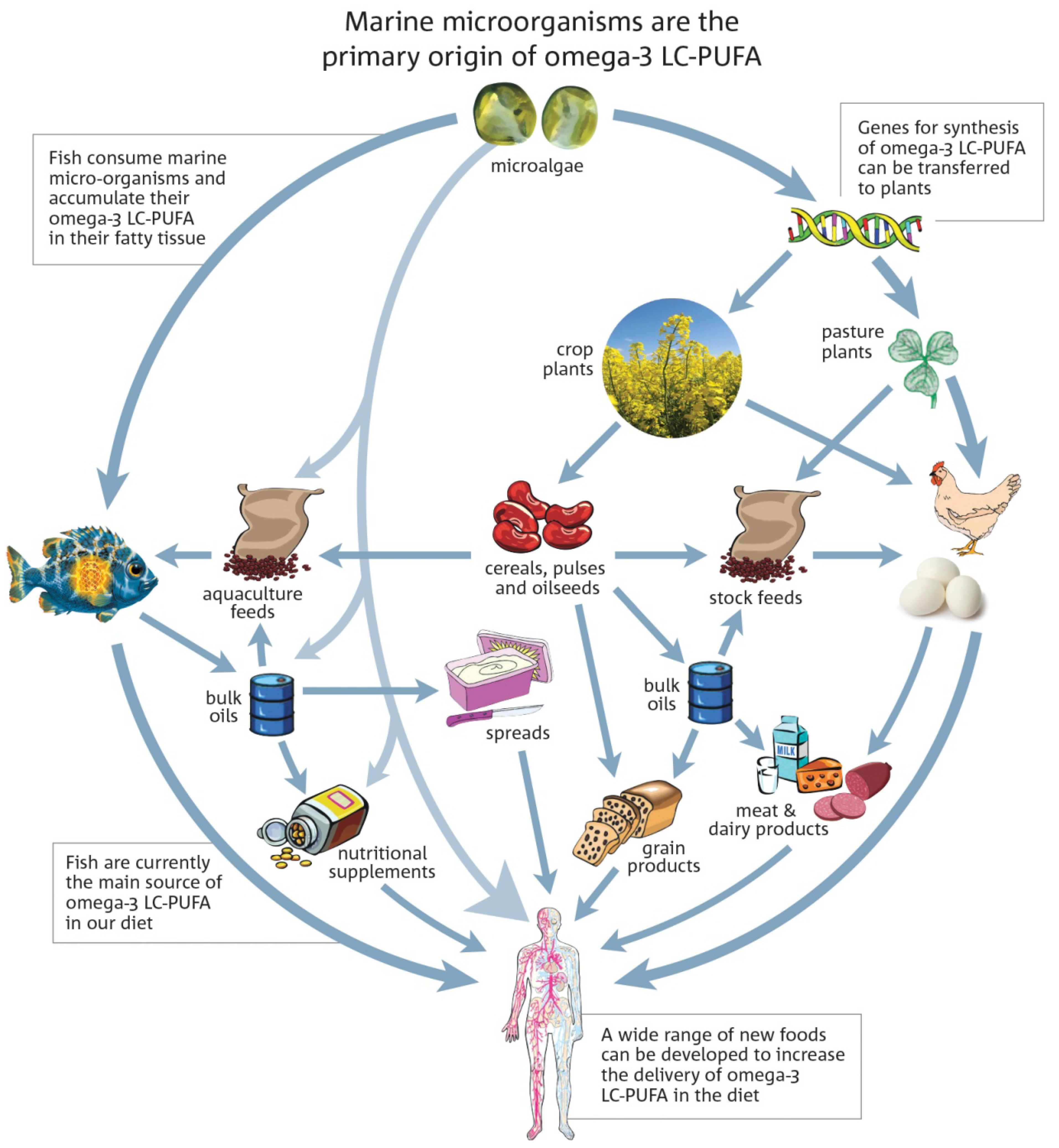
5. Alternative Sources of LC Omega-3 Oils
| Oil Seed and Comparison to Farmed salmon | Reference | SDA% | EPA% | DHA% |
|---|---|---|---|---|
| CSIRO Oil Seeds (includes model plants) | [51] | 10 | ||
| [59] | 5 | 1 | ||
| [51] | 1 | 26 | ||
| [53] | 15 | 14 | ||
| [58] | 5 | 2 | 15 | |
| [59] | 9 | 3 | 13 | |
| BASF Mustard | [61] | 15 | 1.5 | |
| Monsanto-Soy | [56] | 20 | ||
| Dupont-Soy | [55,57] | 20 | 3 | |
| Farmed salmon | ||||
| fed fish oil (FO) | [63,64] | 10 | 17 | |
| fed plant oil/chicken fat-FO | [65] | 1–5 | 5 |
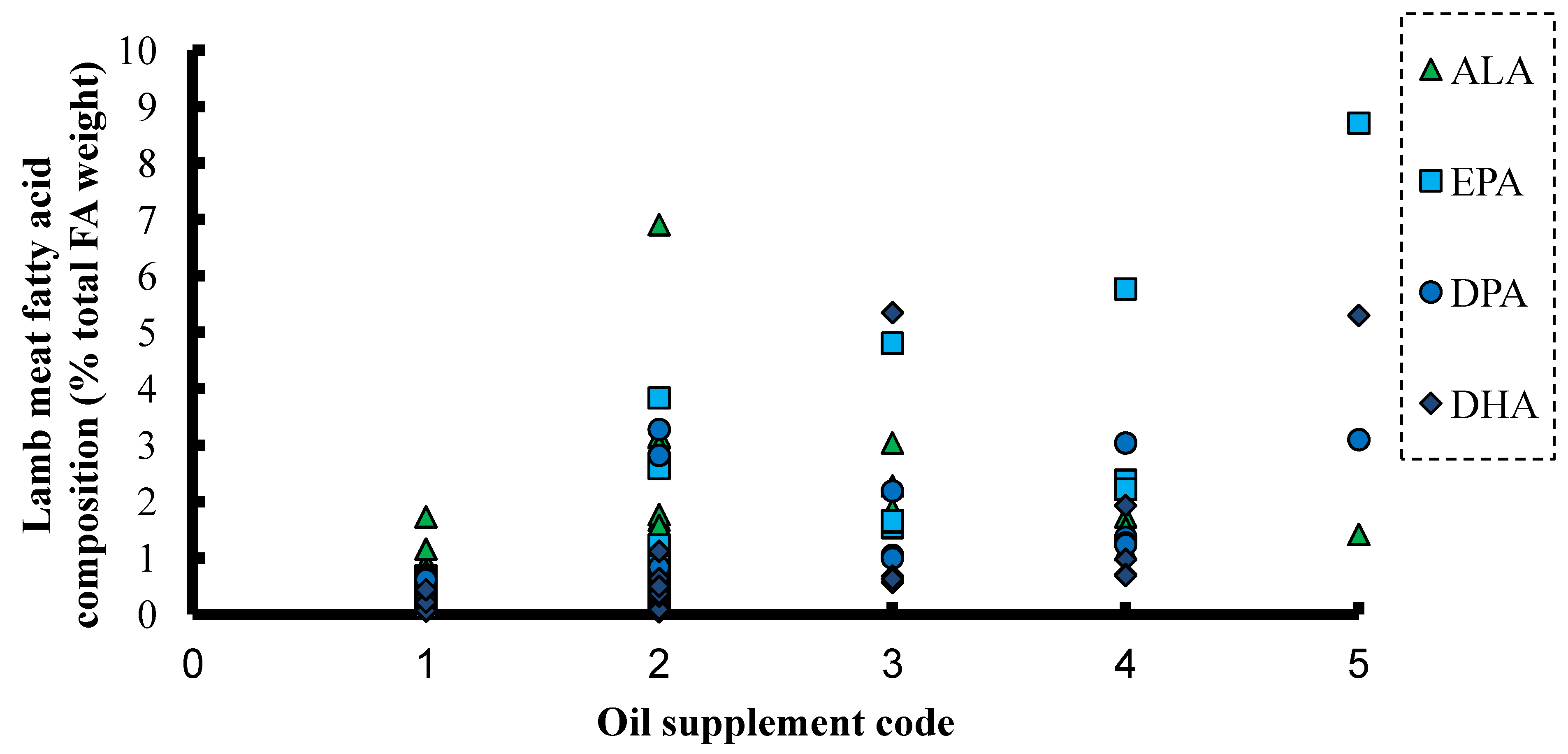
6. ALA and SDA in Animal Feeds


7. ALA and SDA in Aquafeeds
8. SDA Oils in Animal Models and Humans
9. Current Practices with Commercial Aquafeeds and Future Sources of LC Omega-3 Oils

10. TAG Structure of Plant-Based LC Omega-3 Oils for Optimum Bioactivity and Food Processing
11. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Calder, P.C.; Yaqoob, P. Marine omega-3 fatty acids and coronary heart disease. Curr. Opin. Cardiol. 2012, 27, 412–419. [Google Scholar] [CrossRef]
- Greene, J.; Ashburn, B.A.; Razzouk, L.; Smith, D.A. Fish oils, coronary heart disease, and the environment. Am. J. Public Health 2013, 103, 1568–1576. [Google Scholar] [CrossRef]
- Kimmig, L.M.; Karalis, D.G. Do omega-3 polyunsaturated fatty acids prevent cardiovascular disease? A review of the randomized clinical trials. Lipid Insights 2013, 6, 13–20. [Google Scholar]
- Roncaglioni, M.C.; Tombesi, M.; Avanzini, F.; Barlera, S.; Caimi, V.; Longoni, P.; Marzona, I.; Milani, V.; Silletta, M.G.; Tognoni, G.; et al. n-3 Fatty acids in patients with multiple cardiovascular risk factors. N. Engl. J. Med. 2013, 368, 1800–1808. [Google Scholar] [CrossRef]
- Cleland, L.G.; Caughey, G.E.; James, M.J.; Proudman, S.M. Reduction of cardiovascular risk factors with longterm fish oil treatment in early rheumatoid arthritis. J. Rheumatol. 2006, 33, 1973–1979. [Google Scholar]
- Proudman, S.M.; Cleland, L.G.; James, M.J. Dietary omega-3 fats for treatment of inflammatory joint disease: Efficacy and utility. Rheum. Dis. Clin. N. Am. 2008, 34, 469–479. [Google Scholar] [CrossRef]
- Stall, L.A.; Begg, D.P.; Weisinger, R.S.; Sinclair, A.J. The role of omega-3 fatty acids in mood disorders. Curr. Opin. Investig. Drugs 2008, 9, 57–64. [Google Scholar]
- Jeffrey, B.G.; Weisinger, H.S.; Neuringer, M.; Mitchell, D.C. The role of docosahexaenoic acid in retinal function. Lipids 2001, 36, 859–871. [Google Scholar] [CrossRef]
- Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional modulation of cognitive function and mental health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2007, 20, 275–285. [Google Scholar] [CrossRef]
- Williams, C.M.; Burdge, G. Long-chain n-3 PUFA: Plant V. marine sources. Pro. Nutr. Soc. 2006, 65, 42–50. [Google Scholar] [CrossRef]
- Abeywardena, M.Y.; Patten, G.S. Role of ω3 long-chain polyunsaturated fatty acids in reducing cardio-metabolic risk factors. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 232–246. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic acid supplementation and conversion to n-3 long chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fatty Acids. 2009, 80, pp. 85–91. Available online: http://info.babymilkaction.org/sites/info.babymilkaction.org/files/Brenna%202008%20ISSFAL%20statement%20ALA%20supplementaiton%20and%20conversion%20to%20LC%20PUFAS%20in%20humans.pdf (accessed on 02 October 2013).
- Scientific Advisory Committee on Nutrition (SACN) and Committee on Toxicity (COT). Advice on Fish. Consumption: Benefits and Risks; TSO: Norwich, UK, 2004; p. 204. [Google Scholar]
- National Heart Foundation Australia. Position Statement: FISH, Fish Oils, n-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Updated November 2008. Pro-067, 2nd Edition. National Heart Foundation of Australia. Available online: http://www.heartfoundation.org.au/healthy-eating/Pages/fish-oil-program.aspx (accessed on 2 October 2013).
- American Dietetics Association and Dietitians of Canada. Position of the American Dietetics Association and Dietitians of Canada: Dietary fatty acids. J. Am. Diet. Assoc. 2007, 107, 1599–1611. [Google Scholar]
- FAO/WHO. Interim summary of conclusions and dietary recommendations on total fat and fatty acids. In Proceedings of Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition, 10–14 November, 2008; FAO/WHO: Geneva, Switzerland.
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; American Heart Association Committee. Fish consumption, fish oil, omega-3 fatty acids and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- NHMRC. Nutrient Reference Values for Australia and New Zealand; NHMRC: Canberra, Australia, 2006. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). EFSA J. 2010, 8, 1461. [Google Scholar]
- Leaf, A. Historical overview of n-3 fatty acids and coronary heart disease. Am. J. Clin. Nutr. 2008, 87, 1978S–1980S. [Google Scholar]
- Bays, H.E. Safety considerations with omega-3 fatty acid therapy. Am. J. Cardiol. 2007, 99, 35C–43C. [Google Scholar] [CrossRef]
- Nichols, P.D.; Petrie, J.; Singh, S. Long-chain omega-3 oils—An update on sustainable sources. Nutrients 2010, 2, 572–285. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Mori, T.A.; Woodman, R.J. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 95–104. [Google Scholar] [CrossRef]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y. Similarities and differences between the effects of EPA and DHA on markers of atherosclerosis in human subjects. Proc. Nutr. Soc. 2012, 71, 322–331. [Google Scholar] [CrossRef]
- Wei, M.Y.; Jacobson, T.A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr. Athereoscler. Rep. 2011, 13, 474–483. [Google Scholar] [CrossRef]
- Neff, L.M.; Culliner, J.; Cunnigham-Rundles, S.; Seidman, C.; Meehan, D.; Maturi, J.; Wittkowski, K.M.; Levine, B.; Breslow, J.L. Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. J. Nutr. 2011, 141, 207–213. [Google Scholar] [CrossRef]
- Sekikawa, A.; Kadowaki, T.; El-Saed, A.; Okamura, T.; Sutton-Tyrrell, K.; Nakamura, Y.; Evans, R.W.; Mitsunami, K.; Edmundowicz, D.; Nishio, Y.; et al. Differential association of docosahexaenoic and eicosapentaenoic acids with carotid intima-media thickness. Stroke 2012, 42, 2538–2543. [Google Scholar]
- Ramadeen, A.; Connelly, K.A.; Leong-Poi, H.; Hu, X.; Fujii, H.; Laurent, G.; Domenichiello, A.F.; Bazinet, R.P.; Dorian, P. Docosahexaenoic acid, but not eicosapentaenoic acid, supplementation reduces vulnerability to atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2012, 5, 978–983. [Google Scholar] [CrossRef]
- Wu, J.H.; Lemaitre, R.N.; King, I.B.; Song, X.; Sacks, F.M.; Rimm, E.B.; Heckbert, S.R.; Siscovick, D.S.; Mozaffarian, D. Association of plasma phospholipid long-chain ω-3 fatty acids with incident atrial fibrillation in older adults: The cardiovascular health study. Circulation 2012, 125, 1084–1093. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Mursu, J.; Voutilainen, S.; Tuomainen, T.P. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation 2009, 120, 2315–2321. [Google Scholar] [CrossRef]
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef]
- Milte, C.M.; Parletta, N.; Buckley, J.D.; Coates, A.M.; Young, R.M.; Howe, P.R. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: A randomized controlled tria. Nutrition 2012, 28, 670–677. [Google Scholar] [CrossRef]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1134–1143. [Google Scholar] [CrossRef]
- Hall, J.C.E.; Priestley, J.V.; Perry, V.H.; Michael-Titus, A.T. Docosahexaenoic acid, but not eicosapentaenoic acid, reduces the early inflammatory response following compression spinal cord injury in the rat. J. Neurochem. 2012, 121, 738–750. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Hasan, M.R.; Metian, M. Demand and supply of feed ingredients for farmed fish and crustaceans: Trends and prospects; FAO Fisheries and Aquaculture Technical Paper No. 564; FAO: Rome, Italy, 2008. [Google Scholar]
- Bimbo, A.P. Production of marine oils. In Omega-3 Oils. Applications in Functional Foods; Hernandez, E.M., Hosokawa, M., Eds.; AOCS Press: Urbana, IL, USA, 2011; pp. 73–105. [Google Scholar]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding aquaculture in an era of finite resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef]
- Belarbi, E.H.; Molina, E.; Chisti, Y. A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzyme Microb. Technol. 2000, 26, 516–529. [Google Scholar] [CrossRef]
- Barlow, S.M. Fish meal and fish oil: Sustainable feed ingredients for aquafeeds. Global Aquac. Advoc. 2000, 3, 85–86. [Google Scholar]
- Smith, A.D.M.; Brown, C.J.; Bulman, C.M.; Fulton, E.A.; Johnson, P.; Kaplan, I.C.; Lozano-Montes, H.; Mackinson, S.; Marzloff, M.; Shannon, L.J.; et al. Impacts of fishing low-trophic level species on marine ecosystems. Science 2011, 333, 1147–1150. [Google Scholar] [CrossRef]
- Pikitch, E.; Boersma, P.D.; Boyd, I.L.; Conover, D.O.; Cury, P.; Essington, T.; Heppell, S.S.; Houde, E.D.; Mangel, M.; Pauly, D.; et al. Little Fish, Big Impact: Managing a Crucial Link in Ocean. Food Webs; Lenfest Ocean Program: Washington, DC, USA, 2012; p. 120. [Google Scholar]
- Barclay, W.; Weaver, C.; Metz, J.; Hansen, J. Development of a docosahexaenoic acid production technology using Schizochytrium: Historical perspective and update. In Single Cell Oils, Microbial and Algal Oils, 2nd ed.; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Urbana, IL, USA, 2010; pp. 75–96. [Google Scholar]
- Lam, M.K.; Lee, K.T. Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef]
- Zhou, X.-R.; Robert, S.S.; Petrie, J.R.; Frampton, D.M.; Mansour, M.P.; Blackburn, S.I.; Nichols, P.D.; Green, A.G.; Singh, S.P. Isolation and characterization of genes from the marine microalga Pavlova salina encoding front-end desaturases involved in docosahexaenoic acid biosynthesis. Phytochemistry 2007, 68, 785–796. [Google Scholar] [CrossRef]
- Robert, S.S.; Petrie, J.R.; Zhou, X.-R.; Mansour, M.P.; Blackburn, S.I.; Green, A.G.; Singh, S.P.; Nichols, P.D. Isolation and characterisation of a Δ5-fatty acid elongase from the marine microalga Pavlova salina. Mar. Biotechnol. 2009, 11, 410–418. [Google Scholar] [CrossRef]
- Petrie, J.R.; Liu, Q.; Mackenzie, A.M.; Shrestha, P.; Mansour, M.P.; Robert, S.S.; Frampton, D.F.; Blackburn, S.I.; Nichols, P.D.; Singh, S.P. Isolation and characterisation of a high-efficiency desaturase and elongases from microalgae for transgenic LC-PUFA production. Mar. Biotechnol. 2010, 12, 430–438. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Liu, Q.; Mansour, M.P.; Wood, C.C.; Zhou, X.R.; Nichols, P.D.; Green, A.G.; Singh, S.P. Rapid expression of transgenes driven by seed-specific constructs in leaf tissue: DHA production. Plant Methods 2009, 6, 8. [Google Scholar]
- Petrie, J.R.; Shrestha, P.; Mansour, M.P.; Nichols, P.D.; Liu, Q.; Singh, S.P. Metabolic engineering of omega-3 long-chain polyunsaturated fatty acids in plants using an acyl-CoA Δ6-desaturase with ω3-preference from the marine microalga Micromonas pusilla. Metab. Eng. 2010, 12, 233–240. [Google Scholar] [CrossRef]
- Wood, C.C.; Petrie, J.R.; Shrestha, P.; Mansour, M.P.; Nichols, P.D.; Green, A.G.; Singh, S.P. A leaf-based assay using interchangeable design principles to rapidly assemble multistep recombinant pathways. Plant. Biotechnol. J. 2009, 7, 914–920. [Google Scholar] [CrossRef]
- Petrie, J.R.; Singh, S.P. Expanding the docosahexaenoic acid food web for sustainable production: Engineering lower plant pathways into higher plants. AoB Plants 2011, 2011. [Google Scholar] [CrossRef]
- Qi, B.X.; Fraser, T.; Mugford, S.; Dobson, G.; Sayanova, O.; Butler, J.; Napier, J.A.; Stobart, A.K.; Lazarus, C.M. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat. Biotechnol. 2004, 22, 739–745. [Google Scholar] [CrossRef]
- Venegas-Caleron, M.; Sayanova, O.; Napier, J.A. An alternative to fish oils: Metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog. Lipid Res. 2010, 49, 108–119. [Google Scholar] [CrossRef]
- Kinney, A.J.; Cahoon, E.B.; Damude, H.G.; Hitz, W.D.; Kolar, C.W.; Liu, Z.-B. Production of very Long Chain Polyunsaturated Fatty Acids in Oilseed Plants. International Patent Application WO 2004/071467, 26 August 2004. [Google Scholar]
- Cheng, B.; Wu, G.; Vrinten, P.; Falk, K.; Bauer, J.; Qiu, X. Towards the production of high levels of eicosapentaenoic acid in transgenic plants: The effects of different host species, genes and promoters. Transgenic Res. 2010, 19, 221–229. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Zhou, X.-R.; Mansour, M.P.; Liu, Q.; Belide, S.; Nichols, P.D.; Singh, S.P. Metabolic engineering plant seeds with fish oil-like levels of DHA. PLoS One 2012, 7, e49165. [Google Scholar] [CrossRef]
- Petrie, J.R.; Shrestha, P.; Belide, S.; Kennedy, Y.; Lester, G.; Liu, Q.; Divi, U.K.; Mulder, R.J.; Mansour, M.P.; Nichols, P.D.; et al. Metabolic engineering Camelina sativa with fish oil like levels of DHA. PLoS One 2014, 9, e85061. [Google Scholar] [CrossRef]
- Robert, S.S.; Singh, S.P.; Zhou, X.-R.; Mansour, M.P.; Liu, Q.; Belide, S.; Nichols, P.D.; Singh, S.P. Metabolic engineering of Arabidopsis to produce nutritionally important DHA in seed oil. Funct. Plant. Biol. 2005, 32, 473–479. [Google Scholar] [CrossRef]
- Wu, G.; Truksa, M.; Datla, N.; Vrinten, P.; Bauer, J.; Zank, T.; Cirpus, P.; Heinz, E.; Qiu, X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat. Biotechnol. 2005, 23, 1013–1017. [Google Scholar] [CrossRef]
- Finney, A.J. Metabolic engineering in plants for human health and nutrition. Curr. Opin. Biotechnol. 2006, 17, 130–138. [Google Scholar] [CrossRef]
- Miller, M.R.; Bridle, A.R.; Nichols, P.D.; Carter, C.G. Increased elongase and desaturase gene expression with stearidonic acid enriched diet does not enhance long-chain (n-3) content of seawater Atlantic salmon (Salmo salar L.). J. Nutr. 2008, 138, 2179–2185. [Google Scholar] [CrossRef]
- Codabaccus, M.B.; Bridle, A.R.; Nichols, P.D.; Carter, C.G. Effect of feeding Atlantic salmon (Salmo salar L.) a diet enriched with stearidonic acid from parr to smolt on growth and n-3 LC-PUFA biosynthesis. Br. J. Nutr. 2011, 105, 1772–1782. [Google Scholar] [CrossRef]
- Abeywardena, M.Y.; Wijesundera, C.; CSIRO Animal, Food and Health Sciences, Australia. 2013; Unpublished work.
- Rymer, C.; Gibbs, R.A.; Givens, D.I. Comparison of algal and fish sources on the oxidative stability of poultry meat and its enrichment with omega-3 polyunsaturated fatty acids. Poult. Sci. 2010, 89, 150–159. [Google Scholar] [CrossRef]
- Rymer, C.; Hartnell, G.F.; Givens, D.I. The effect of feeding modified soybean oil enriched with C18:4n-3 to broilers on the deposition of n-3 fatty acids in chicken meat. Br. J. Nutr. 2011, 105, 866–878. [Google Scholar] [CrossRef]
- Poureslami, R.; Raes, K.; Huyghebaert, G.; de Smet, S. Effects of diet, age and gender on the polyunsaturated fatty acid composition of broiler anatomical compartments. Br. Poult. J. 2010, 51, 81–91. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Guo, Y.; Jiao, P.; Long, F. Effects of dietary lipids and Clostridium butyricum on fat deposition and meat quality of broiler chickens. Poult. Sci. 2010, 89, 254–260. [Google Scholar] [CrossRef]
- Jia, W.; Rogiewicz, A.; Bruce, H.L.; Slominski, B.A. Feeding flaxseed enhances deposition of omega-3 fatty acids in broiler meat portions in different manner. Can. J. Anim. Sci. 2010, 90, 203–206. [Google Scholar] [CrossRef]
- Lopez-Ferrer, S.; Baucells, M.D.; Barroeta, A.C.; Gashorn, M.A. n-3 Enrichment of chicken meat using fish oil: Alternative substitution with rapeseed and linseed oils. Poult. Sci. 1999, 78, 356–365. [Google Scholar] [CrossRef]
- Lopez-Ferrer, S.; Baucells, M.D.; Barroeta, A.C.; Gashorn, M.A. n-3 Enrichment of chicken meat. 1. Use of very long-chain fatty acids in chicken diets and their influence on meat quality: Fish oil. Poult. Sci. 2001, 80, 741–752. [Google Scholar] [CrossRef]
- Roth-Maier, D.A.; Eder, K.; Kirchgessner, M. Liver performance and fatty acid composition of meat in broiler chickens fed diets with various amounts of ground or whole flaxseed. J. Anim. Physiol. Anim. Nutr. 1998, 79, 260–268. [Google Scholar] [CrossRef]
- Schreiner, M.H.W.; Hulan, H.W.; Razzazi-Fazeli, E.; Böhm, J.; Moreira, R.G. Effect of different sources of dietary omega-3 fatty acids on general performance and fatty acid profiles of thigh, breast liver and portal blood of broilers. J. Sci. Food Agric. 2005, 85, 219–226. [Google Scholar] [CrossRef]
- Bas, P.; Berthelot, V.; Pottier, E.; Normand, J. Effect of level of linseed on fatty acid composition of muscles and adipose tissues of lambs with emphasis on trans fatty acids. Meat Sci. 2007, 77, 678–688. [Google Scholar] [CrossRef]
- Nute, G.R.; Richardson, R.J.; Wood, J.D.; Hughes, S.I.; Wilkinson, R.G.; Cooper, S.L.; Sinclair, L.A. Effect of dietary oil source on the flavour and colour and lipid stability of lamb meat. Meat Sci. 2004, 77, 547–555. [Google Scholar]
- Kitessa, S.M.; Gulati, S.K.; Ashes, J.R.; Scott, T.W.; Fleck, E. Effect of feeding tuna oil supplement protected against hydrogenation in the rumen on growth and n-3 fatty acid content of lamb fat and muscle. Aus. J. Agric. Res. 2001, 52, 433–437. [Google Scholar] [CrossRef]
- Kitessa, S.M.; Williams, A.; Gulati, S.; Boghossian, V.; Reynolds, J.; Pearce, K.L. Influence of duration of supplementation with ruminally protected linseed oil on the fatty acid composition of feedlot lambs. Anim. Feed Sci. Technol. 2009, 151, 228–239. [Google Scholar] [CrossRef]
- Kitessa, S.; Liu, S.M.; Briegel, J.; Pethick, D.; Gardner, G.; Ferguson, M.; Allingham, P.; Nattrass, G.; McDonagh, M.; Eric Ponnampalam, E.; et al. Effects of intensive or pasture finishing in spring and linseed supplementation in autumn on the omega-3 content of lamb meat and its carcass distribution. Anim. Prod. Sci. 2010, 50, 130–137. [Google Scholar] [CrossRef]
- Jerónimo, E.E.; Alves, S.P.; Prates, J.A.M.; Santos-Silva, J.; Bessa, R.J. Effect of dietary replacement of sunflower oil with linseed oil on intramuscular fatty acids of lamb meat. Meat Sci. 2009, 83, 499–505. [Google Scholar] [CrossRef]
- Diaz, M.T.; Caneque, V.; Sanchez, C.I.; Lauzurica, S.; Perez, C.; Fernandez, C.; Alvarez, I.; de la Fuente, J. Nutritional and sensory aspects of light lamb meat enriched in n-3 fatty acids during refrigerated storage. Food Chem. 2011, 124, 147–155. [Google Scholar] [CrossRef]
- Berthlot, V.; Bas, P.; Pottier, E.; Normand, J. The effect of maternal linseed supplementation and/or lamb linseed supplementation on muscle and subcutaneous adipose tissue fatty acid composition of indoor lambs. Meat Sci. 2012, 90, 548–557. [Google Scholar] [CrossRef]
- Wachira, A.M.; Sinclair, L.A.; Wilkinson, G.; Enser, M.; Wood, J.D.; Fisher, A.V. Effects of dietary fat source and breed on the carcass composition, n-3 polyunsaturated fatty acid and conjugated linoleic acid content of sheep meat and adipose tissue. Br. J. Nutr. 2002, 88, 697–709. [Google Scholar] [CrossRef]
- Elmore, J.S.; Cooper, S.L.; Enser, M.; Mottram, D.S.; Sinclair, A.L.; Wilkinson, R.G.; Wood, J.D. Dietary manipulation of fatty acid composition in lamb meat and its effect on the volatile aroma compounds of grilled lamb. Meat Sci. 2005, 69, 233–242. [Google Scholar] [CrossRef]
- FAO. How to Feed the World in 2050. Available online: http://www.fao.org (accessed on 21 October 2013).
- Miller, M.R.; Nichols, P.D.; Carter, C.G. Replacement of dietary fish oil with a stearidonic acid containing oil for Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. 2007, 146, 197–206. [Google Scholar] [CrossRef]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. Omega-3 oil sources for use in aquaculture—Alternatives to the unsustainable harvest of wild fish. Nutr. Res. Rev. 2008, 21, 85–96. [Google Scholar] [CrossRef]
- Codabaccus, B.M.; Bridle, A.R.; Nichols, P.D.; Carter, C.G. An extended feeding history with a stearidonic acid enriched diet from parr to smolt increases n-3 long-chain polyunsaturated fatty acids biosynthesis in white muscle and liver of Atlantic salmon (Salmo salar L.). Aquaculture 2011, 322–323, 65–73. [Google Scholar]
- Tu, W.C.; Muhlhausler, B.S.; James, M.J.; Stone, D.A.; Gibson, R.A. Dietary alpha-linolenic acid does not enhance accumulation of omega-3 long-chain polyunsaturated fatty acids in barramundi (Lates calcarifer). Comp. Biochem. Physiol. 2013, 164, 29–37. [Google Scholar]
- Alhazzaa, R.; Bridle, A.R.; Nichols, P.D.; Carter, C.G. Replacing dietary fish oil with Echium oil enriched barramundi with C18 PUFA rather than long-chain PUFA. Aquaculture 2011, 312, 162–171. [Google Scholar] [CrossRef]
- Alhazzaa, R.; Bridle, A.R.; Nichols, P.D.; Carter, C.G. Up-regulated desaturase and elongase gene expression promoted accumulation of polyunsaturated fatty acid (PUFA) but not long-chain PUFA in Lates calcarifer, a tropical euryhaline fish, fed a stearidonic acid- and γ-linoleic acid-enriched diet. J. Agric. Food Chem. 2011, 59, 8423–8434. [Google Scholar] [CrossRef]
- Bell, J.G.; Strachan, F.; Good, J.E.; Tocher, D.R. Effect of dietary echium oil on growth, fatty acid composition and metabolism, gill prostaglandin production and macrophage activity in Atlantic cod (Gadus morhua L.). Aquac. Res. 2006, 37, 606–617. [Google Scholar] [CrossRef]
- Bharadwaj, A.S.; Hart, S.D.; Brown, B.J.; Li, Y.; Watkins, B.A.; Brown, P.B. Dietary source of stearidonic acid promotes higher muscle DHA concentrations than linolenic acid in hybrid striped bass. Lipids 2010, 45, 21–27. [Google Scholar] [CrossRef]
- Cleveland, B.C.; Francis, D.S.; Turchini, G.M. Echium oil provides no benefit over linseed oil for (n-3) long-chain PUFA biosynthesis in rainbow trout. J. Nutr. 2012, 142, 1–8. [Google Scholar] [CrossRef]
- Diaz-Lopez, M.; Perez, M.J.; Acosta, N.G.; Tocher, D.R.; Jerez, S.; Lorenzo, A.; Rodriguez, C. Effect of dietary substitution of fish oil by echium oil on growth, plasma parameters and body lipid composition in gilthead seabream (Sparus aurata L.). Aquac. Nutr. 2009, 15, 500–512. [Google Scholar] [CrossRef]
- Alhazzaa, R.; Bridle, A.R.; Carter, C.G.; Nichols, P.D. Sesamin modulation of lipid class and fatty acid profile in early juvenile teleost, Lates calcarifer, fed different dietary oils. Food Chem. 2012, 134, 2057–2065. [Google Scholar] [CrossRef]
- Seierstad, S.L.; Poppe, T.T.; Koppang, E.O.; Svindland, A.; Rosenlund, G; Frøyland, L.; Larsen, S. Influence of dietary lipid composition on cardiac pathology in farmed Atlantic salmon, Salmo salar L. J. Fish. Dis. 2005, 28, 677–690. [Google Scholar] [CrossRef]
- James, M.J.; Ursin, V.M.; Cleland, L.G. Metabolism of stearidonic acid in human subjects: Comparison with the metabolism of other n-3 fatty acids. Am. J. Clin. Nutr. 2003, 77, 1140–1145. [Google Scholar]
- Harris, W.S.; Lemke, S.L.; Hansen, S.N.; Goldstein, D.A.; di Rienzo, M.A.; Su, H.; Nemeth, M.A.; Taylor, M.L.; Ahmed, G.; George, C. Stearidonic acid-enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids 2008, 43, 805–811. [Google Scholar] [CrossRef]
- Lemke, S.L.; Vicini, J.L.; Su, H.; Goldstein, D.A.; Nemeth, M.A.; Krul, E.S.; Harris, W.S. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: randomized, double-blind clinical study of efficacy and safety. Am. J. Clin. Nutr. 2010, 92, 766–775. [Google Scholar] [CrossRef]
- Walker, C.G.; Jebb, S.A.; Calder, P.C. Stearidonic acid as a supplemental source of ω-3 polyunsaturated fatty acids to enhance status for improved human health. Nutrition 2013, 29, 363–369. [Google Scholar] [CrossRef]
- Abeywardena, M.Y.; Kitessa, S.; Nichols, P.D. Dietary Echium oil rich in stearidonic (18:4ω3) acid does not increase cardiac membrane EPA or DHA. In Proceedings of the Nutrition Society of Australia, 34th Annual meeting, Perth, Australia, 30 November–3 December 2010.
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.E.; Willett, W.C.; Ma, J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef]
- Harris, W.S.; von Schacky, C. The omega-3 index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Harris, W.S.; Sands, S.A.; Windsor, S.L.; Ali, H.A.; Stevens, T.L.; Magalski, A.; Porter, C.B.; Borkon, A.M. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: Correlation with erythrocytes and response to supplementation. Circulation 2004, 110, 1645–1649. [Google Scholar] [CrossRef]
- Smullen, R.; Ridley Corporation, Australia. Personal communication, 2013.
- Turchini, G.M.; Ng, W.K.; Tocher, D. Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press: Boca Raton, FL, USA, 2010; p. 533. [Google Scholar]
- Nichols, P.D.; Petrie, J.P.; Singh, S.P. Readily available sources of long-chain omega-3 oils: Is farmed Australian seafood a better source of the good oil than wild-caught seafood? Nutrients 2014, 6, 1063–1079. [Google Scholar] [CrossRef]
- Codabaccus, B.M.; Carter, C.G.; Bridle, A.R.; Nichols, P.D. The “n-3 LC-PUFA sparing effect” of modified dietary n-3 LC-PUFA content and DHA to EPA ratio in Atlantic salmon smolt. Aquaculture 2012, 356–357, 135–140. [Google Scholar]
- Fraser, B.; Perlmutter, P.; Wijesundera, C. Practical synthesis of triacylglycerol regioisomers containing long-chain polyunsaturated fatty acids. J. Am. Oil Chem. Soc. 2007, 84, 11–21. [Google Scholar] [CrossRef]
- Wijesundera, C.; Ceccato, C.; Watkins, P.; Fagan, P.; Fraser, B.; Thienthong, N.; Perlmutter, P. Docosahexaenoic acid is more stable to oxidation when located at the sn-2 position of triacylglycerol compared to sn-1(3). J. Am. Oil Chem. Soc. 2008, 85, 543–548. [Google Scholar] [CrossRef]
- Hayes, K.C. Synthetic and modified glycerides: Effects on plasma lipids. Curr. Opin. Lipidol. 2001, 12, 55–60. [Google Scholar] [CrossRef]
- Sundram, K.; Karupaiah, T.; Hayes, K.C. Stearic acid-rich interesterified fat and trans-rich fat raise the LDL/HDL ratio and plasma glucose relative to palm olein in humans. Nutr. Metab. 2007, 4. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.M.; Martin, N.C.; Robinson, L.E.; Ahmadi, L.; Marangoni, A.G.; Wright, A.J. Influence of interesterification of a stearic acid-rich spreadable fat on acute metabolic risk factors. Lipids 2009, 44, 17–26. [Google Scholar] [CrossRef]
- Hunter, J.E. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 2001, 36, 655–668. [Google Scholar] [CrossRef]
- Berry, S.E.E. Triglycerol structure and interesterification of palmitic and stearic acid-rich fats: An overview and implications for cardiovascular disease. Nutr. Res. Rev. 2009, 22, 3–17. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kitessa, S.M.; Abeywardena, M.; Wijesundera, C.; Nichols, P.D. DHA-Containing Oilseed: A Timely Solution for the Sustainability Issues Surrounding Fish Oil Sources of the Health-Benefitting Long-Chain Omega-3 Oils. Nutrients 2014, 6, 2035-2058. https://doi.org/10.3390/nu6052035
Kitessa SM, Abeywardena M, Wijesundera C, Nichols PD. DHA-Containing Oilseed: A Timely Solution for the Sustainability Issues Surrounding Fish Oil Sources of the Health-Benefitting Long-Chain Omega-3 Oils. Nutrients. 2014; 6(5):2035-2058. https://doi.org/10.3390/nu6052035
Chicago/Turabian StyleKitessa, Soressa M., Mahinda Abeywardena, Chakra Wijesundera, and Peter D. Nichols. 2014. "DHA-Containing Oilseed: A Timely Solution for the Sustainability Issues Surrounding Fish Oil Sources of the Health-Benefitting Long-Chain Omega-3 Oils" Nutrients 6, no. 5: 2035-2058. https://doi.org/10.3390/nu6052035
APA StyleKitessa, S. M., Abeywardena, M., Wijesundera, C., & Nichols, P. D. (2014). DHA-Containing Oilseed: A Timely Solution for the Sustainability Issues Surrounding Fish Oil Sources of the Health-Benefitting Long-Chain Omega-3 Oils. Nutrients, 6(5), 2035-2058. https://doi.org/10.3390/nu6052035