Single Sodium Pyruvate Ingestion Modifies Blood Acid-Base Status and Post-Exercise Lactate Concentration in Humans
1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Procedures
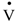 O2max, participants performed a graded cycle ergometry test on an electromagnetically-braked, cycle ergometer (ER 900 Jaeger, Viasys Healthcare GmbH, Hoechberg, Germany). The height of the ergometer seat was individually adjusted, and the participants were allowed a 5-min warm-up period at an intensity of 1.5 W·kg−1 with a pedaling cadence of 60 rpm. After the warm-up period, the work rate was increased by 25 W·min−1 until volitional exhaustion [16]. Breath by breath pulmonary gas exchange was measured by Oxycon-Pro analyzer (Viasys Healthcare GmbH, Hoechberg, Germany), and the O2 and CO2 analyzers were calibrated prior to each test using standard gases of known concentrations in accordance with manufacturer guidelines. The heart rates were monitored continuously by telemetry (S-625, Polar Electro-Oy, Kempele, Finland) during each test session and the first 5 min of passive recovery in a seated position. After the
O2max, participants performed a graded cycle ergometry test on an electromagnetically-braked, cycle ergometer (ER 900 Jaeger, Viasys Healthcare GmbH, Hoechberg, Germany). The height of the ergometer seat was individually adjusted, and the participants were allowed a 5-min warm-up period at an intensity of 1.5 W·kg−1 with a pedaling cadence of 60 rpm. After the warm-up period, the work rate was increased by 25 W·min−1 until volitional exhaustion [16]. Breath by breath pulmonary gas exchange was measured by Oxycon-Pro analyzer (Viasys Healthcare GmbH, Hoechberg, Germany), and the O2 and CO2 analyzers were calibrated prior to each test using standard gases of known concentrations in accordance with manufacturer guidelines. The heart rates were monitored continuously by telemetry (S-625, Polar Electro-Oy, Kempele, Finland) during each test session and the first 5 min of passive recovery in a seated position. After the 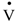 O2max test, subjects visited the laboratory for a practice ride, which familiarized the subjects with the experiment protocol and confirmed the power output (~90%
O2max test, subjects visited the laboratory for a practice ride, which familiarized the subjects with the experiment protocol and confirmed the power output (~90% 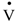 O2max).
O2max).2.3. Measurements
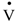 O2max. The respiratory gas analysis and the volume measurements were performed breath by breath with a face-mask connected to the analyzer. The breath-by-breath pulmonary
O2max. The respiratory gas analysis and the volume measurements were performed breath by breath with a face-mask connected to the analyzer. The breath-by-breath pulmonary 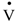 O2 was measured continuously throughout the exercise by an Oxycon-Pro gas analyzing system (Viasys Healthcare GmbH, Hoechberg, Germany). The data were first manually filtered to remove outlying breaths, defined as breaths deviating by more than three standard deviations from the preceding five breaths. The data were subsequently interpolated to provide second-by-second values, and then, the slow component amplitude was estimated by calculating the difference between the mean
O2 was measured continuously throughout the exercise by an Oxycon-Pro gas analyzing system (Viasys Healthcare GmbH, Hoechberg, Germany). The data were first manually filtered to remove outlying breaths, defined as breaths deviating by more than three standard deviations from the preceding five breaths. The data were subsequently interpolated to provide second-by-second values, and then, the slow component amplitude was estimated by calculating the difference between the mean 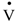 O2 during the last 60 s of the exercise and the mean
O2 during the last 60 s of the exercise and the mean 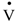 O2 during the 60-s period on third minute of exercise [19].
O2 during the 60-s period on third minute of exercise [19].2.4. Blood Analysis
2.5. Statistics
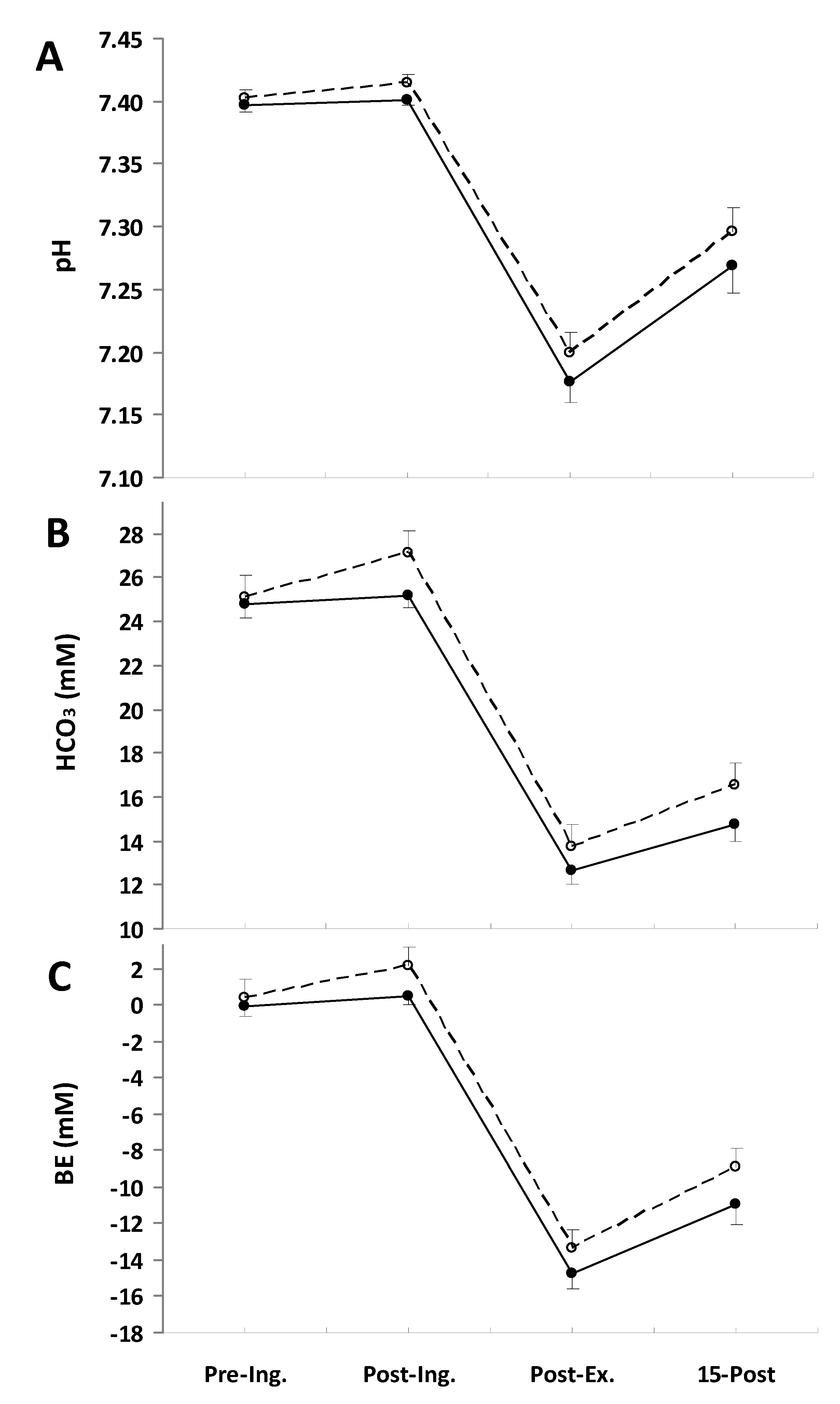
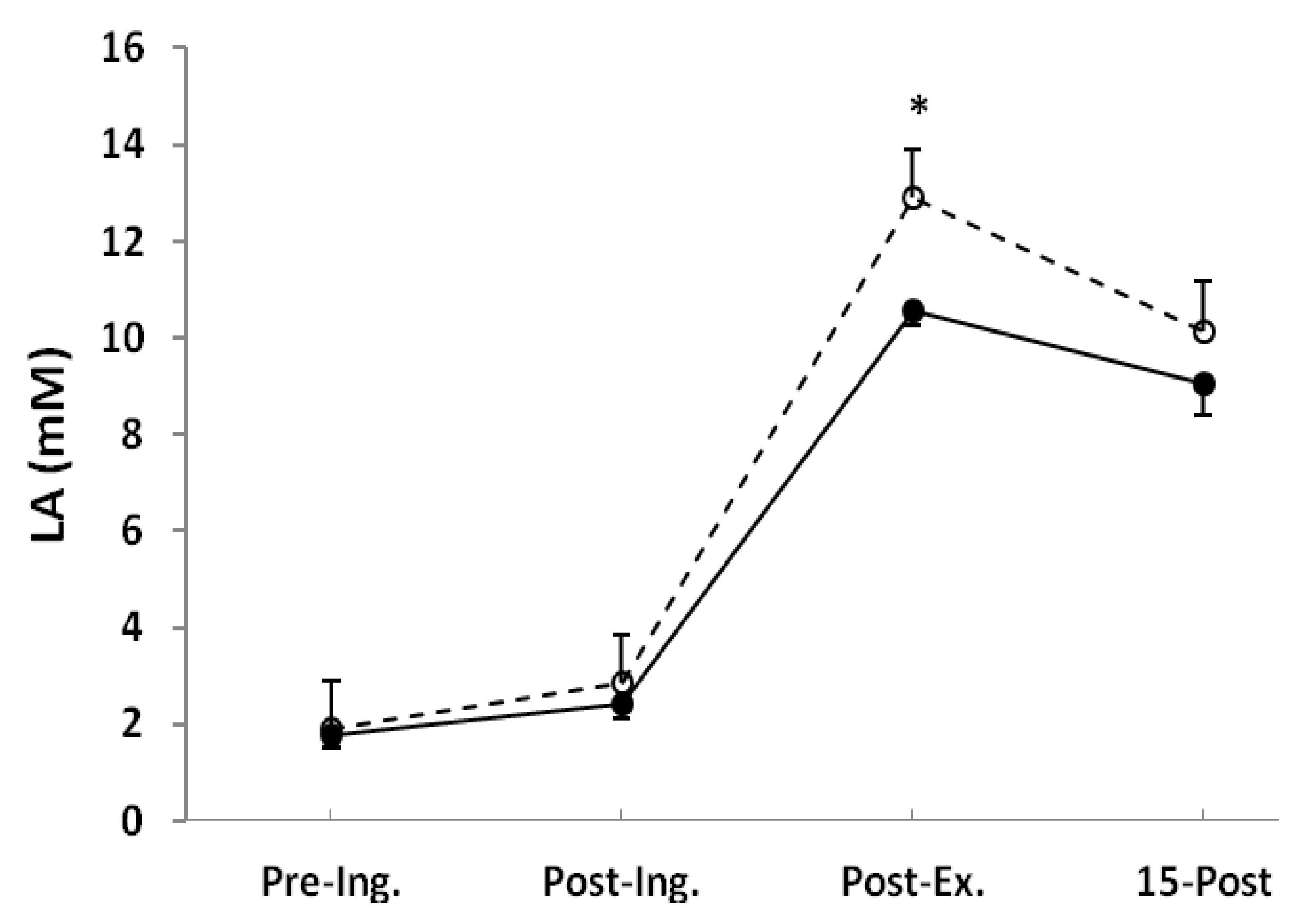
3. Results
| Placebo | NaP | |
|---|---|---|
| PYR (μM) # | ||
| Rest | 101 ± 18 | 110 ± 33 |
| 60 min after ingestion | 149 ± 22 | 191 ± 51 |
| 3 min after exercise | 329 ± 34 | 344 ± 34 |
| 15 min after exercise | 354 ± 42 | 300 ± 69 |
| ALA (μM) # | ||
| Rest | 296 ± 21 | 286 ± 28 |
| 60 min after ingestion | 285 ± 24 | 331 ± 23 |
| 3 min after exercise | 375 ± 53 | 385 ± 34 |
| 15 min after exercise | 383 ± 21 | 427 ± 45 |
| GLU (mM) # | ||
| Rest | 52.0 ± 1.4 | 51.7 ± 1.4 |
| 60 min after ingestion | 51.8 ± 0.8 | 51.7 ± 1.5 |
| 3 min after exercise | 63.3 ± 2.0 | 63.3 ± 2.8 |
| 15 min after exercise | 58.8 ± 1.4 | 57.2 ± 2.9 |
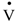 O2 response during the exercise (Table 2). Consistent with this, the slow component amplitude was not significantly different between treatments. There were also no marked differences in
O2 response during the exercise (Table 2). Consistent with this, the slow component amplitude was not significantly different between treatments. There were also no marked differences in 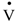 CO2 during the final minute of the exercise, being 4.08 ± 0.10 L·min−1 in the placebo trial and 4.03 ± 0.09 L·min−1 in the NaP trial; therefore, the respiratory exchange ratio (RER) was also not altered by the NaP ingestion (Table 2). Furthermore, no effects of NaP were noted in
CO2 during the final minute of the exercise, being 4.08 ± 0.10 L·min−1 in the placebo trial and 4.03 ± 0.09 L·min−1 in the NaP trial; therefore, the respiratory exchange ratio (RER) was also not altered by the NaP ingestion (Table 2). Furthermore, no effects of NaP were noted in 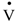 E (Table 2).
E (Table 2).| Placebo | NaP | |
|---|---|---|
| O2 uptake, L min−1 | ||
| Baseline | 1.07 ± 0.02 | 1.01 ± 0.05 |
| End-exercise | 3.52 ± 0.06 | 3.44 ± 0.06 |
| Slow component amplitude | 0.53 ± 0.04 | 0.50 ± 0.04 |
| CO2 output, L min−1 | ||
| Baseline | 0.81 ± 0.03 | 0.78 ± 0.04 |
| End-exercise | 4.08 ± 0.10 | 4.03 ± 0.09 |
| Minute ventilation, L min−1 | ||
| Baseline | 23 ± 1 | 22 ± 1 |
| End-exercise | 121 ± 5 | 116 ± 6 |
| Respiratory exchange ratio | ||
| Baseline | 0.76 ± 0.02 | 0.77 ± 0.02 |
| End-exercise | 1.16 ± 0.02 | 1.17 ± 0.02 |
| Heart rate, beats min−1 | ||
| Baseline | 86 ± 3 | 87 ± 4 |
| End-exercise | 171 ± 2 | 171 ± 3 |
4. Discussion
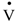 O2 slow component amplitude have been reported to improve the tolerance of severe intensity exercise [43,44]. However, the results of the pre-exercise alkalinization by oral sodium bicarbonate ingestion are equivocal. Some authors found a significant reduction of the slow component [28,45], whereas others observed no effect [46,47]. In the present study, NaP did not influence the slow component amplitude.
O2 slow component amplitude have been reported to improve the tolerance of severe intensity exercise [43,44]. However, the results of the pre-exercise alkalinization by oral sodium bicarbonate ingestion are equivocal. Some authors found a significant reduction of the slow component [28,45], whereas others observed no effect [46,47]. In the present study, NaP did not influence the slow component amplitude.5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spriet, L.L.; Howlett, R.A.; Heigenhauser, G.J. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med. Sci. Sports Exerc. 2000, 32, 756–763. [Google Scholar] [CrossRef]
- Felig, P.; Wahren, J. Amino acid metabolism in exercising man. J. Clin. Investig. 1971, 50, 2703–2714. [Google Scholar] [CrossRef]
- Regitz, V.; Azumi, T.; Stephan, H.; Naujocks, S.; Schaper, W. Biochemical mechanism of infarct size reduction by pyruvate. Cardiovasc. Res. 1981, 15, 652–658. [Google Scholar] [CrossRef]
- Kerr, P.M.; Suleiman, M.S.; Halestrap, A.P. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am. J. Physiol. 1999, 276, H496–H502. [Google Scholar]
- Mongan, P.D.; Fontana, J.L.; Chen, R.; Bunger, R. Intravenous pyruvate prolongs survival during hemorrhagic shock in swine. Am. J. Physiol. 1999, 277, H2253–H2263. [Google Scholar]
- Flaherty, D.C.; Hoxha, B.; Sun, J.; Gurji, H.; Simecka, J.W.; Mallet, R.T.; Olivencia-Yurvati, A.H. Pyruvate-fortified fluid resuscitation improves hemodynamic stability while suppressing systemic inflammation and myocardial oxidative stress after hemorrhagic shock. Mil. Med. 2010, 175, 166–172. [Google Scholar] [CrossRef]
- Hu, S.; Bai, X.D.; Liu, X.Q.; Wang, H.B.; Zhong, Y.X.; Fang, T.; Zhou, F.Q. Pyruvate Ringer’s solution corrects lactic acidosis and prolongs survival during hemorrhagic shock in rats. J. Emerg. Med. 2013, 45, 885–893. [Google Scholar] [CrossRef]
- Zweier, J.L.; Jacobus, W.E. Substrate-induced alterations of high energy phosphate metabolism and contractile function in the perfused heart. J. Biol. Chem. 1987, 262, 8015–8021. [Google Scholar]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar]
- Karetzky, M.S.; Cain, S.M. Effect of sodium pyruvate infusion on acid-base balance and gas exchange in the dog. Am. J. Physiol. 1969, 217, 1472–1476. [Google Scholar]
- Morrison, M.A.; Spriet, L.L.; Dyck, D.J. Pyruvate ingestion for 7 days does not improve aerobic performance in well-trained individuals. J. Appl. Physiol. 2000, 89, 549–556. [Google Scholar]
- Ebersole, K.T.; Stout, J.R.; Eckerson, J.M.; Housh, T.J.; Evetovich, T.K.; Smith, D.B. The Effect of Pyruvate Supplementation on Critical Power. J. Strength Cond. Res. 2000, 14, 132–134. [Google Scholar]
- Olek, R.; Luszczyk, M.; Kujach, S.; Ziemann, E.; Pieszko, M.; Pischel, I.; Laskowski, R.; Gdansk University of Physical Education and Sport, Gdansk, Poland. Unpublished work. 2014.
- Siegler, J.C.; Midgley, A.W.; Polman, R.C.; Lever, R. Effects of various sodium bicarbonate loading protocols on the time-dependent extracellular buffering profile. J. Strength Cond. Res. 2010, 24, 2551–2557. [Google Scholar] [CrossRef]
- Turner, M.; Page, R.; Mitchell, N.; Siegler, J. The effects of Energised Greens upon blood acid-base balance during resting conditions. J. Int. Soc. Sports Nutr. 2011, 8. [Google Scholar] [CrossRef]
- Olek, R.A.; Safranow, K.; Jakubowska, K.; Olszewska, M.; Chlubek, D.; Laskowski, R. Allopurinol intake does not modify the slow component of
O2 kinetics and oxidative stress induced by severe intensity exercise. Physiol. Res. 2012, 61, 89–96. [Google Scholar]
- Kalman, D.; Colker, C.M.; Wilets, I.; Roufs, J.B.; Antonio, J. The effects of pyruvate supplementation on body composition in overweight individuals. Nutrition 1999, 15, 337–340. [Google Scholar]
- Koh-Banerjee, P.K.; Ferreira, M.P.; Greenwood, M.; Bowden, R.G.; Cowan, P.N.; Almada, A.L.; Kreider, R.B. Effects of calcium pyruvate supplementation during training on body composition, exercise capacity, and metabolic responses to exercise. Nutrition 2005, 21, 312–319. [Google Scholar] [CrossRef]
- Bearden, S.E.; Moffatt, R.J. VO2 slow component: To model or not to model? Med. Sci. Sports Exerc. 2001, 33, 677–680. [Google Scholar] [CrossRef]
- Grocott, M.P.; Martin, D.S.; Levett, D.Z.; McMorrow, R.; Windsor, J.; Montgomery, H.E. Arterial blood gases and oxygen content in climbers on Mount Everest. N. Engl. J. Med. 2009, 360, 140–149. [Google Scholar] [CrossRef]
- Maughan, R.J. A simple, rapid method for the determination of glucose, lactate, pyruvate, alanine, 3-hydroxybutyrate and acetoacetate on a single 20-mul blood sample. Clin. Chim. Acta 1982, 122, 231–240. [Google Scholar] [CrossRef]
- Mongan, P.D.; Karaian, J.; van der Schuur, B.M.; Via, D.K.; Sharma, P. Pyruvate prevents poly-ADP ribose polymerase (PARP) activation, oxidative damage, and pyruvate dehydrogenase deactivation during hemorrhagic shock in swine. J. Surg. Res. 2003, 112, 180–188. [Google Scholar] [CrossRef]
- Zhou, F.Q. Pyruvate in the correction of intracellular acidosis: A metabolic basis as a novel superior buffer. Am. J. Nephrol. 2005, 25, 55–63. [Google Scholar] [CrossRef]
- Poole, R.C.; Halestrap, A.P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol. 1993, 264, C761–C782. [Google Scholar]
- Constantin-Teodosiu, D.; Simpson, E.J.; Greenhaff, P.L. The importance of pyruvate availability to PDC activation and anaplerosis in human skeletal muscle. Am. J. Physiol. 1999, 276, E472–E478. [Google Scholar]
- Sahlin, K.; Katz, A.; Henriksson, J. Redox state and lactate accumulation in human skeletal muscle during dynamic exercise. Biochem. J. 1987, 245, 551–556. [Google Scholar]
- Stephens, T.J.; McKenna, M.J.; Canny, B.J.; Snow, R.J.; McConell, G.K. Effect of sodium bicarbonate on muscle metabolism during intense endurance cycling. Med. Sci. Sports Exerc. 2002, 34, 614–621. [Google Scholar] [CrossRef]
- Berger, N.J.; McNaughton, L.R.; Keatley, S.; Wilkerson, D.P.; Jones, A.M. Sodium bicarbonate ingestion alters the slow but not the fast phase of VO2 kinetics. Med. Sci. Sports Exerc. 2006, 38, 1909–1917. [Google Scholar] [CrossRef]
- Lindh, A.M.; Peyrebrune, M.C.; Ingham, S.A.; Bailey, D.M.; Folland, J.P. Sodium bicarbonate improves swimming performance. Int. J. Sports Med. 2008, 29, 519–523. [Google Scholar]
- Vanhatalo, A.; McNaughton, L.R.; Siegler, J.; Jones, A.M. Effect of induced alkalosis on the power-duration relationship of “all-out” exercise. Med. Sci. Sports Exerc. 2010, 42, 563–570. [Google Scholar]
- Henderson, G.C.; Horning, M.A.; Lehman, S.L.; Wolfel, E.E.; Bergman, B.C.; Brooks, G.A. Pyruvate shuttling during rest and exercise before and after endurance training in men. J. Appl. Physiol. 2004, 97, 317–325. [Google Scholar]
- Stellingwerff, T.; Leblanc, P.J.; Hollidge, M.G.; Heigenhauser, G.J.; Spriet, L.L. Hyperoxia decreases muscle glycogenolysis, lactate production, and lactate efflux during steady-state exercise. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1180–E1190. [Google Scholar] [CrossRef]
- Bangsbo, J.; Gibala, M.J.; Krustrup, P.; Gonzalez-Alonso, J.; Saltin, B. Enhanced pyruvate dehydrogenase activity does not affect muscle O2 uptake at onset of intense exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R273–R280. [Google Scholar]
- Babij, P.; Matthews, S.M.; Rennie, M.J. Changes in blood ammonia, lactate and amino acids in relation to workload during bicycle ergometer exercise in man. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 50, 405–411. [Google Scholar] [CrossRef]
- Bassini, A.; Magalhaes-Neto, A.M.; Sweet, E.; Bottino, A.; Veiga, C.; Tozzi, M.B.; Pickard, M.B.; Cameron, L.C. Caffeine decreases systemic urea in elite soccer players during intermittent exercise. Med. Sci. Sports Exerc. 2013, 45, 683–690. [Google Scholar] [CrossRef]
- Stanko, R.T.; Diven, W.F.; Robertson, R.J.; Spina, R.J.; Galbreath, R.W.; Reilly, J.J., Jr.; Goss, F.L. Amino acid arterial concentration and muscle exchange during submaximal arm and leg exercise: The effect of dihydroxyacetone and pyruvate. J. Sports Sci. 1993, 11, 17–23. [Google Scholar] [CrossRef]
- Sahlin, K.; Katz, A.; Broberg, S. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am. J. Physiol. 1990, 259, C834–C841. [Google Scholar]
- Timmons, J.A.; Gustafsson, T.; Sundberg, C.J.; Jansson, E.; Greenhaff, P.L. Muscle acetyl group availability is a major determinant of oxygen deficit in humans during submaximal exercise. Am. J. Physiol. 1998, 274, E377–E380. [Google Scholar]
- Howlett, R.A.; Heigenhauser, G.J.; Hultman, E.; Hollidge-Horvat, M.G.; Spriet, L.L. Effects of dichloroacetate infusion on human skeletal muscle metabolism at the onset of exercise. Am. J. Physiol. 1999, 277, E18–E25. [Google Scholar]
- Koppo, K.; Wilkerson, D.P.; Bouckaert, J.; Wilmshurst, S.; Campbell, I.T.; Jones, A.M. Influence of DCA on pulmonary
O2 kinetics during moderate-intensity cycle exercise. Med. Sci. Sports Exerc. 2004, 36, 1159–1164. [Google Scholar]
- Bangsbo, J.; Gibala, M.J.; Howarth, K.R.; Krustrup, P. Tricarboxylic acid cycle intermediates accumulate at the onset of intense exercise in man but are not essential for the increase in muscle oxygen uptake. Pflugers Arch. 2006, 452, 737–743. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Korzeniewski, B. Physiological background of the change point in VO2 and the slow component of oxygen uptake kinetics. J. Physiol. Pharmacol. 2001, 52, 167–184. [Google Scholar]
- Bailey, S.J.; Winyard, P.G.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Jones, A.M. Acute l-arginine supplementation reduces the O2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J. Appl. Physiol. 2010, 109, 1394–1403. [Google Scholar] [CrossRef]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef]
- Kolkhorst, F.W.; Rezende, R.S.; Levy, S.S.; Buono, M.J. Effects of sodium bicarbonate on VO2 kinetics during heavy exercise. Med. Sci. Sports Exerc. 2004, 36, 1895–1899. [Google Scholar] [CrossRef]
- Santalla, A.; Perez, M.; Montilla, M.; Vicente, L.; Davison, R.; Earnest, C.; Lucia, A. Sodium bicarbonate ingestion does not alter the slow component of oxygen uptake kinetics in professional cyclists. J. Sports Sci. 2003, 21, 39–47. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Duda, K.; Majerczak, J.; Domanski, J.; Emmerich, J. Metabolic alkalosis induced by pre-exercise ingestion of NaHCO3 does not modulate the slow component of VO2 kinetics in humans. J. Physiol. Pharmacol. 1997, 48, 211–223. [Google Scholar]
- Stanko, R.T.; Robertson, R.J.; Galbreath, R.W.; Reilly, J.J., Jr.; Greenawalt, K.D.; Goss, F.L. Enhanced leg exercise endurance with a high-carbohydrate diet and dihydroxyacetone and pyruvate. J. Appl. Physiol. 1990, 69, 1651–1656. [Google Scholar]
- Stanko, R.T.; Robertson, R.J.; Spina, R.J.; Reilly, J.J., Jr.; Greenawalt, K.D.; Goss, F.L. Enhancement of arm exercise endurance capacity with dihydroxyacetone and pyruvate. J. Appl. Physiol. 1990, 68, 119–124. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Olek, R.A.; Kujach, S.; Wnuk, D.; Laskowski, R. Single Sodium Pyruvate Ingestion Modifies Blood Acid-Base Status and Post-Exercise Lactate Concentration in Humans. Nutrients 2014, 6, 1981-1992. https://doi.org/10.3390/nu6051981
Olek RA, Kujach S, Wnuk D, Laskowski R. Single Sodium Pyruvate Ingestion Modifies Blood Acid-Base Status and Post-Exercise Lactate Concentration in Humans. Nutrients. 2014; 6(5):1981-1992. https://doi.org/10.3390/nu6051981
Chicago/Turabian StyleOlek, Robert A., Sylwester Kujach, Damian Wnuk, and Radoslaw Laskowski. 2014. "Single Sodium Pyruvate Ingestion Modifies Blood Acid-Base Status and Post-Exercise Lactate Concentration in Humans" Nutrients 6, no. 5: 1981-1992. https://doi.org/10.3390/nu6051981
APA StyleOlek, R. A., Kujach, S., Wnuk, D., & Laskowski, R. (2014). Single Sodium Pyruvate Ingestion Modifies Blood Acid-Base Status and Post-Exercise Lactate Concentration in Humans. Nutrients, 6(5), 1981-1992. https://doi.org/10.3390/nu6051981





