Intake of Tibetan Hull-Less Barley is Associated with a Reduced Risk of Metabolic Related Syndrome in Rats Fed High-Fat-Sucrose Diets
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Diets
| Components | Basal Diet (g/100 g) | High-Fat-Sucrose Diet (g/100 g) | |
|---|---|---|---|
| Refined Wheat | W-THB | ||
| Whole wheat flour | 30 | - | - |
| Corn starch | 36 | - | - |
| Refined wheat flour | - | 50.4 | - |
| Whole Tibetan hull-less barley (W-THB) | - | - | 50.4 |
| Lard | - | 10 | 10 |
| York powder | - | 5 | 5 |
| Cholesterol | - | 0.5 | 0.5 |
| Sodium cholate | - | 0.1 | 0.1 |
| Sucrose | - | 8 | 8 |
| Cellulose | 5 | 5 | 5 |
| Soybean oil | 1 | - | - |
| Soybean meal | 23 | 16 | 16 |
| Vitamin mix | 4 | 4 | 4 |
| Mineral mix | 1 | 1 | 1 |
| Calculated energy | |||
| Protein (%) | 25.5 | 21.9 | 21.5 |
| Carbohydrate (%) | 63.2 | 42.7 | 42.0 |
| Fat (%) | 11.2 | 35.5 | 36.0 |
| Energy (kcal/g) | 3.60 | 3.94 | 3.97 |
2.3. Experimental Procedures
2.4. Serum Lipid Profile
2.5. Histological Observations of Hepatic Tissue
2.6. Hepatic Glycogen
2.7. Assessment of Insulin Resistance
2.8. Statistical Analyses
3. Results
3.1. Feed Intake, and Body, Fat, and Tissue Weights
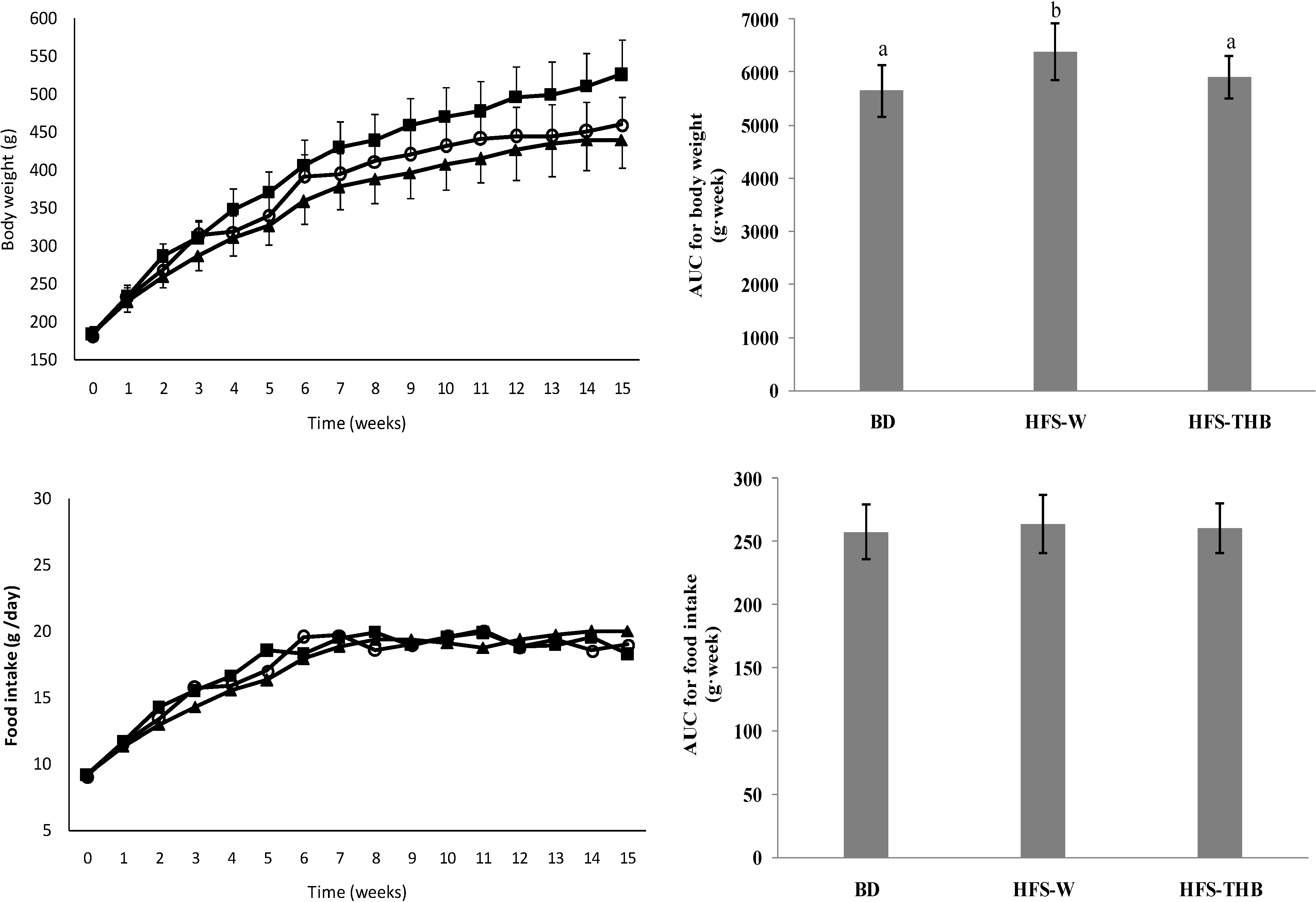
| Tissues | Basal Diet (n = 10) | High-Fat-Sucrose Diet | |
|---|---|---|---|
| Refined Wheat (n = 14) | Whole THB (n = 14) | ||
| Liver (g) | 11.2 ± 1.71 a | 13.3 ± 1.40 b | 12.1 ± 1.58 a,b |
| Kidney (g) | 2.78 ± 0.30 a | 3.23 ± 0.17 b | 3.28 ± 0.33 b |
| Epididymal fat (g) | 5.69 ± 2.13 a | 10.4 ± 4.90 b | 6.45 ± 1.56 a |
| Perirenal fat (g) | 1.90 ± 0.52 a | 3.90 ± 1.67 b | 3.19 ± 0.74 b |
| Liver (% #) | 2.48 ± 0.31 | 2.57 ± 0.12 | 2.59 ± 0.36 |
| Kidney (%) | 0.621 ± 0.079 a | 0.626 ± 0.053 a | 0.689 ± 0.047 b |
| Epididymal fat (%) | 1.24 ± 0.35 a | 1.96 ± 0.79 b | 1.38 ± 0.29 a |
| Perirenal fat (%) | 0.420 ± 0.100 a | 0.735 ± 0.262 b | 0.683 ± 0.148 b |
3.2. Plasma Cholesterol, TG, and Lipoprotein Concentrations
| Lipid Profiles | Basal Diet (n = 10) | High-Fat-Sucrose Diet | |
|---|---|---|---|
| Refined Wheat (n = 14) | Whole THB (n = 14) | ||
| Total cholesterol | 1.46 ± 0.32 | 1.47 ± 0.34 | 1.50 ± 0.23 |
| TG | 0.69 ± 0.24 a | 1.15 ± 0.23 b | 0.84 ± 0.12 a,b |
| LDL-C | 0.17 ± 0.03 | 0.14 ± 0.04 | 0.15 ± 0.03 |
| HDL-C | 1.39 ± 0.36 b | 1.02 ± 0.22 a | 1.40 ± 0.18 b |
3.3. Histology of Liver
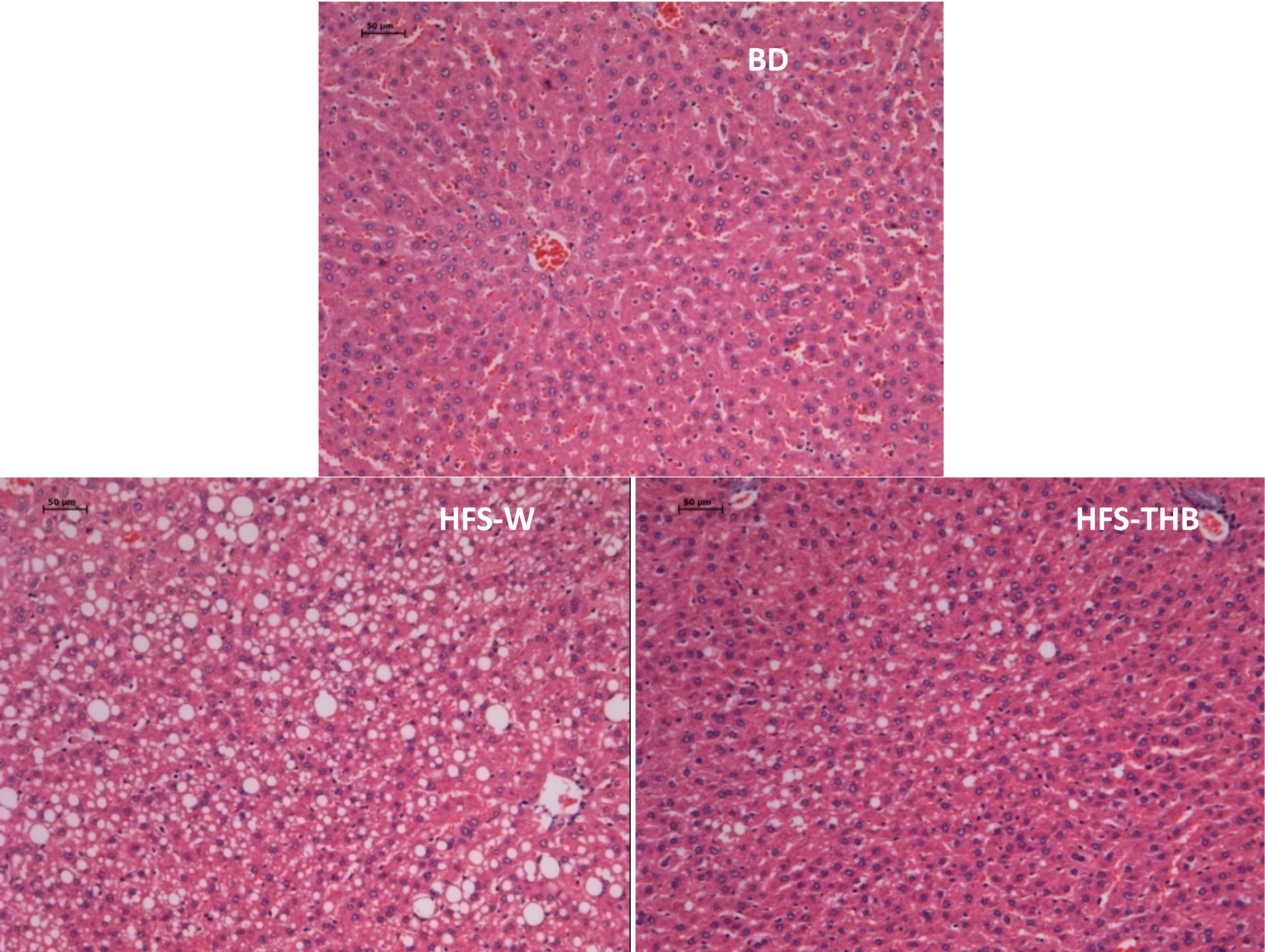
3.4. Hepatic Glycogen
3.5. Insulin Resistance, Plasma Glucose, and Plasma Insulin
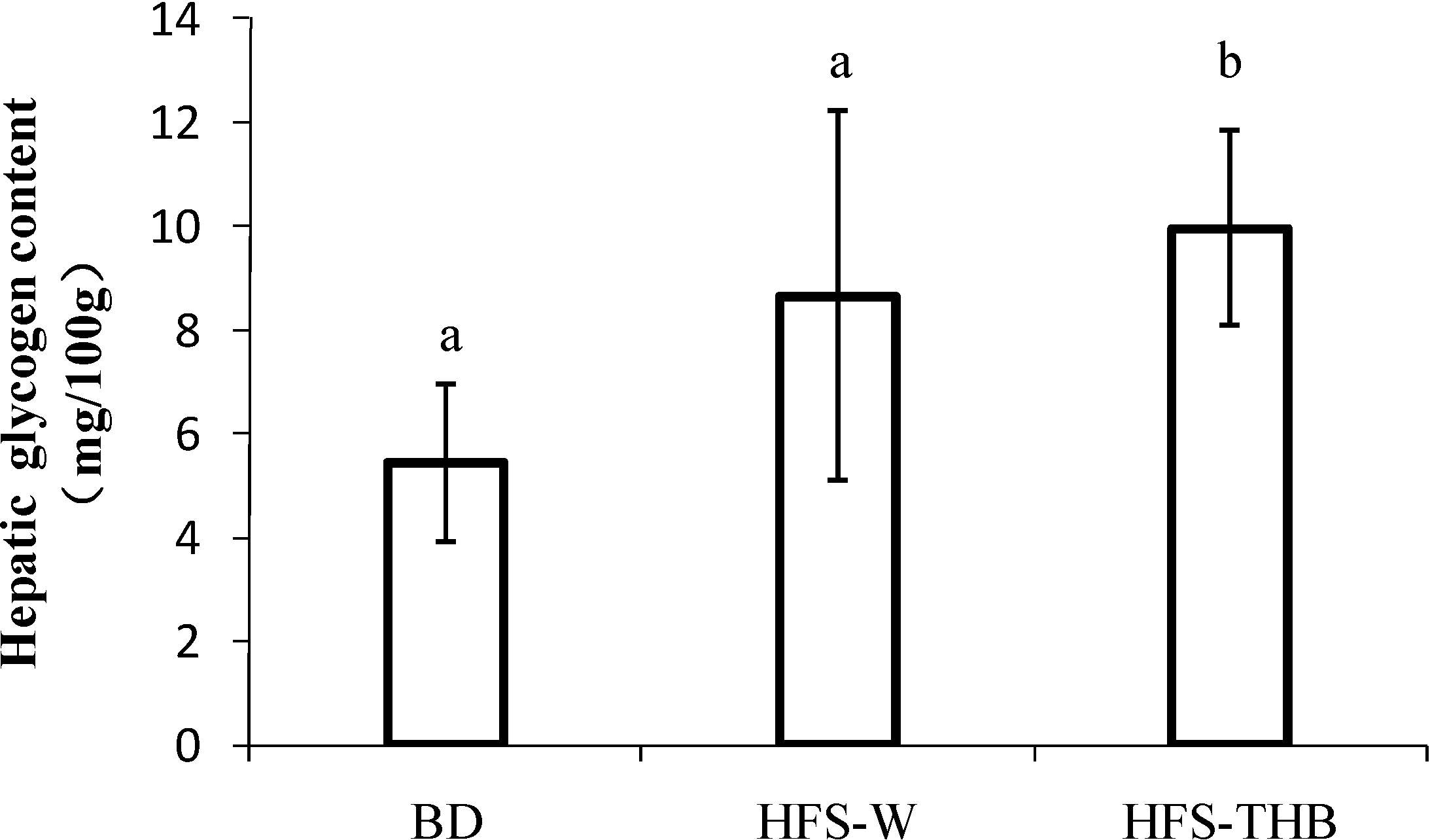

| Group | P Value | ||
|---|---|---|---|
| BD | HFS-W | HFS-THB | |
| BD | - | 0.035 | 0.043 |
| HFS-W | - | 0.069 | |
| HFS-THB | - | ||
4. Discussion
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Albert, K.G.M.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- Björck, I.; Östman, E.; Kristensen, M.; Anson, N.M.; Price, R.K.; Haenen, G.R.M.M; Havenaar, R.; Knudsen, K.E.B.; Frid, A.; Mykkänen, H.; et al. Cereal grains for nutrition and health benefits: Overview of results from in vitro, animal and human studies in the HEALTHGRAIN project. Trends Food Sci. Tech. 2012, 25, 87–100. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Mirmiran, P.; Azizi, F. Whole-grain consumption and the metabolic syndrome: A favorable association in Tehranian adults. Eur. J. Clin. Nutr. 2005, 59, 353–362. [Google Scholar] [CrossRef]
- Sahyoun, N.R.; Jacques, P.F.; Zhang, X.L.; Juan, W.; Mckeown, N.M. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am. J. Clin. Nutr. 2006, 8, 124–131. [Google Scholar]
- Pereira, M.A.; Jacobs, D.R., Jr.; Pins, J.J.; Raatz, S.K.; Gross, M.D.; M.D., J.L.; Seaquist, E.R. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am. J. Clin. Nutr. 2002, 75, 848–855. [Google Scholar]
- USDA. Nutrition and Your Health: Dietary Guildelines for Americans; U.S. Department of Agriculture, U.S. Department of Health and Human Services, U.S. Government of Printing Office: Washington, DC, USA, 2005.
- USDHHS. Healthy People 2010: Volumes I and II; U.S. Department of Health and Human Services, U.S. Government of Printing Office: Washington, DC, USA, 2000.
- Dani, C.; Oliboni, L.S.; Vanderlinde, R.; Bonatto, D.; Salvador, M.; Henriques, J.A.P. Phenolic content and antioxidant activities of white and purple juices manufactured with organically- or conventionally-produced grapes. Food Chem. Toxicol. 2007, 45, 2574–2580. [Google Scholar] [CrossRef]
- Charles, M.T.; Mercier, J.; Makhlouf, J.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit I. Role of pre- and post-challenge accumulation of the phytoalexin-rishitin. Postharvest Biol. Tec. 2008, 47, 10–20. [Google Scholar] [CrossRef]
- Gong, L.X.; Jin, J.; Wu, L.J.; Wu, X.Q.; Zhang, Y. Tibetan hull-less barley (Hordeum vulgare L.) as a potential source of antioxidants. Cereal Chem. 2012, 89, 290–295. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, J.; Chen, J. Analysis of β-glucan content in barley cultivars from different locations of China. Food Chem. 2002, 79, 251–254. [Google Scholar] [CrossRef]
- Gou, A.C.; Li, L.; Guo, C.H. Discussion on the importance of highland barley production from Tibetan compatriot the food construction variety. Chin. Agric. Sci. Bull. 2005, 21, 142–145. [Google Scholar]
- Xiong, M.; Zhang, H.; Chen, J. Effect of Tibetan hull-less barley on plasma lipid and body weight in dyslipidaemia patients. Med. Sci. Technol. 2005, 5, 45. [Google Scholar]
- Li, Z.; Tong, J.; Gao, J. The influence of taking naked oat fruit on glycometabolism of healty adults. J. Qinghai Med. Coll. 2009, 30, 46–50. [Google Scholar]
- Mikušová, L.; Šturdík, E.; Holubková, A. Whole grain cereal food in prevention of obesity. Acta Chim. Slovaca 2011, 4, 95–114. [Google Scholar]
- Jang, Y.; Lee, J.H.; Kim, O.Y.; Park, H.Y.; Lee, S.Y. Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: Randomized controlled clinical trial. Arterioscl. Throm. Vas. 2001, 21, 2065–2071. [Google Scholar] [CrossRef]
- Klaus, R.; Roggen, K.; Dellweg, S.; Heise, T.; tom Dieck, H. Improvement of insulin resistance after diet with a whol-grain based dietary product: Results of a randomized, controlled cross-over study in obese subjects with elevated fasting blood glucose. Br. J. Nutr. 2007, 98, 929–936. [Google Scholar]
- Maclntosh, C.G.; Holt, S.H.; Brand-Miller, J.C. The degree of fat saturation does not alter glycemic, insulinemic or satiety responses to a starchy staple in healthy men. J. Nutr. 2003, 133, 2577–2580. [Google Scholar]
- Brownlee, I.A.; Moore, C.; Chatfield, M.; Richardson, D.P.; Ashby, P.; Kuznesof, S.A.; Jebb, S.A.; Seal, C.J. Markers of cardiovascular risk are not changed by increased whole-grain intake: The WHOLEheart study, a randomized, controlled dietary intervention. Br. J. Nutr. 2010, 104, 125–134. [Google Scholar] [CrossRef]
- Katcher, H.I.; Legro, R.S.; Kunselman, A.R.; Gillies, P.J.; Demers, L.M.; Bagshaw, D.M.; Kris-Etherton, P.M. The effects of a whole grain enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 79–90. [Google Scholar]
- Leinonen, K.S.; Poutanen, K.S.; Mykkänen, H.M. Rye bread decreases serum total and LDL cholesterol in men with moderately elevated serum cholesterol. J. Nutr. 2000, 130, 164–170. [Google Scholar]
- Andersson, A.; Tengblad, S.; Karlström, B.; Kamal-Eldin, A.; Landberg, R.; Basu, S.; Åman, P.; Vessby, B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation on healthy, moderately overweight subjects. J. Nutr. 2007, 137, 1401–1407. [Google Scholar]
- The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Part 1. Worldwide Definition for Use in Clinical Practice. Available online: http://www.idf.org (accessed on 3 April 2007).
- Oron-Herman, M.; Kamari, Y.; Grossman, E.; Yeger, G.; Peleg, E.; Shabtay, Z.; Shamiss, A.; Sharabi, Y. Metabolic syndrome: Comparison of the two commonly used animal models. Am. J. Hpertens. 2008, 21, 1018–1022. [Google Scholar] [CrossRef]
- He, Y.N.; Zhai, F.Y.; Wang, Z.H.; Wang, H.J.; Hu, Y.S.; Yang, X.G. The status and trend for dietary pattern of energy, protein and fat in Chinese residents. Acta Nutr. Sin. 2005, 27, 358–365. [Google Scholar]
- Han, S.F.; Zhang, H.; Zhai, C.K. Protective potentials of wild rice (Zizania latifolia (Grised) Turcz) against obesity and liptoxicity induced by a high-fat/cholesterol diet in rats. Food Chem. Toxicol. 2012, 50, 2263–2269. [Google Scholar] [CrossRef]
- Han, S.; Zhang, H.; Qin, L.; Zhai, C. Effects of dietary carbohydrate replaced with wild rice (Zizania latifolia (Grised) Turcz) on insulin resistance in rats fed with a high-fat/cholesterol diet. Nutrients 2013, 5, 552–564. [Google Scholar] [CrossRef]
- Fardet, A. New hypothesis for the health-protective mechanisms of whole grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef]
- Koh-Banerjee, P.; Rimm, E.B. Whole grain consumption and weight gain: A review of the epidemiological evidenc, potential mechanisms and opportunities for future research. Proc. Nutr. Soc. 2003, 62, 25–29. [Google Scholar] [CrossRef]
- Zechner, R.; Kienesberger, P.C.; Haemmerle, G.; Zimmermann, R.; Lass, A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 2009, 50, 3–21. [Google Scholar]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Lzgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Youn, M.; Csallany, A.S.; Gallaher, D.D. Whole grain consumption has a modest effect on the development of diabetes in the Goto-Kakisaki rat. Brit. J. Nutr. 2010, 107, 192–201. [Google Scholar]
- Boden, G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997, 46, 3–10. [Google Scholar] [CrossRef]
- Chen, X.; Iqubal, N.; Boden, G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J. Clin. Investig. 1999, 103, 365–372. [Google Scholar] [CrossRef]
- Oakes, N.D.; Cooney, G.J.; Camilleri, S.; Chisholm, D.J.; Kraegen, E.W. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes 1997, 46, 1768–1774. [Google Scholar] [CrossRef]
- Lam, T.K.T.; Carpentier, A.; Lewis, G.F.; van de Werve, G.; Fantus, I.G.; Giacca, A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am. J. Physiol. Endocrinol Metab. 2003, 284, E863–E873. [Google Scholar]
- Tashi, N. Report on Barley Gramplasm Appraisal and Breeding; Department of Crop Sciences, College of Agriculture, University of Saskathchewan: Saskatoon, SK, Canada, 1993. [Google Scholar]
- Choi, J.S.; Kim, H.; Jung, M.H.; Hong, S.; Song, J. Consumption of barley β-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010, 54, 1004–1013. [Google Scholar] [CrossRef]
- Qian, J.W.; Jiang, S.P.; Su, W.T.; Su, D.H.; Pi, N.N.; Xie, Y.; Gao, P. Analysis of fatty acid composition of hull-less barley bran oil and its effect on lipid metabolism in hyperlipidemai rats. Sichuan J. Zool. 2009, 28, 739–742. [Google Scholar]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr. 2001, 20, 5–19. [Google Scholar] [CrossRef]
- Kris-Etherton, P.; Daniels, S.R.; Eckel, R.H.; Engler, M.; Howard, B.V.; Krauss, R.M.; Lichtenstein, A.H.; Sacks, F.; Jeor, S.S.; Stampfer, M. Summary of the scientific conference on dietary fatty acids and cardiovascular health. Circulation 2001, 103, 1034–1039. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gong, L.; Gong, L.; Zhang, Y. Intake of Tibetan Hull-Less Barley is Associated with a Reduced Risk of Metabolic Related Syndrome in Rats Fed High-Fat-Sucrose Diets. Nutrients 2014, 6, 1635-1648. https://doi.org/10.3390/nu6041635
Gong L, Gong L, Zhang Y. Intake of Tibetan Hull-Less Barley is Associated with a Reduced Risk of Metabolic Related Syndrome in Rats Fed High-Fat-Sucrose Diets. Nutrients. 2014; 6(4):1635-1648. https://doi.org/10.3390/nu6041635
Chicago/Turabian StyleGong, Lingxiao, Lingyun Gong, and Ying Zhang. 2014. "Intake of Tibetan Hull-Less Barley is Associated with a Reduced Risk of Metabolic Related Syndrome in Rats Fed High-Fat-Sucrose Diets" Nutrients 6, no. 4: 1635-1648. https://doi.org/10.3390/nu6041635




