Alternative Sources of Omega-3 Fats: Can We Find a Sustainable Substitute for Fish?
Abstract
:1. Introduction
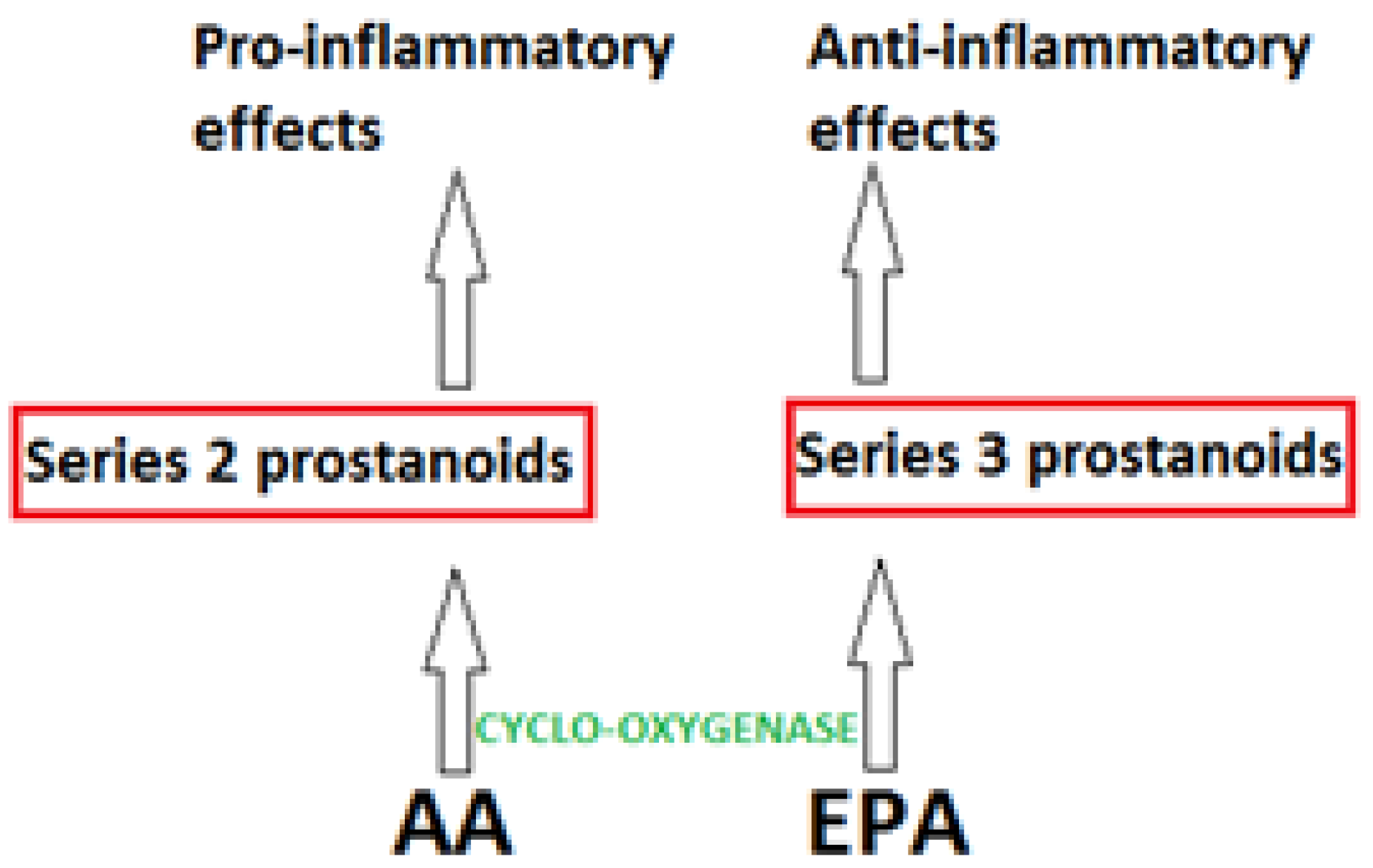
2. Mechanisms of Fatty Acids in Inflammation
3. Inflammation, Omega-3 Pufa and Health Benefits
4. Do Plant Oils Offer an Alternative to Fish Oils?
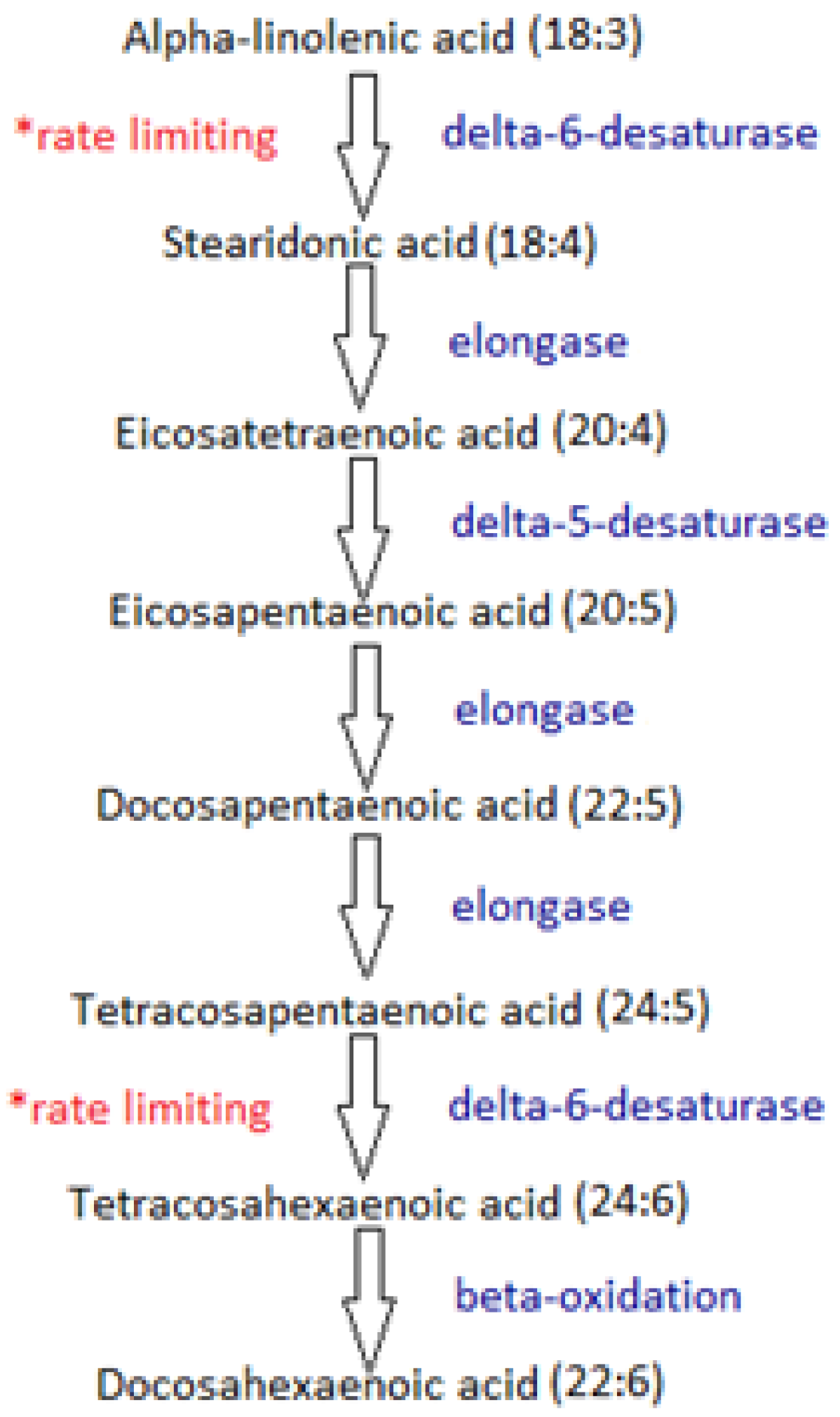
5. Stearidonic Acid: Can We Bypass the Rate-Limiting Step?
5.1. The Relationship of SDA Supplementation with Health and Disease
5.2. Can SDA Substitute as an Animal Feed?
5.3. The Differential Effects of EPA and DHA in Inflammation
6. Algal Oils as a Source of EPA and DHA
7. Conclusions
Acknowledgments
Conflict of Interest
References
- Calder, P. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505–1519. [Google Scholar]
- Guil-Guerrero, J.L. Stearidonic acid (18:4n-3): Metabolism, nutritional importance, medical uses and natural sources. Eur. J. Lipid. Sci. Technol. 2007, 109, 1226–1236. [Google Scholar] [CrossRef]
- Simopoulos, A. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Sadovy, Y.; Reynolds, J.D. Extinction vulnerability in marine populations. Fish Fish. 2003, 4, 25–64. [Google Scholar] [CrossRef]
- Calder, P. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res. 2008, 52, 885–897. [Google Scholar] [CrossRef]
- Burdge, G.; Calder, P. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Surette, M.; Edens, M.; Chilton, F.; Tramposch, K. Dietary echium oil increases plasma and neutrophil long-chain (n-3) fatty acids and lowers serum triacylglycerols in hypertriglyceridemic humans. J. Nutr. 2004, 134, 1406–1411. [Google Scholar]
- Calder, P. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592–599. [Google Scholar] [CrossRef]
- Nie, D.; Che, M.; Grignon, D.; Tang, K.; Honn, K. Role of eicosanoids in prostate cancer progression. Cancer Metast. Rev. 2001, 20, 195–206. [Google Scholar] [CrossRef]
- Berridge, M. Inositol trisphosphate and diacylglycerol as second messengers. Biochem. J. 1984, 220, 345–360. [Google Scholar]
- Egert, S. Influence of three rapeseed oil-rich diets, fortified with α-linolenic acid, eicosapentaenoic acid or docosahexaenoic acid on the composition and oxidizability of low-density lipoproteins: Results of a controlled study in healthy volunteers. Eur. J. Clin. Nutr. 2007, 61, 314–325. [Google Scholar] [CrossRef]
- Miles, E.A.; Banerjee, T.; Dooper, M.M.B.W.; M’Rabet, L.; Graus, Y.M.F.; Calder, P.C. The influence of different combinations of γ-linolenic acid, stearidonic acid and epa on immune function in healthy young male subjects. Br. J. Nutr. 2004, 91, 893. [Google Scholar] [CrossRef]
- Vendramini-Costa, D.; Carvalho, J. Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef]
- Calder, P. Polyunsaturated fatty acids, inflammation, and immunity. Lipids 2001, 36, 1007–1024. [Google Scholar] [CrossRef]
- Sharma, R.; Zucknick, M.; London, R.; Kacevska, M.; Liddle, C.; Clarke, S. Systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. Clin. Colorectal Cancer 2008, 7, 331–337. [Google Scholar] [CrossRef]
- Robert, G.; Descazeaud, A.; Nicolaïew, N.; Terry, S.; Sirab, N.; Vacherot, F.; Maillé, P.; Allory, Y.; de la Taille, A. Inflammation in benign prostatic hyperplasia: A 282 patients’ immunohistochemical analysis. Prostate 2009, 69, 1774–1780. [Google Scholar] [CrossRef]
- Yen, D.; Cheung, J.; Scheerens, H.; Poulet, F.; McClanahan, T.; McKenzie, B.; Kleinschek, M.; Owyang, A.; Mattson, J.; Blumenschein, W.; et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006, 116, 1310–1316. [Google Scholar] [CrossRef]
- Elson, C.; Cong, Y.; Weaver, C.; Schoeb, T.; McClanahan, T.; Fick, R.; Kastelein, R. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology 2007, 132, 2359–2370. [Google Scholar] [CrossRef]
- Panayi, G.; Lanchbury, J.; Kingsley, G. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992, 35, 729–735. [Google Scholar] [CrossRef]
- Krutmann, J.; Grewe, M. Sequential activation of Th1 and Th2 cells in the immunopathogenesis of atopic eczema—The 2-phase model. Allergologie 1996, 19, 449–451. [Google Scholar]
- Hansson, G. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Ishihara, K.; Komatsu, W.; Saito, H.; Shinohara, K. Comparison of the effects of dietary alpha-linolenic, stearidonic, and eicosapentaenoic acids on production of inflammatory mediators in mice. Lipids 2002, 37, 481–486. [Google Scholar] [CrossRef]
- Van Horssen, R.; Ten Hagen, T.; Eggermont, A. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef]
- Mohammed, A.; Janakiram, N.; Brewer, M.; Duff, A.; Lightfoot, S.; Brush, R.; Anderson, R.; Rao, C. Endogenous n-3 polyunsaturated fatty acids delay progression of pancreatic ductal adenocarcinoma in Fat-1-p48Cre/+-LSL-KrasG12D/+ mice. Neoplasia 2012, 14, 1249–1259. [Google Scholar]
- Chua, M.; Sio, M.; Sorongon, M.; Dy, J. Relationship of dietary intake of omega-3 and omega-6 fatty acids with risk of prostate cancer development: A meta-analysis of prospective studies and review of literature. Prostate Cancer 2012, 2012, 826254. [Google Scholar]
- Williams, C.D.; Whitley, B.; Hoyo, C.; Grant, D.; Iraggi, J.; Newman, K.; Gerber, L.; Taylor, L.; McKeever, M.; Freedland, S. A High ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr. Res. 2011, 31, 1–8. [Google Scholar]
- Oh, K.; Willett, W.; Fuchs, C.; Giovannucci, E. Dietary marine n-3 fatty acids in relation to risk of distal colorectal adenoma in women. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 835–841. [Google Scholar] [CrossRef]
- Daniel, C.; McCullough, M.; Patel, R.; Jacobs, E.; Flanders, W.; Thun, M.; Calle, E. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 516–525. [Google Scholar] [CrossRef]
- Kobayashi, N.; Barnard, J.; Henning, S.; Elashoff, D.; Reddy, S.; Cohen, P.; Leung, P.; Hong-Gonzalez, J.; Freedland, S.; Said, J.; et al. Effect of altering dietary w-6/w-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin. Cancer Res. 2006, 12, 4670. [Google Scholar]
- Murff, H.; Shu, X.; Li, H.; Yang, G.; Wu, X.; Cai, H.; Wen, W.; Gao, Y.; Zheng, W. Dietary polyunsaturated fatty acids and breast cancer risk in chinese women, a prospective cohort study. Int. J. Cancer Suppl. 2011, 128, 1434–1441. [Google Scholar] [CrossRef]
- Musa-Veloso, K.; Binns, M.; Kocenas, A.; Chung, C.; Rice, H.; Oppedal-Olsen, H.; Lloyd, H.; Lemke, S. Impact of low v. moderate intakes of long-chain n-3 fatty acids on risk of coronary heart disease. Br. J. Nutr. 2011, 106, 1129–1141. [Google Scholar] [CrossRef]
- Jiang, W.; Oken, H.; Fiuzat, M.; Shaw, L.; Martsberger, C.; Kuchibhatla, M.; Kaddurah-Daouk, R.; Steffens, D.; Baillie, R.; Cuffe, M.; et al. Plasma omega-3 polyunsaturated fatty acids and survival in patients with chronic heart failure and major depressive disorder. J. Cardiovasc. Trans. Res. 2012, 5, 92–99. [Google Scholar] [CrossRef]
- Ibrahim, A.; Mbodji, K.; Hassan, A.; Aziz, M.; Boukhettala, N.; Coeffier, M.; Savoye, G.; Dechelotte, P.; Marion-Letellier, R. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin. Nutr. 2011, 30, 678–687. [Google Scholar] [CrossRef]
- Uchiyama, K.; Nakamura, M.; Odahara, S.; Koido, S.; Katahira, K.; Shiraishi, H.; Ohkusa, T.; Fujise, K.; Tajiri, H. n-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm. Bowel. Dis. 2010, 16, 1696–1707. [Google Scholar] [CrossRef]
- Miller, M.; Nichols, P.; Carter, C. n-3 oil sources for use in aquaculture—Alternatives to the unsustainable harvest of wild fish. Nutr. Res. Rev. 2008, 21, 85–96. [Google Scholar] [CrossRef]
- Kuhnt, K.; Degen, C.; Jaudszus, A.; Jahreis, G. Searching for health beneficial n-3 and n-6 fatty acids in plant seeds. Eur. J. Lipid. Sci. Technol. 2012, 114, 153–160. [Google Scholar] [CrossRef]
- Bell, J.; Tocher, D.; Henderson, R.; Dick, J.; Crampton, V. Altered fatty acid compositions in atlantic salmon (Salmo Salar L.) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet. J. Nutr. 2003, 133, 2793–2801. [Google Scholar]
- Torstensen, B.E.; Lie, Ø.; Frøyland, L. Lipid metabolism and tissue composition in atlantic salmon (Salmo Salar L.)—Effects of capelin oil, palm oil, and oleic acid-enriched sunflower oil as dietary lipid sources. Lipids 2000, 35, 653–664. [Google Scholar] [CrossRef]
- Seierstad, S.; Seljeflot, I.; Johansen, O.; Hansen, R.; Haugen, M.; Rosenlund, G.; Froyland, L.; Arnesen, H. Dietary intake of differently fed salmon; the influence on markers of human atherosclerosis. Eur. J. Clin. Nutr. 2005, 35, 52–59. [Google Scholar]
- Stulc, T.; Ceska, R. Cholesterol lowering and the vessel wall: New insights and future perspectives. Physiol. Res. 2001, 50, 461–471. [Google Scholar]
- Bell, J.G.; Henderson, R.J.; Tocher, D.R.; Sargent, J.R. Replacement of dietary fish oil with increasing levels of linseed oil: Modification of flesh fatty acid compositions in Atlantic salmon (Salmo Salar L.) using a fish oil finishing diet. Lipids 2004, 39, 223–232. [Google Scholar] [CrossRef]
- Codabaccus, M.; Bridle, A.; Nichols, P.; Carter, C. Restoration of fillet n-3 long-chain polyunsaturated fatty acid is improved by a modified fish oil finishing diet strategy for Atlantic salmon (Salmo Salar L.) smolts fed palm fatty acid distillate. J. Agric. Food Chem. 2012, 60, 458–466. [Google Scholar] [CrossRef]
- James, M.; Ursin, V.; Cleland, L. Metabolism of stearidonic acid in human subjects: Comparison with the metabolism of other n-3 fatty acids. Am. J. Clin. Nutr. 2003, 77, 1140–1145. [Google Scholar]
- Surette, M. Dietary omega-3 PUFA and health: Stearidonic acid-containing seed oils as effective and sustainable alternatives to traditional marine oils. Mol. Nutr. Food Res. 2013, 57, 748–759. [Google Scholar]
- Lemke, S.; Vicini, J.; Su, H.; Goldstein, D.; Nemeth, M.; Krul, E.; Harris, W. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: Randomized, double-blind clinical study of efficacy and safety. Am. J. Clin. Nutr. 2010, 92, 766–775. [Google Scholar] [CrossRef]
- Harris, W.; DiRienzo, M.; Sands, S.; George, C.; Jones, P.; Eapen, A. Stearidonic acid increases the red blood cell and heart eicosapentaenoic acid content in dogs. Lipids 2007, 42, 325–333. [Google Scholar] [CrossRef]
- Hammond, B.; Lemen, J.; Ahmed, G.; Miller, K.; Kirkpatrick, J.; Fleeman, T. Safety assessment of SDA soybean oil: Results of a 28-day gavage study and a 90-day/one generation reproduction feeding study in rats. Regul. Toxicol. Pharmacol. 2008, 52, 311–323. [Google Scholar] [CrossRef]
- Krul, E.; Lemke, S.; Mukherjea, R.; Taylor, M.; Goldstein, D.; Su, H.; Liu, P.; Lawless, A.; Harris, W.; Maki, K. Effects of duration of treatment and dosage of eicosapentaenoic acid and stearidonic acid on red blood cell eicosapentaenoic acid content. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 51–59. [Google Scholar] [CrossRef]
- Von Schacky, C. A review of omega-3 ethyl esters for cardiovascular prevention and treatment of increased blood triacylglyceride levels. Vasc. Health Risk Manag. 2006, 2, 251–262. [Google Scholar] [CrossRef]
- Walker, C.; Jebb, S.; Calder, P. Stearidonic acid as a supplemental source of w-3 polyunsaturated fatty acids to enhance status for improved human health. Nutrition 2013, 29, 363–369. [Google Scholar] [CrossRef]
- Forrest, L.; Boudyguina, E.; Wilson, M.; Parks, J. Echium oil reduces atherosclerosis in apoB100-only LDLrKO mice. Atherosclerosis 2012, 220, 118–121. [Google Scholar] [CrossRef]
- Howard, B.; Robbins, D.; Sievers, M.; Lee, E.; Rhoades, D.; Devereux, R.; Cowan, L.; Gray, R.; Welty, T.; Go, O.; et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The strong heart study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 830–835. [Google Scholar] [CrossRef]
- Banz, W.; Davis, J.; Clough, R.; Cheatwood, J. Stearidonic acid: Is there a role in the prevention and management of type 2 diabetes mellitus? J. Nutr. 2012, 142, 635S–640S. [Google Scholar] [CrossRef]
- Wu, D.; Meydani, M.; Leka, L.; Nightinggale, Z.; Handelman, G.; Blumberg, J.; Meydani, S. Effect of dietary supplementation with black currant seed oil on the immune response of healthy elderly subjects. Am. J. Clin. Nutr. 1999, 70, 536–543. [Google Scholar]
- Whelan, J.; Gouffon, J.; Zhao, Y. Effects of dietary stearidonic acid on biomarkers of lipid metabolism. J. Nutr. 2012, 142, 630S–634S. [Google Scholar] [CrossRef]
- Horia, E.; Watkins, B. Comparison of stearidonic acid and α-linoleic acid on PGE2 production and COX-2 protein levels in MDA-MB-231 breast cancer cell cultures. J. Nutr. Biochem. 2005, 16, 184–192. [Google Scholar] [CrossRef]
- Ferrandina, G.; Legge, F.; Ranelletti, F.; Zannoni, G.; Maggiano, N.; Evangelisti, A.; Mancuso, S.; Scambia, G.; Lauriola, L. Cyclooxygenase-2 expression in endometrial carcinoma: Correlation with clinicopathologic parameters and clinical outcome. Cancer 2002, 95, 801–807. [Google Scholar] [CrossRef]
- Kelavkar, U.; Hutzley, J.; Dhir, R.; Kim, P.; Allen, K.; McHugh, K. Prostate tumour growth and recurrence can be modulated by the ω-6:ω-3 ratio in diet: Athymic mouse xenograft model simulating radical prostatectomy. Neoplasia 2006, 8, 112–124. [Google Scholar] [CrossRef]
- Kitessa, S.M.; Young, P. Echium oil is better than rapeseed oil in enriching poultry meat with n-3 polyunsaturated fatty acids, including eicosapentaenoic acid and docosapentaenoic acid. Br. J. Nutr. 2009, 101, 709. [Google Scholar] [CrossRef]
- Cleveland, B.; Francis, D.; Turchini, G. Echium oil provides no benefit over linseed oil for (n-3) long-chain PUFA biosynthesis in rainbow trout. J. Nutr. 2012, 142, 1449–1455. [Google Scholar] [CrossRef]
- Miller, M.; Nichols, P.; Carter, C. Replacement of dietary fish oil for Atlantic salmon parr (Salmo Salar L.) with a stearidonic acid containing oil has no effect on omega-3 long-chain polyunsaturated fatty acid concentrations. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 197–206. [Google Scholar] [CrossRef]
- Kitessa, S.M.; Young, P.; Nattrass, G.; Gardner, G.; Pearce, K.; Pethick, D.W. When balanced for precursor fatty acid supply echium oil is not superior to linseed oil in enriching lamb tissues with long-chain n-3 PUFA. Br. J. Nutr. 2012, 108, 71. [Google Scholar] [CrossRef]
- Verlengia, R.; Gorjao, R.; Kanunfre, C.; Bordin, S.; de Lima, T.; Martins, E.; Curi, R. Comparative effects of eicosapentaenoic acid and docosahexaenoic acid on proliferation, cytokine production and pleiotropic gene expression in Jurkat cells. J. Nutr. Biochem. 2004, 15, 657–665. [Google Scholar] [CrossRef]
- Moore, K.; de Waal Malefyt, R.; Coffman, R.; O-Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Weldon, S.; Mullen, A.; Loscher, C.; Hurley, L.; Roche, H. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007, 18, 250–258. [Google Scholar] [CrossRef]
- Lewis, A.; Varghese, S.; Xu, H.; Alexander, H. Interleukin-1 and cancer progression: The emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J. Transl. Med. 2006, 4, 48–60. [Google Scholar] [CrossRef]
- Naugler, W.; Karin, M. The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 2008, 14, 109–119. [Google Scholar] [CrossRef]
- Adarme-Vega, T.; Lim, D.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11. [Google Scholar] [CrossRef]
- Subhadra, B.; Grinson-George. Algal Biorefinery-Based Industry: An Approach to Address Fuel and Food Insecurity for a Carbon-Smart World. J. Sci. Food. Agric. 2011, 91, 2–13. [Google Scholar] [CrossRef]
- Doughman, D.; Krupanidhi, S.; Sanjeeve, C. Omega-3 fatty acids for nutrition and medicine considering microalgae oil as a vegetarian source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef]
- Kyle, D. The large-scale production and use of a single-cell oil highly enriched in docosahexaenoic acid. ACS Symp. Ser. 2001, 788, 92–107. [Google Scholar]
- Sijtsma, L.; de Swaaf, M. Biotechnological production and applications of the ω-3 polyunsaturated fatty acid docosahexaenoic acid. Appl. Microbiol. Biotechnol. 2004, 64, 146–153. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lenihan-Geels, G.; Bishop, K.S.; Ferguson, L.R. Alternative Sources of Omega-3 Fats: Can We Find a Sustainable Substitute for Fish? Nutrients 2013, 5, 1301-1315. https://doi.org/10.3390/nu5041301
Lenihan-Geels G, Bishop KS, Ferguson LR. Alternative Sources of Omega-3 Fats: Can We Find a Sustainable Substitute for Fish? Nutrients. 2013; 5(4):1301-1315. https://doi.org/10.3390/nu5041301
Chicago/Turabian StyleLenihan-Geels, Georgia, Karen S. Bishop, and Lynnette R. Ferguson. 2013. "Alternative Sources of Omega-3 Fats: Can We Find a Sustainable Substitute for Fish?" Nutrients 5, no. 4: 1301-1315. https://doi.org/10.3390/nu5041301
APA StyleLenihan-Geels, G., Bishop, K. S., & Ferguson, L. R. (2013). Alternative Sources of Omega-3 Fats: Can We Find a Sustainable Substitute for Fish? Nutrients, 5(4), 1301-1315. https://doi.org/10.3390/nu5041301