A Randomised Cross-Over Pharmacokinetic Bioavailability Study of Synthetic versus Kiwifruit-Derived Vitamin C
Abstract
:1. Introduction
2. Study Design and Methods
2.1. Participants
2.2. Study Design
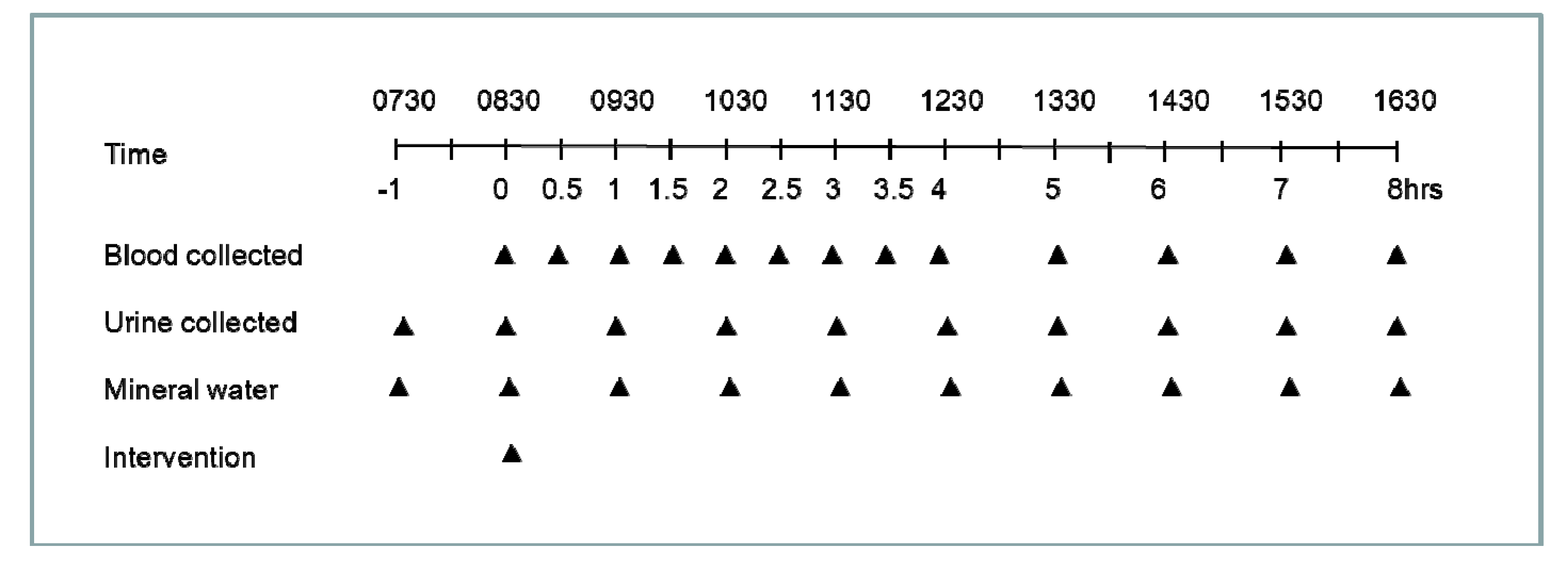
2.3. Interventions
2.4. Sample Collection and Processing
2.5. Plasma and Urine Analysis
2.6. Data and Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Vitamin C Uptake into Plasma
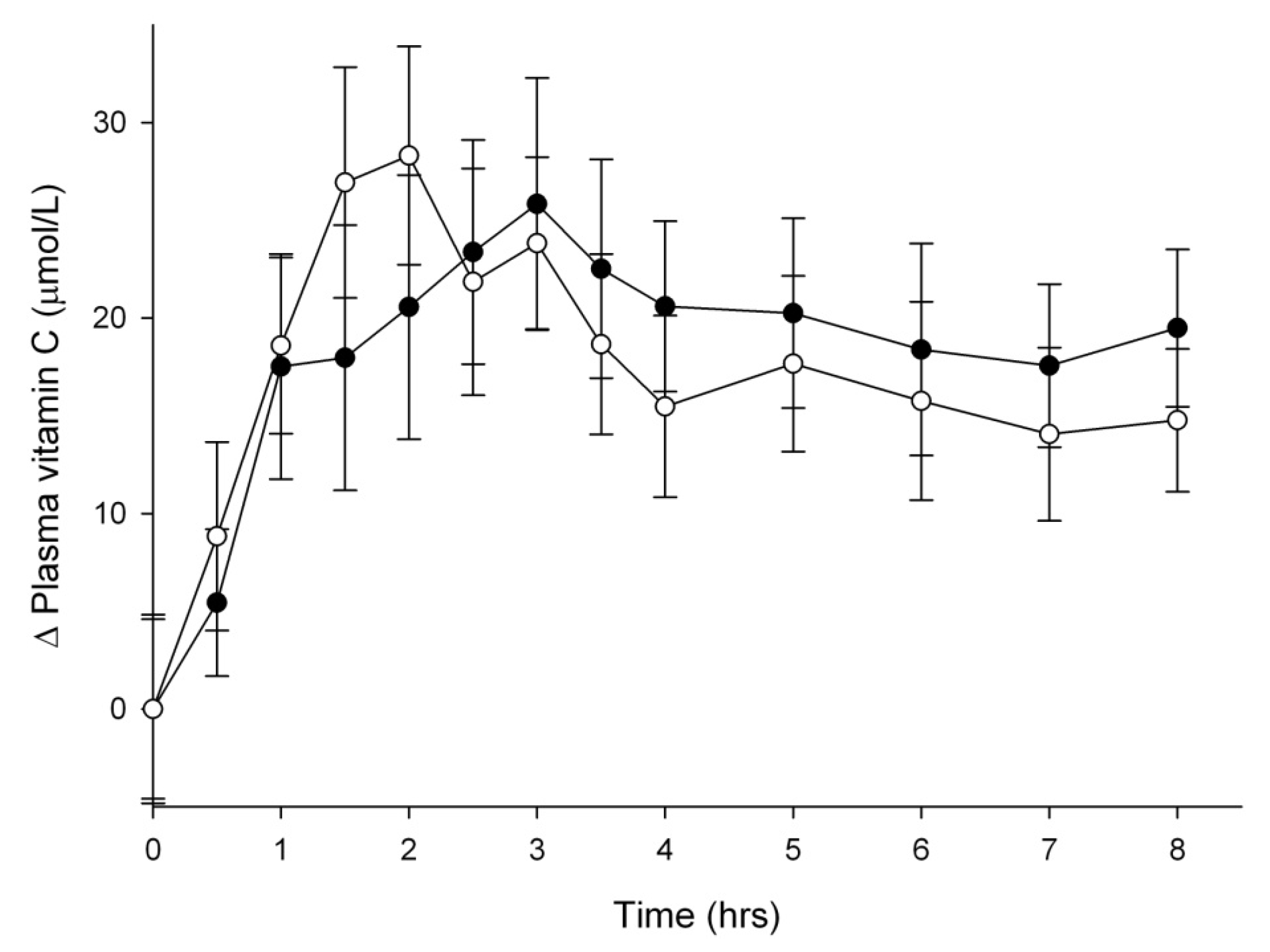
| Vitamin C (200 mg) a | Kiwifruit (1.5 Sungold) a | P value b | |
|---|---|---|---|
| Plasma AUC (h × µmol/L) | 220 ± 23 | 237 ± 13 | 0.483 |
| Plasma ascorbate (mg) c | 211 ± 18 | 227 ± 16 | 0.496 |
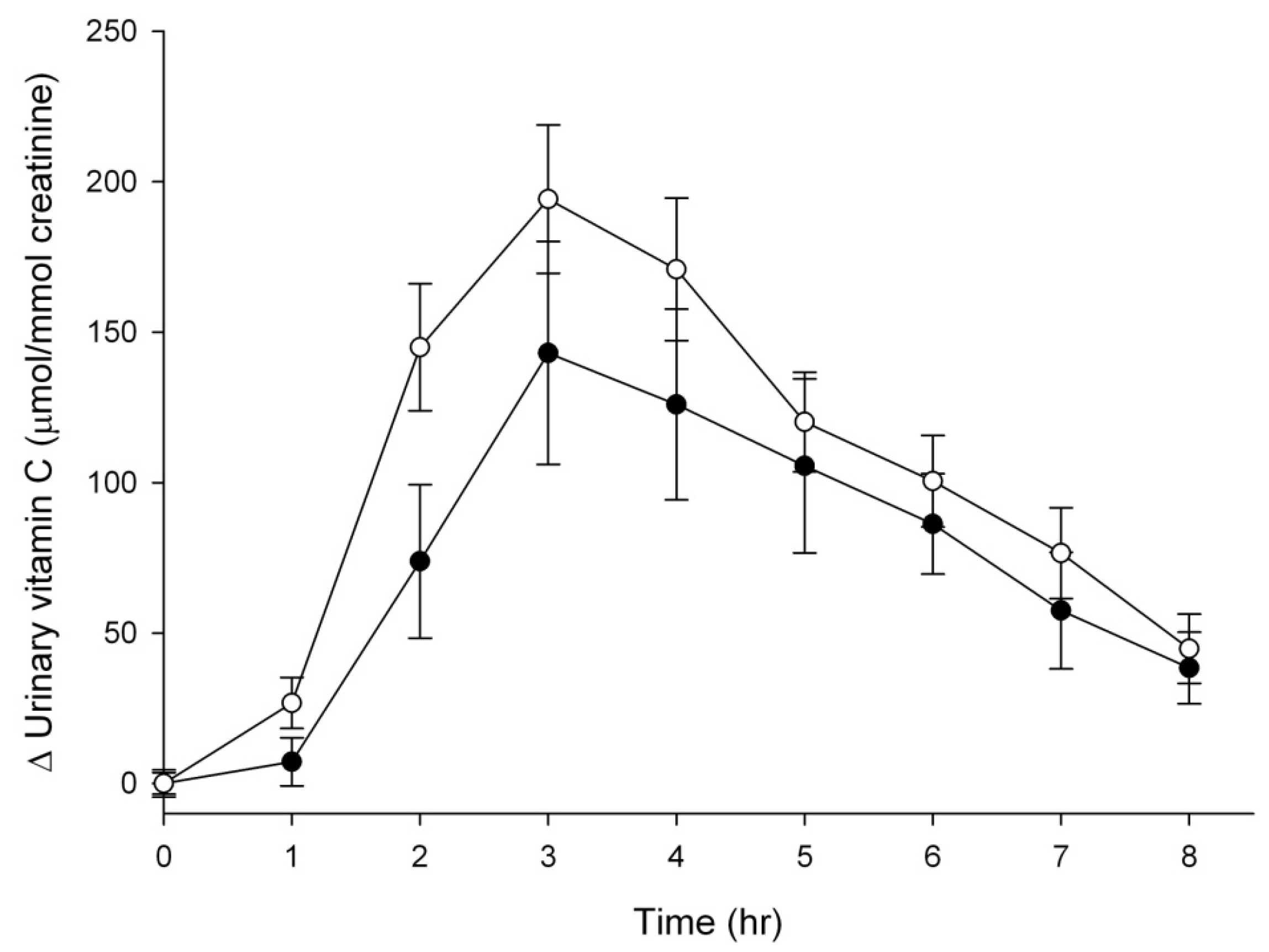
| Urinary AUC (h × µmol/mmol creatinine) | 618 ± 133 | 856 ± 118 | 0.004 |
| Urinary ascorbate (mg) | 74 ± 14 | 101 ± 10 | 0.033 |
3.3. Vitamin C Excretion in Urine
4. Discussion
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- Tsao, C.S. An Overview of Ascorbic Acid Chemistry and Biochemistry. In Vitamin C in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 25–58. [Google Scholar]
- Sauberlich, H.E. A History of Scurvy and Vitamin C. In Vitamin C in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 1–24. [Google Scholar]
- Ivanov, V.; Carr, A.C.; Frei, B. Red wine antioxidants bind to human lipoproteins and protect them from metal ion-dependent and -independent oxidation. J. Agric. Food Chem. 2001, 49, 4442–4449. [Google Scholar] [CrossRef]
- Beker, B.Y.; Sonmezoglu, I.; Imer, F.; Apak, R. Protection of ascorbic acid from copper(II)-catalyzed oxidative degradation in the presence of flavonoids: Quercetin, catechin and morin. Int. J. Food Sci. Nutr. 2011, 62, 504–512. [Google Scholar] [CrossRef]
- Clemetson, C.A.; Andersen, L. Plant polyphenols as antioxidants for ascorbic acid. Ann. N. Y. Acad. Sci. 1966, 136, 341–376. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? Faseb J. 1999, 13, 1007–1024. [Google Scholar]
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.V.; Chen, X.Z.; Wang, Y.; Brubaker, R.F.; Hediger, M.A. A family of mammalian Na+-dependent l-ascorbic acid transporters. Nature 1999, 399, 70–75. [Google Scholar] [CrossRef]
- Savini, I.; Rossi, A.; Pierro, C.; Avigliano, L.; Catani, M.V. SVCT1 and SVCT2: Key proteins for vitamin C uptake. Amino Acids 2008, 34, 347–355. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Kamp, F.; Jandel, D.; Hoenicke, I.; Pietrzk, K.; Gross, R.; Trugo, N.M.; Donangelo, C.M. Bioavailability of iron, zinc, folate, and vitamin C in the IRIS multi-micronutrient supplement: Effect of combination with a milk-based cornstarch porridge. Food Nutr. Bull. 2003, 24 (3 Suppl.), 20–26. [Google Scholar]
- Mangels, A.R.; Block, G.; Frey, C.M.; Patterson, B.H.; Taylor, P.R.; Norkus, E.P.; Levander, O.A. The bioavailability to humans of ascorbic acid from oranges, orange juice and cooked broccoli is similar to that of synthetic ascorbic acid. J. Nutr. 1993, 123, 1054–1061. [Google Scholar]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Simcock, J.W.; Vissers, M.C. A randomised steady-state bioavailability study of synthetic and natural (kiwifruit-derived) vitamin C. Nutrients 2013, 5, 3684–3695. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Bozonet, S.M.; Pearson, J.F.; Braithwaite, L.J. Dietary ascorbate affects steady state tissue levels in vitamin C-deficient mice: Tissue deficiency after sub-optimal intake and superior bioavailability from a food source (kiwifruit). Am. J. Clin. Nutr. 2011, 93, 292–301. [Google Scholar] [CrossRef]
- Uchida, E.; Kondo, Y.; Amano, A.; Aizawa, S.; Hanamura, T.; Aoki, H.; Nagamine, K.; Koizumi, T.; Maruyama, N.; Ishigami, A. Absorption and excretion of ascorbic acid alone and in acerola (Malpighia emarginata) juice: Comparison in healthy Japanese subjects. Biol. Pharm. Bull. 2011, 34, 1744–1747. [Google Scholar] [CrossRef]
- Bates, C.J.; Jones, K.S.; Bluck, L.J. Stable isotope-labelled vitamin C as a probe for vitamin C absorption by human subjects. Br. J. Nutr. 2004, 91, 699–705. [Google Scholar] [CrossRef]
- Jones, E.; Hughes, R.E. The influence of bioflavonoids on the absorption of vitamin C. IRCS Med. Sci. 1984, 12, 320. [Google Scholar]
- Pelletier, O.; Keith, M.O. Bioavailability of synthetic and natural ascorbic acid. J. Am. Diet. Assoc. 1974, 64, 271–275. [Google Scholar]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.K.; King, J.; Cantilena, L.R. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Kondo, Y.; Higashi, C.; Iwama, M.; Ishihara, K.; Handa, S.; Mugita, H.; Maruyama, N.; Koga, H.; Ishigami, A. Bioavailability of vitamin C from mashed potatoes and potato chips after oral administration in healthy Japanese men. Br. J. Nutr. 2012, 107, 885–892. [Google Scholar] [CrossRef]
- Carr, A.C.; Pullar, J.M.; Moran, S.; Vissers, M.C.M. Bioavailability of vitamin C from kiwifruit in non-smoking males: Determination of ‘healthy’ and ‘optimal’ intakes. J. Nutr. Sci. 2012, 1, e14. [Google Scholar] [CrossRef]
- Sato, Y.; Uchiki, T.; Iwama, M.; Kishimoto, Y.; Takahashi, R.; Ishigami, A. Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution. Biol. Pharm. Bull. 2010, 33, 364–369. [Google Scholar] [CrossRef]
- Carter, B.; Monsivais, P.; Drewnowski, A. Absorption of folic acid and ascorbic acid from nutrient comparable beverages. J. Food Sci. 2010, 75, H289–H293. [Google Scholar] [CrossRef]
- Nadler, S.B.; Hidalgo, J.H.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar]
- Guarnieri, S.; Riso, P.; Porrini, M. Orange juice vs. vitamin C: Effect on hydrogen peroxide-induced DNA damage in mononuclear blood cells. Br. J. Nutr. 2007, 97, 639–643. [Google Scholar] [CrossRef]
- Nelson, E.W.; Streiff, R.R.; Cerda, J.J. Comparative bioavailability of folate and vitamin C from a synthetic and a natural source. Am. J. Clin. Nutr. 1975, 28, 1014–1019. [Google Scholar]
- Vinson, J.A.; Bose, P. Comparative bioavailability of synthetic and natural vitamin C in guinea pigs. Nutr. Rep. Int. 1983, 27, 875–879. [Google Scholar]
- Vinson, J.A.; Bose, P. Comparative bioavailability to humans of ascorbic acid alone or in a citrus extract. Am. J. Clin. Nutr. 1988, 48, 601–604. [Google Scholar]
- Graumlich, J.F.; Ludden, T.M.; Conry-Cantilena, C.; Cantilena, L.R., Jr.; Wang, Y.; Levine, M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm. Res. 1997, 14, 1133–1139. [Google Scholar] [CrossRef]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Simcock, J.W.; Vissers, M.C. Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am. J. Clin. Nutr. 2013, 97, 800–807. [Google Scholar]
- Sims, I.M.; Monro, J.A. Fiber: Composition, structures, and functional properties. Adv. Food Nutr. Res. 2013, 68, 81–99. [Google Scholar] [CrossRef]
- Keltz, F.R.; Kies, C.; Fox, H.M. Urinary ascorbic acid excretion in the human as affected by dietary fiber and zinc. Am. J. Clin. Nutr. 1978, 31, 1167–1171. [Google Scholar]
- Lee, D.E.; Shin, B.J.; Hur, H.J.; Kim, J.H.; Kim, J.; Kang, N.J.; Kim, D.O.; Lee, C.Y.; Lee, K.W.; Lee, H.J. Quercetin, the active phenolic component in kiwifruit, prevents hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Br. J. Nutr. 2010, 104, 164–170. [Google Scholar] [CrossRef]
- Song, J.; Kwon, O.; Chen, S.; Daruwala, R.; Eck, P.; Park, J.B.; Levine, M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and glucose. J. Biol. Chem. 2002, 277, 15252–15260. [Google Scholar]
- Corpe, C.P.; Tu, H.; Eck, P.; Wang, J.; Faulhaber-Walter, R.; Schnermann, J.; Margolis, S.; Padayatty, S.; Sun, H.; Wang, Y.; Nussbaum, R.L.; Espey, M.G.; Leine, M. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J. Clin. Investig. 2010, 120, 1069–1083. [Google Scholar] [CrossRef]
- Yalcin, O.; Karatas, F.; Erulas, F.A.; Ozdemir, E. The levels of glutathione peroxidase, vitamin A, E, C and lipid peroxidation in patients with transitional cell carcinoma of the bladder. BJU Int. 2004, 93, 863–866. [Google Scholar] [CrossRef]
- Ochoa-Brust, G.J.; Fernandez, A.R.; Villanueva-Ruiz, G.J.; Velasco, R.; Trujillo-Hernandez, B.; Vasquez, C. Daily intake of 100 mg ascorbic acid as urinary tract infection prophylactic agent during pregnancy. Acta Obstet. Gynecol. Scand. 2007, 86, 783–787. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carr, A.C.; Bozonet, S.M.; Vissers, M.C.M. A Randomised Cross-Over Pharmacokinetic Bioavailability Study of Synthetic versus Kiwifruit-Derived Vitamin C. Nutrients 2013, 5, 4451-4461. https://doi.org/10.3390/nu5114451
Carr AC, Bozonet SM, Vissers MCM. A Randomised Cross-Over Pharmacokinetic Bioavailability Study of Synthetic versus Kiwifruit-Derived Vitamin C. Nutrients. 2013; 5(11):4451-4461. https://doi.org/10.3390/nu5114451
Chicago/Turabian StyleCarr, Anitra C., Stephanie M. Bozonet, and Margreet C. M. Vissers. 2013. "A Randomised Cross-Over Pharmacokinetic Bioavailability Study of Synthetic versus Kiwifruit-Derived Vitamin C" Nutrients 5, no. 11: 4451-4461. https://doi.org/10.3390/nu5114451






