Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action
Abstract
:1. Introduction
2. Polyphenols and Cancer
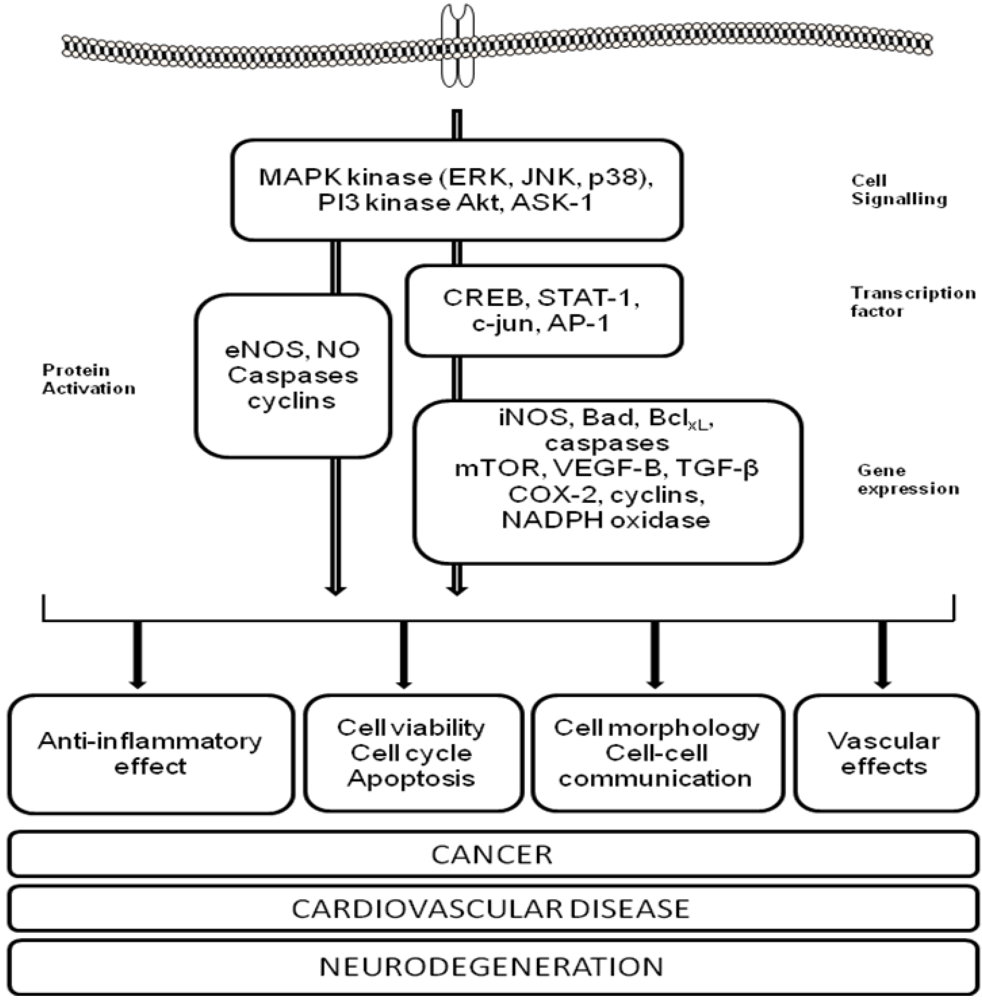
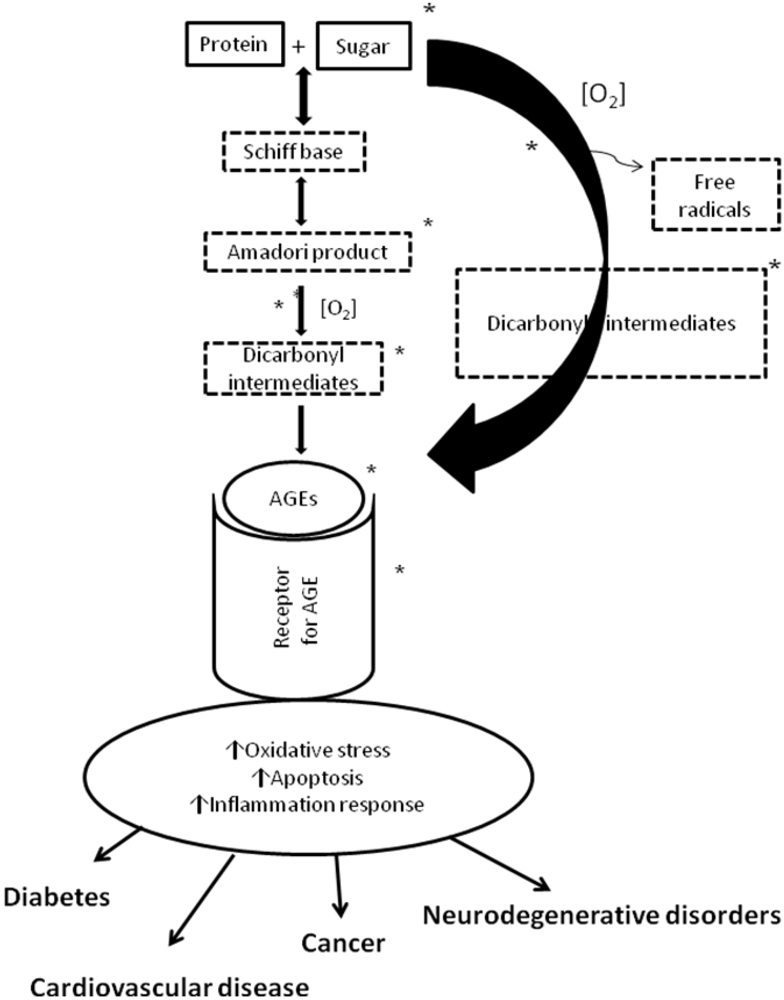
3. Polyphenols and Cardiovascular Disease
4. Polyphenols and Neurodegeneration
5. Summary
Acknowledgements
References
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Checkoway, H.; Powers, K.; Smith-Weller, T.; Franklin, G.M.; Longstreth, W.T., Jr.; Swanson, P.D. Parkinson’s disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am. J. Epidemiol. 2002, 155, 732–738. [Google Scholar] [PubMed]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar]
- Jeong, Y.J.; Choi, Y.J.; Kwon, H.M.; Kang, S.W.; Park, H.S.; Lee, M.; Kang, Y.H. Differential inhibition of oxidized LDL-induced apoptosis in human endothelial cells treated with different flavonoids. Br. J. Nutr. 2005, 93, 581–591. [Google Scholar]
- Fuhrman, B.; Volkova, N.; Coleman, R.; Aviram, M. Grape powder polyphenols attenuate atherosclerosis development in apolipoprotein E deficient (E0) mice and reduce macrophage atherogenicity. J. Nutr. 2005, 135, 722–728. [Google Scholar]
- Hubbard, G.P.; Wolffram, S.; de Vos, R.; Bovy, A.; Gibbins, J.M.; Lovegrove, J.A. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: A pilot study. Br. J. Nutr. 2006, 96, 482–488. [Google Scholar]
- Ludwig, A.; Lorenz, M.; Grimbo, N.; Steinle, F.; Meiners, S.; Bartsch, C.; Stangl, K.; Baumann, G.; Stangl, V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 2004, 316, 659–665. [Google Scholar]
- Hallund, J.; Bugel, S.; Tholstrup, T.; Ferrari, M.; Talbot, D.; Hall, W.L.; Reimann, M.; Williams, C.M.; Wiinberg, N. Soya isoflavone-enriched cereal bars affect markers of endothelial function in postmenopausal women. Br. J. Nutr. 2006, 95, 1120–1126. [Google Scholar]
- Hodgson, J.; Croft, K. Dietary flavonoids: Effects on endothelial function and blood pressure. J. Sci. Food Agric. 2006, 86, 2492–2498. [Google Scholar]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [PubMed]
- Mantena, S.K.; Baliga, M.S.; Katiyar, S.K. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis 2006, 27, 1682–1691. [Google Scholar]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Montedoro, G.F.; Morozzi, G. Cancer chemoprevention by hydroxytyrosol isolated from virgin olive oil through G1 cell cycle arrest and apoptosis. Eur. J. Cancer Prev. 2002, 11, 351–358. [Google Scholar]
- Fini, L.; Hotchkiss, E.; Fogliano, V.; Graziani, G.; Romano, M.; De Vol, E.B.; Qin, H.; Selgrad, M.; Boland, C.R.; Ricciardiello, L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis 2008, 29, 139–146. [Google Scholar]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessi, M.A.; Spencer, J.P. Hydroxytyrosol inhibits the proliferation of human colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Mol. Nutr. Food Res. 2009, 53, 897–903. [Google Scholar]
- Wang, W.; Heideman, L.; Chung, C.S.; Pelling, J.C.; Koehler, K.J.; Birt, D.F. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol. Carcinog. 2000, 28, 102–110. [Google Scholar]
- Piao, M.; Mori, D.; Satoh, T.; Sugita, Y.; Tokunaga, O. Inhibition of endothelial cell proliferation, in vitro angiogenesis, and the down-regulation of cell adhesion-related genes by genistein. Combined with a cDNA microarray analysis. Endothelium 2006, 13, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar]
- D’Archivio, M.; Santangelo, C.; Scazzocchio, B.; Vari, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory effects of polyphenols on apoptosis induction: Relevance for cancer prevention. Int. J. Mol. Sci. 2008, 9, 213–228. [Google Scholar]
- Guo, W.; Kong, E.; Meydani, M. Dietary polyphenols, inflammation, and cancer. Nutr. Cancer 2009, 61, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Nifli, A.P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 79–113. [Google Scholar]
- Stoner, G.D.; Mukhtar, H. Polyphenols as cancer chemopreventive agents. J. Cell. Biochem. Suppl. 1995, 22, 169–180. [Google Scholar]
- Franceschi, S.; Parpinel, M.; La Vecchia, C.; Favero, A.; Talamini, R.; Negri, E. Role of different types of vegetables and fruit in the prevention of cancer of the colon, rectum, and breast. Epidemiology 1998, 9, 338–341. [Google Scholar] [PubMed]
- La Vecchia, C.; Chatenoud, L.; Franceschi, S.; Soler, M.; Parazzini, F.; Negri, E. Vegetables and fruit and human cancer: Update of an Italian study. Int. J. Cancer 1999, 82, 151–152. [Google Scholar]
- Benetou, V.; Orfanos, P.; Lagiou, P.; Trichopoulos, D.; Boffetta, P.; Trichopoulou, A. Vegetables and fruits in relation to cancer risk: Evidence from the Greek EPIC cohort study. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 387–392. [Google Scholar]
- Feskanich, D.; Ziegler, R.G.; Michaud, D.S.; Giovannucci, E.L.; Speizer, F.E.; Willett, W.C.; Colditz, G.A. Prospective study of fruit and vegetable consumption and risk of lung cancer among men and women. J. Natl. Cancer Inst. 2000, 92, 1812–1823. [Google Scholar]
- Zhang, S.M.; Hunter, D.J.; Rosner, B.A.; Giovannucci, E.L.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Intakes of fruits, vegetables, and related nutrients and the risk of non-Hodgkin’s lymphoma among women. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 477–485. [Google Scholar] [PubMed]
- Gonzalez, C.A.; Pera, G.; Agudo, A.; Bueno-de-Mesquita, H.B.; Ceroti, M.; Boeing, H.; Schulz, M.; Del Giudice, G.; Plebani, M.; Carneiro, F.; et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Int. J. Cancer 2006, 118, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Favero, A.; Parpinel, M.; Franceschi, S. Diet and risk of breast cancer: Major findings from an Italian case-control study. Biomed. Pharmacother. 1998, 52, 109–115. [Google Scholar]
- Larsson, S.C.; Andersson, S.O.; Johansson, J.E.; Wolk, A. Fruit and vegetable consumption and risk of bladder cancer: A prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 2519–2522. [Google Scholar]
- Larsson, S.C.; Hakansson, N.; Naslund, I.; Bergkvist, L.; Wolk, A. Fruit and vegetable consumption in relation to pancreatic cancer risk: A prospective study. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 301–305. [Google Scholar]
- Botterweck, A.A.; van den Brandt, P.A.; Goldbohm, R.A. A prospective cohort study on vegetable and fruit consumption and stomach cancer risk in The Netherlands. Am. J. Epidemiol. 1998, 148, 842–853. [Google Scholar]
- Boffetta, P.; Couto, E.; Wichmann, J.; Ferrari, P.; Trichopoulos, D.; Bueno-de-Mesquita, H.B.; van Duijnhoven, F.J.; Buchner, F.L.; Key, T.; Boeing, H.; et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2010, 102, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.E. Primary prevention of colorectal cancer: Lifestyle, nutrition, exercis. Recent Results Cancer Res. 2005, 166, 177–211. [Google Scholar]
- Li, Q.; Zhao, H.F.; Zhang, Z.F.; Liu, Z.G.; Pei, X.R.; Wang, J.B.; Cai, M.Y.; Li, Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience 2009, 159, 1208–1215. [Google Scholar]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Duthie, S.J.; Dobson, V.L. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur. J. Nutr. 1999, 38, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Calomme, M.; Pieters, L.; Vlietinck, A.; Vanden Berghe, D. Inhibition of bacterial mutagenesis by Citrus flavonoids. Planta Med. 1996, 62, 222–226. [Google Scholar]
- Plaumann, B.; Fritsche, M.; Rimpler, H.; Brandner, G.; Hess, R.D. Flavonoids activate wild-type p53. Oncogene 1996, 13, 1605–1614. [Google Scholar]
- van Erk, M.J.; Roepman, P.; van der Lende, T.R.; Stierum, R.H.; Aarts, J.M.; van Bladeren, P.J.; van Ommen, B. Integrated assessment by multiple gene expression analysis of quercetin bioactivity on anticancer-related mechanisms in colon cancer cells in vitro. Eur. J. Nutr. 2005, 44, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.A.; Wang, Z.Y.; Athar, M.; Bickers, D.R.; Mukhtar, H. Inhibition of the skin tumorigenicity of (±)-7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene by tannic acid, green tea polyphenols and quercetin in Sencar mice. Cancer Lett. 1988, 42, 7–12. [Google Scholar]
- Takada, M.; Nakamura, Y.; Koizumi, T.; Toyama, H.; Kamigaki, T.; Suzuki, Y.; Takeyama, Y.; Kuroda, Y. Suppression of human pancreatic carcinoma cell growth and invasion by epigallocatechin-3-gallate. Pancreas 2002, 25, 45–48. [Google Scholar]
- Inoue, M.; Tajima, K.; Mizutani, M.; Iwata, H.; Iwase, T.; Miura, S.; Hirose, K.; Hamajima, N.; Tominaga, S. Regular consumption of green tea and the risk of breast cancer recurrence: Follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett. 2001, 167, 175–182. [Google Scholar]
- Takada, M.; Ku, Y.; Habara, K.; Ajiki, T.; Suzuki, Y.; Kuroda, Y. Inhibitory effect of epigallocatechin-3-gallate on growth and invasion in human biliary tract carcinoma cells. World J. Surg. 2002, 26, 683–686. [Google Scholar]
- Rieger-Christ, K.M.; Hanley, R.; Lodowsky, C.; Bernier, T.; Vemulapalli, P.; Roth, M.; Kim, J.; Yee, A.S.; Le, S.M.; Marie, P.J.; Libertino, J.A.; Summerhayes, I.C. The green tea compound, (−)-epigallocatechin-3-gallate downregulates N-cadherin and suppresses migration of bladder carcinoma cells. J. Cell. Biochem. 2007, 102, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.; Mathur, P.S.; Greene, G.L. Inhibition of mammary tumorigenesis in the C3(1)/SV40 mouse model by green tea. Breast Cancer Res. Treat. 2008, 107, 359–369. [Google Scholar]
- Larsen, C.A.; Dashwood, R.H. Suppression of Met activation in human colon cancer cells treated with (−)-epigallocatechin-3-gallate: Minor role of hydrogen peroxide. Biochem. Biophys. Res. Commun. 2009, 389, 527–530. [Google Scholar]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar]
- Llor, X.; Pons, E.; Roca, A.; Alvarez, M.; Mane, J.; Fernandez-Banares, F.; Gassull, M.A. The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin. Nutr. 2003, 22, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, R.; Fernandez-Banares, F.; Navarro, E.; Castella, E.; Mane, J.; Alvarez, M.; Pastor, C.; Cabre, E.; Gassull, M.A. Effect of olive oil on early and late events of colon carcinogenesis in rats: Modulation of arachidonic acid metabolism and local prostaglandin E(2) synthesis. Gut 2000, 46, 191–199. [Google Scholar]
- Solanas, M.; Hurtado, A.; Costa, I.; Moral, R.; Menendez, J.A.; Colomer, R.; Escrich, E. Effects of a high olive oil diet on the clinical behavior and histopathological features of rat DMBA-induced mammary tumors compared with a high corn oil diet. Int. J. Oncol. 2002, 21, 745–753. [Google Scholar]
- Gill, C.I.; Boyd, A.; McDermott, E.; McCann, M.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; McGlynn, H.; Rowland, I. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer 2005, 117, 1–7. [Google Scholar] [PubMed]
- Hashim, Y.Z.; Rowland, I.R.; McGlynn, H.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; Kaisalo, L.; Wahala, K.; Gill, C.I. Inhibitory effects of olive oil phenolics on invasion in human colon adenocarcinoma cells in vitro. Int. J. Cancer 2008, 122, 495–500. [Google Scholar] [PubMed]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessi, M.A.; Spencer, J.P. Inhibition of p38/CREB phosphorylation and COX-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem. Biophys. Res. Commun. 2007, 362, 606–611. [Google Scholar]
- Adams, L.S.; Chen, S. Phytochemicals for breast cancer prevention by targeting aromatase. Front. Biosci. 2009, 14, 3846–3863. [Google Scholar]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar]
- Hopfner, M.; Schuppan, D.; Scherubl, H. Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J. Gastroenterol. 2008, 14, 1–14. [Google Scholar]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar]
- Wang, W.; Wang, X.; Peng, L.; Deng, Q.; Liang, Y.; Qing, H.; Jiang, B. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci. 2010, 101, 112–119. [Google Scholar]
- Corona, G.; Spencer, J.P.; Dessi, M.A. Extra virgin olive oil phenolics: Absorption, metabolism, and biological activities in the GI tract. Toxicol. Ind. Health 2009, 25, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Sebolt-Leopold, J.S.; Herrera, R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer 2004, 4, 937–947. [Google Scholar]
- Tsatsanis, C.; Androulidaki, A.; Venihaki, M.; Margioris, A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006, 38, 1654–1661. [Google Scholar]
- Guichard, C.; Pedruzzi, E.; Fay, M.; Marie, J.C.; Braut-Boucher, F.; Daniel, F.; Grodet, A.; Gougerot-Pocidalo, M.A.; Chastre, E.; Kotelevets, L.; Lizard, G.; Vandewalle, A.; Driss, F.; Ogier-Denis, E. Dihydroxyphenylethanol induces apoptosis by activating serine/threonine protein phosphatase PP2A and promotes the endoplasmic reticulum stress response in human colon carcinoma cells. Carcinogenesis 2006, 27, 1812–1827. [Google Scholar]
- Lee, S.Y.; Munerol, B.; Pollard, S.; Youdim, K.A.; Pannala, A.S.; Kuhnle, G.G.; Debnam, E.S.; Rice-Evans, C.; Spencer, J.P. The reaction of flavanols with nitrous acid protects against N-nitrosamine formation and leads to the formation of nitroso derivatives which inhibit cancer cell growth. Free Radic. Biol. Med. 2006, 40, 323–334. [Google Scholar]
- Adhami, V.M.; Malik, A.; Zaman, N.; Sarfaraz, S.; Siddiqui, I.A.; Syed, D.N.; Afaq, F.; Pasha, F.S.; Saleem, M.; Mukhtar, H. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin. Cancer Res. 2007, 13, 1611–1619. [Google Scholar] [PubMed]
- Banerjee, S.; Manna, S.; Mukherjee, S.; Pal, D.; Panda, C.K.; Das, S. Black tea polyphenols restrict benzopyrene-induced mouse lung cancer progression through inhibition of Cox-2 and induction of caspase-3 expression. Asian Pac. J. Cancer Prev. 2006, 7, 661–666. [Google Scholar]
- Kumar, N.; Shibata, D.; Helm, J.; Coppola, D.; Malafa, M. Green tea polyphenols in the prevention of colon cancer. Front. Biosci. 2007, 12, 2309–2315. [Google Scholar]
- Chell, S.; Kadi, A.; Williams, A.C.; Paraskeva, C. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim. Biophys. Acta 2006, 1766, 104–119. [Google Scholar]
- van Heist, J.; Niessen, H.; Hoekman, K.; Schalkwij, C. Advanced glycation end products in human cancer tissues: Detection of Nepsilon-(carboxymethyl)lysine and argpryrimidine. Ann. N. Y. Acad. Sci. 2005, 1043, 725–733. [Google Scholar]
- Bengmark, S. Advanced Glycation and Lipoxidation End Products–Amplifiers of Inflammation: The Role of Food. JPEN J. Parenter. Enteral Nutr. 2007, 31, 430–440. [Google Scholar]
- Kiho, T.; Usui, S.; Hirano, K.; Aizawa, K.; Inakuma, T. Tomato paste fraction inhibiting the formation of advance glycation end-products. Biosci. Biotechnol. Biochem. 2004, 1, 200–205. [Google Scholar]
- Lo, C.-Y.; Li, S.; Tan, D.; Pan, M.-H.; Sang, S.; HO, C.-T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006, 50, 1118–1128. [Google Scholar]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.-Y.; Yang, C.; HO, C.-T. Tea polyphenol (−)-Epigallocatechin-3-Gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar]
- Kuniyasu, H.; Oue, N.; Wakikawa, A.; Shigeishi, N.; Matsutani, N.; Kuraoka, K.; Ito, R.; Yokozaki, H.; Yasui, W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J. Pathol. 2001, 196, 163–170. [Google Scholar]
- Sparvero, L.; Asafu-Adjei, D.; Kang, R.; Tang, D.; Amin, N.; Im, J.; Rutledge, R.; Lin, B.; Amoscato, A.; Zeh, H.; Lotze, M. RAGE (Receptor for Advanced Glycation Endproducts), RAGE Ligands, and their role in Cancer and Inflammation. J. Transl. Med. 2009, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Ku, Y.; Toyama, H.; Suzuki, Y.; Kuroda, Y. Suppressive effects of tea polyphenol and conformational changes with receptor for advanced glycation en products (RAGE) expression in human hepatoma cells. Hepatogastroenterology 2002, 49, 928–931. [Google Scholar]
- WHO: Cardiovascular diseases (CVDs). Fact sheet No317. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed on 10 September 2009).
- Ambrose, J.A.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar]
- Jia, C.P.; Chen, M.J.; Huang, S.Z.; Zeng, Y.T. A study of inductive effect of hemin on expression of the beta-globin genes in K562 cells. Yi Chuan 2002, 24, 399–402. [Google Scholar]
- Tanasescu, M.; Leitzmann, M.F.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Exercise type and intensity in relation to coronary heart disease in men. JAMA 2002, 288, 1994–2000. [Google Scholar]
- Twisk, J.; Gillian-Daniel, D.L.; Tebon, A.; Wang, L.; Barrett, P.H.; Attie, A.D. The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Invest. 2000, 105, 521–532. [Google Scholar]
- Arts, I.C.; Jacobs, D.R., Jr.; Harnack, L.J.; Gross, M.; Folsom, A.R. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology 2001, 12, 668–675. [Google Scholar]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D. Antioxidant flavonols and coronary heart disease risk. Lancet 1997, 349, 699. [Google Scholar]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [PubMed]
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in Finland: A cohort study. BMJ 1996, 312, 478–481. [Google Scholar]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar]
- Nakachi, K.; Matsuyama, S.; Miyake, S.; Suganuma, M.; Imai, K. Preventive effects of drinking green tea on cancer and cardiovascular disease: Epidemiological evidence for multiple targeting prevention. Biofactors 2000, 13, 49–54. [Google Scholar]
- Rein, D.; Paglieroni, T.G.; Pearson, D.A.; Wun, T.; Schmitz, H.H.; Gosselin, R.; Keen, C.L. Cocoa and wine polyphenols modulate platelet activation and function. J. Nutr. 2000, 130, 2120S–2126S. [Google Scholar]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Yochum, L.; Kushi, L.H.; Meyer, K.; Folsom, A.R. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am. J. Epidemiol. 1999, 149, 943–949. [Google Scholar]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar]
- Peters, U.; Poole, C.; Arab, L. Does tea affect cardiovascular disease? A meta-analysis. Am. J. Epidemiol. 2001, 154, 495–503. [Google Scholar]
- Di Castelnuovo, A.; Rotondo, S.; Iacoviello, L.; Donati, M.B.; De Gaetano, G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation 2002, 105, 2836–2844. [Google Scholar]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [PubMed]
- Lin, J.; Rexrode, K.M.; Hu, F.; Albert, C.M.; Chae, C.U.; Rimm, E.B.; Stampfer, M.J.; Manson, J.E. Dietary intakes of flavonols and flavones and coronary heart disease in US women. Am. J. Epidemiol. 2007, 165, 1305–1313. [Google Scholar]
- Rimm, E.B.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann. Intern. Med. 1996, 125, 384–389. [Google Scholar]
- Sesso, H.D.; Gaziano, J.M.; Liu, S.; Buring, J.E. Flavonoid intake and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2003, 77, 1400–1408. [Google Scholar]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81, 292S–297S. [Google Scholar]
- Rein, D.; Lotito, S.; Holt, R.R.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G. Epicatechin in human plasma: In vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr. 2000, 130, 2109–2114. [Google Scholar] [PubMed]
- Stein, J.H.; Keevil, J.G.; Wiebe, D.A.; Aeschlimann, S.; Folts, J.D. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 1999, 100, 1050–1055. [Google Scholar]
- Wan, Y.; Vinson, J.A.; Etherton, T.D.; Proch, J.; Lazarus, S.A.; Kris-Etherton, P.M. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am. J. Clin. Nutr. 2001, 74, 596–602. [Google Scholar]
- Desch, S.; Schmidt, J.; Kobler, D.; Sonnabend, M.; Eitel, I.; Sareban, M.; Rahimi, K.; Schuler, G.; Thiele, H. Effect of cocoa products on blood pressure: Systematic review and meta-analysis. Am. J. Hypertens. 2010, 23, 97–103. [Google Scholar]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [PubMed]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schomig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar]
- Taubert, D.; Roesen, R.; Schomig, E. Effect of cocoa and tea intake on blood pressure: A meta-analysis. Arch. Intern. Med. 2007, 167, 626–634. [Google Scholar]
- Park, Y.K.; Kim, J.S.; Kang, M.H. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: Double-blind, placebo controlled intervention trial. Biofactors 2004, 22, 145–147. [Google Scholar]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003, 290, 1030–1031. [Google Scholar]
- Heiss, C.; Finis, D.; Kleinbongard, P.; Hoffmann, A.; Rassaf, T.; Kelm, M.; Sies, H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J. Cardiovasc. Pharmacol. 2007, 49, 74–80. [Google Scholar]
- Heiss, C.; Kleinbongard, P.; Dejam, A.; Perre, S.; Schroeter, H.; Sies, H.; Kelm, M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2005, 46, 1276–1283. [Google Scholar]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.K.; Milbury, P.; Paul, S.M.; Blumberg, J.; Mietus-Snyder, M.L. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar]
- Wang-Polagruto, J.F.; Villablanca, A.C.; Polagruto, J.A.; Lee, L.; Holt, R.R.; Schrader, H.R.; Ensunsa, J.L.; Steinberg, F.M.; Schmitz, H.H.; Keen, C.L. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. 2), S177–S186; discussion S206-S209. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Mulder, T.P.; Draijer, R.; Desideri, G.; Molhuizen, H.O.; Ferri, C. Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J. Hypertens. 2009, 27, 774–781. [Google Scholar]
- Widlansky, M.E.; Hamburg, N.M.; Anter, E.; Holbrook, M.; Kahn, D.F.; Elliott, J.G.; Keaney, J.F., Jr.; Vita, J.A. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Nutr. 2007, 26, 95–102. [Google Scholar]
- Cuevas, A.M.; Guasch, V.; Castillo, O.; Irribarra, V.; Mizon, C.; San Martin, A.; Strobel, P.; Perez, D.; Germain, A.M.; Leighton, F. A high-fat diet induces and red wine counteracts endothelial dysfunction in human volunteers. Lipids 2000, 35, 143–148. [Google Scholar]
- Papamichael, C.; Karatzis, E.; Karatzi, K.; Aznaouridis, K.; Papaioannou, T.; Protogerou, A.; Stamatelopoulos, K.; Zampelas, A.; Lekakis, J.; Mavrikakis, M. Red wine’s antioxidants counteract acute endothelial dysfunction caused by cigarette smoking in healthy nonsmokers. Am. Heart J. 2004, 147, E5. [Google Scholar]
- Pearson, D.A.; Paglieroni, T.G.; Rein, D.; Wun, T.; Schramm, D.D.; Wang, J.F.; Holt, R.R.; Gosselin, R.; Schmitz, H.H.; Keen, C.L. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb. Res. 2002, 106, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.; Paglieroni, T.G.; Wun, T.; Pearson, D.A.; Schmitz, H.H.; Gosselin, R.; Keen, C.L. Cocoa inhibits platelet activation and function. Am. J. Clin. Nutr. 2000, 72, 30–35. [Google Scholar]
- Keevil, J.G.; Osman, H.E.; Reed, J.D.; Folts, J.D. Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. J. Nutr. 2000, 130, 53–56. [Google Scholar] [PubMed]
- Mathur, S.; Devaraj, S.; Grundy, S.M.; Jialal, I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J. Nutr. 2002, 132, 3663–3667. [Google Scholar]
- Mao, T.K.; van de Water, J.; Keen, C.L.; Schmitz, H.H.; Gershwin, M.E. Modulation of TNF-alpha secretion in peripheral blood mononuclear cells by cocoa flavanols and procyanidins. Dev. Immunol. 2002, 9, 135–141. [Google Scholar]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Kirkpatrick, N.J.; Polagruto, J.A.; Ensunsa, J.L.; Schmitz, H.H.; Keen, C.L. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003, 73, 857–869. [Google Scholar]
- Hollenberg, N.K.; Martinez, G.; McCullough, M.; Meinking, T.; Passan, D.; Preston, M.; Rivera, A.; Taplin, D.; Vicaria-Clement, M. Aging, acculturation, salt intake, and hypertension in the Kuna of Panama. Hypertension 1997, 29, 171–176. [Google Scholar] [PubMed]
- Stensvold, I.; Tverdal, A.; Solvoll, K.; Foss, O.P. Tea consumption. relationship to cholesterol, blood pressure, and coronary and total mortality. Prev. Med. 1992, 21, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Lu, F.H.; Wu, J.S.; Wu, C.H.; Chang, C.J. The protective effect of habitual tea consumption on hypertension. Arch. Intern. Med. 2004, 164, 1534–1540. [Google Scholar]
- Negishi, H.; Xu, J.W.; Ikeda, K.; Njelekela, M.; Nara, Y.; Yamori, Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J. Nutr. 2004, 134, 38–42. [Google Scholar]
- Uchida, S.; Ozaki, M.; Akashi, T.; Yamashita, K.; Niwa, M.; Taniyama, K. Effects of (−)-epigallocatechin-3-O-gallate (green tea tannin) on the life span of stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. Suppl. 1995, 22, S302–S303. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.J.; Keaney, J.F., Jr.; Holbrook, M.; Gokce, N.; Swerdloff, P.L.; Frei, B.; Vita, J.A. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 2001, 104, 151–156. [Google Scholar]
- Hodgson, J.M.; Puddey, I.B.; Burke, V.; Beilin, L.J.; Jordan, N. Effects on blood pressure of drinking green and black tea. J. Hypertens. 1999, 17, 457–463. [Google Scholar]
- Hodgson, J.M.; Puddey, I.B.; Burke, V.; Watts, G.F.; Beilin, L.J. Regular ingestion of black tea improves brachial artery vasodilator function. Clin. Sci. (Lond.) 2002, 102, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Vorster, H.; Jerling, J.C.; Magee, E.; Mulligan, A.; Runswick, S.A.; Cummings, J.H. Effect of black tea drinking on blood lipids, blood pressure and aspects of bowel habit. Br. J. Nutr. 1997, 78, 41–55. [Google Scholar]
- Knekt, P.; Isotupa, S.; Rissanen, H.; Heliovaara, M.; Jarvinen, R.; Hakkinen, S.; Aromaa, A.; Reunanen, A. Quercetin intake and the incidence of cerebrovascular disease. Eur. J. Clin. Nutr. 2000, 54, 415–417. [Google Scholar]
- Andrade, A.C.; Cesena, F.H.; Consolim-Colombo, F.M.; Coimbra, S.R.; Benjo, A.M.; Krieger, E.M.; Luz, P.L. Short-term red wine consumption promotes differential effects on plasma levels of high-density lipoprotein cholesterol, sympathetic activity, and endothelial function in hypercholesterolemic, hypertensive, and healthy subjects. Clinics (Sao Paulo) 2009, 64, 435–442. [Google Scholar] [PubMed]
- Spaak, J.; Merlocco, A.C.; Soleas, G.J.; Tomlinson, G.; Morris, B.L.; Picton, P.; Notarius, C.F.; Chan, C.T.; Floras, J.S. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diamete. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H605–H612. [Google Scholar]
- Hansen, A.S.; Marckmann, P.; Dragsted, L.O.; Finne Nielsen, I.L.; Nielsen, S.E.; Gronbaek, M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur. J. Clin. Nutr. 2005, 59, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Burke, V.; Puddey, I.B. Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J. Hypertens. 2005, 23, 47–54. [Google Scholar]
- Agewall, S.; Wright, S.; Doughty, R.N.; Whalley, G.A.; Duxbury, M.; Sharpe, N. Does a glass of red wine improve endothelial function? Eur. Heart J. 2000, 21, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kim, S.; Eto, M.; Iijima, K.; Ako, J.; Yoshizumi, M.; Akishita, M.; Kondo, K.; Itakura, H.; Hosoda, K.; Toba, K.; Ouchi, Y. Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery. Am. J. Cardiol. 2001, 88, 1457–1460. [Google Scholar]
- Karatzi, K.; Papamichael, C.; Aznaouridis, K.; Karatzis, E.; Lekakis, J.; Matsouka, C.; Boskou, G.; Chiou, A.; Sitara, M.; Feliou, G.; Kontoyiannis, D.; Zampelas, A.; Mavrikakis, M. Constituents of red wine other than alcohol improve endothelial function in patients with coronary artery disease. Coron. Artery Dis. 2004, 15, 485–490. [Google Scholar]
- Whelan, A.P.; Sutherland, W.H.; McCormick, M.P.; Yeoman, D.J.; de Jong, S.A.; Williams, M.J. Effects of white and red wine on endothelial function in subjects with coronary artery disease. Intern. Med. J. 2004, 34, 224–228. [Google Scholar]
- Appeldoorn, M.M.; Venema, D.P.; Peters, T.H.; Koenen, M.E.; Arts, I.C.; Vincken, J.P.; Gruppen, H.; Keijer, J.; Hollman, P.C. Some phenolic compounds increase the nitric oxide level in endothelial cells in vitro. J. Agric. Food Chem. 2009, 57, 7693–7699. [Google Scholar] [PubMed]
- Schmitt, C.A.; Dirsch, V.M. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide 2009, 21, 77–91. [Google Scholar]
- Fitzpatrick, D.F.; Hirschfield, S.L.; Ricci, T.; Jantzen, P.; Coffey, R.G. Endothelium-dependent vasorelaxation caused by various plant extracts. J. Cardiovasc. Pharmacol. 1995, 26, 90–95. [Google Scholar]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Forstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Leikert, J.F.; Rathel, T.R.; Wohlfart, P.; Cheynier, V.; Vollmar, A.M.; Dirsch, V.M. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation 2002, 106, 1614–1617. [Google Scholar]
- Karim, M.; McCormick, K.; Kappagoda, C.T. Effects of cocoa extracts on endothelium-dependent relaxation. J. Nutr. 2000, 130, 2105S–2108S. [Google Scholar]
- Chin-Dusting, J.P.; Fisher, L.J.; Lewis, T.V.; Piekarska, A.; Nestel, P.J.; Husband, A. The vascular activity of some isoflavone metabolites: Implications for a cardioprotective role. Br. J. Pharmacol. 2001, 133, 595–605. [Google Scholar]
- Fitzpatrick, D.F.; Bing, B.; Rohdewald, P. Endothelium-dependent vascular effects of Pycnogenol. J. Cardiovasc. Pharmacol. 1998, 32, 509–515. [Google Scholar]
- Fitzpatrick, D.F.; Hirschfield, S.L.; Coffey, R.G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 1993, 265, H774–H778. [Google Scholar]
- Karamsetty, M.R.; Klinger, J.R.; Hill, N.S. Phytoestrogens restore nitric oxide-mediated relaxation in isolated pulmonary arteries from chronically hypoxic rats. J. Pharmacol. Exp. Ther. 2001, 297, 968–974. [Google Scholar]
- Woodman, O.L.; Chan, E. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol. 2004, 31, 786–790. [Google Scholar]
- Lorenz, M.; Wessler, S.; Follmann, E.; Michaelis, W.; Dusterhoft, T.; Baumann, G.; Stangl, K.; Stangl, V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 2004, 279, 6190–6195. [Google Scholar] [PubMed]
- Stoclet, J.C.; Chataigneau, T.; Ndiaye, M.; Oak, M.H.; El Bedoui, J.; Chataigneau, M.; Schini-Kerth, V.B. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004, 500, 299–313. [Google Scholar]
- Corder, R.; Douthwaite, J.A.; Lees, D.M.; Khan, N.Q.; Viseu Dos Santos, A.C.; Wood, E.G.; Carrier, M.J. Endothelin-1 synthesis reduced by red wine. Nature 2001, 414, 863–864. [Google Scholar]
- Khan, N.Q.; Lees, D.M.; Douthwaite, J.A.; Carrier, M.J.; Corder, R. Comparison of red wine extract and polyphenol constituents on endothelin-1 synthesis by cultured endothelial cells. Clin. Sci. (Lond.) 2002, 103 (Suppl. 48), 72–75. [Google Scholar] [PubMed]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Steffen, Y.; Schewe, T.; Sies, H. Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem. Biophys. Res. Commun. 2007, 359, 828–833. [Google Scholar]
- Pignatelli, P.; Pulcinelli, F.M.; Celestini, A.; Lenti, L.; Ghiselli, A.; Gazzaniga, P.P.; Violi, F. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am. J. Clin. Nutr. 2000, 72, 1150–1155. [Google Scholar]
- Freedman, J.E.; Parker C., III; Li, L.; Perlman, J.A.; Frei, B.; Ivanov, V.; Deak, L.R.; Iafrati, M.D.; Folts, J.D. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 2001, 103, 2792–2798. [Google Scholar] [PubMed]
- Natella, F.; Nardini, M.; Belelli, F.; Pignatelli, P.; Di Santo, S.; Ghiselli, A.; Violi, F.; Scaccini, C. Effect of coffee drinking on platelets: Inhibition of aggregation and phenols incorporation. Br. J. Nutr. 2008, 100, 1276–1282. [Google Scholar]
- Steptoe, A.; Gibson, E.L.; Vuononvirta, R.; Hamer, M.; Wardle, J.; Rycroft, J.A.; Martin, J.F.; Erusalimsky, J.D. The effects of chronic tea intake on platelet activation and inflammation: A double-blind placebo controlled trial. Atherosclerosis 2007, 193, 277–282. [Google Scholar]
- Gresele, P.; Pignatelli, P.; Guglielmini, G.; Carnevale, R.; Mezzasoma, A.M.; Ghiselli, A.; Momi, S.; Violi, F. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J. Nutr. 2008, 138, 1602–1608. [Google Scholar] [PubMed]
- Holt, R.R.; Actis-Goretta, L.; Momma, T.Y.; Keen, C.L. Dietary flavanols and platelet reactivity. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. 2), S187–S196; discussion S206-S209. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Chronopoulos, A.K.; Singh, I.; Francis, M.A.; Moriarty, H.; Pike, M.J.; Turner, A.H.; Mann, N.J.; Sinclair, A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003, 77, 1466–1473. [Google Scholar]
- Peppa, M.; Raptis, S. Advanced glycation end products and cardiovascular disease. Curr. Diabetes Rev. 2008, 4, 92–100. [Google Scholar]
- Schramm, D.; German, J. Potential effects of flavonoids on the etiology of vascular disease. J. Nutr. Biochem. 1998, 9, 560–566. [Google Scholar]
- Huang, S.-M.; Wu, C.-H.; Yen, G.-C. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol. Nutr. Food Res. 2006, 50, 1129–1139. [Google Scholar]
- Kim, J.; Lee, E.; Kim, D.; Yu, B.; Chung, H. Kaempferol modulates pro-inflammatory NF-kB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age (Dordr.) 2010, 32, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Hy, L.X.; Keller, D.M. Prevalence of AD among whites: A summary by levels of severity. Neurology 2000, 55, 198–204. [Google Scholar]
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar]
- de Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar]
- Tanner, C.M.; Goldman, S.M. Epidemiology of Parkinson’s disease. Neurol. Clin. 1996, 14, 317–335. [Google Scholar]
- Barzilai, A.; Melamed, E. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson’s disease. Trends Mol. Med. 2003, 9, 126–132. [Google Scholar]
- Jellinger, K.A. Cell death mechanisms in neurodegeneration. J. Cell. Mol. Med. 2001, 5, 1–17. [Google Scholar]
- Spires, T.L.; Hannan, A.J. Nature, nurture and neurology: Gene-environment interactions in neurodegenerative disease. FEBS Anniversary Prize Lecture delivered on 27 June 2004 at the 29th FEBS Congress in Warsaw. FEBS J. 2005, 272, 2347–2361. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hebert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar]
- Orgogozo, J.M.; Dartigues, J.F.; Lafont, S.; Letenneur, L.; Commenges, D.; Salamon, R.; Renaud, S.; Breteler, M.B. Wine consumption and dementia in the elderly: A prospective community study in the Bordeaux area. Rev. Neurol. (Paris) 1997, 153, 185–192. [Google Scholar] [PubMed]
- Truelsen, T.; Thudium, D.; Gronbaek, M. Amount and type of alcohol and risk of dementia: The Copenhagen City Heart Study. Neurology 2002, 59, 1313–1319. [Google Scholar]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Letenneur, L.; Proust-Lima, C.; Le, G.A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006, 67, 1370–1376. [Google Scholar]
- Dai, Q.; Borenstein, A.R.; Wu, Y.; Jackson, J.C.; Larson, E.B. Fruit and vegetable juices and Alzheimer’s disease: The Kame Project. Am. J. Med. 2006, 119, 751–759. [Google Scholar]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243–255. [Google Scholar] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005, 53, 7029–7034. [Google Scholar]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [PubMed]
- Milbury, P.E.; Kalt, W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J. Agric. Food Chem. 2010, 58, 3950–3956. [Google Scholar]
- Youdim, K.A.; Joseph, J.A. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: A multiplicity of effects. Free Radic. Biol. Med. 2001, 30, 583–594. [Google Scholar]
- Inanami, O.; Watanabe, Y.; Syuto, B.; Nakano, M.; Tsuji, M.; Kuwabara, M. Oral administration of (-)catechin protects against ischemia-reperfusion-induced neuronal death in the gerbil. Free Radic. Res. 1998, 29, 359–365. [Google Scholar]
- Luo, Y.; Smith, J.V.; Paramasivam, V.; Burdick, A.; Curry, K.J.; Buford, J.P.; Khan, I.; Netzer, W.J.; Xu, H.; Butko, P. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA 2002, 99, 12197–12202. [Google Scholar]
- Bastianetto, S.; Zheng, W.H.; Quirion, R. The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: Involvement of its flavonoid constituents and protein kinase C. J. Neurochem. 2000, 74, 2268–2277. [Google Scholar] [PubMed]
- Tchantchou, F.; Xu, Y.; Wu, Y.; Christen, Y.; Luo, Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007, 21, 2400–2408. [Google Scholar]
- Hsieh, H.-M.; Wua, W.-M.; Hu, M.-L. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with D-galactose. Food Chem. Toxicol. 2009, 47, 625–632. [Google Scholar]
- Shan, Q.; Lu, J.; Zheng, Y.; Li, J.; Zhou, Z.; Hu, B.; Zhang, Z.; Fan, S.; Mao, Z.; Wang, Y.-J.; Ma, D. Purple Sweet Potato Color Ameliorates Cognition Deficits and Attenuates Oxidative Damage and Inflammation in AgingMouse Brain Induced by D-Galactose. J. Biomed. Biotechnol. 2009, 2009, 564737. [Google Scholar]
- Munch, G.; Thome, J.; Foley, P.; Schinzel, R.; Riederer, P. Advanced glycation endproducts in ageing and Alzheimer’s disease. Brain Res. Brain Res. Rev. 1997, 23, 134–143. [Google Scholar]
- Ramasamy, R.; Vannucci, S.; Yan, S.; Herold, K.; Yan, S.; Schmidt, A. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 2001, 12, 3871–3875. [Google Scholar]
- Vauzour, D.; Corona, G.; Spencer, J.P. Caffeic acid, tyrosol and p-coumaric acid are potent inhibitors of 5-S-cysteinyl-dopamine induced neurotoxicity. Arch. Biochem. Biophys. 2010, 501, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Vafeiadou, K.; Corona, G.; Pollard, S.E.; Tzounis, X.; Spencer, J.P. Champagne wine polyphenols protect primary cortical neurons against peroxynitrite-induced injury. J. Agric. Food Chem. 2007, 55, 2854–2860. [Google Scholar]
- Spencer, J.P. Food for thought: The role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc. Nutr. Soc. 2008, 67, 238–252. [Google Scholar]
- Spencer, J.P. Flavonoids: Modulators of brain function? Br. J. Nutr. 2008, 99 (E-Suppl. 1), ES60–ES77. [Google Scholar] [PubMed]
- Rendeiro, C.; Spencer, J.P.; Vauzour, D.; Butler, L.T.; Ellis, J.A.; Williams, C.M. The impact of flavonoids on spatial memory in rodents: From behaviour to underlying hippocampal mechanisms. Genes Nutr. 2009, 4, 251–270. [Google Scholar]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar]
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar]
- Macready, A.L.; Kennedy, O.B.; Ellis, J.A.; Williams, C.M.; Spencer, J.P.; Butler, L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227–242. [Google Scholar]
- How, P.S.; Cox, R.; Ellis, J.A.; Spencer, J.P.E. The impact of plant-derived flavonoids on mood, memory and motor skills in UK adults. Proc. Nutr. Soc. 2007, 66, 87A. [Google Scholar]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Prior, R.L.; Cao, G.; Martin, A.; Taglialatela, G.; Bickford, P.C. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J. Neurosci. 1998, 18, 8047–8055. [Google Scholar] [PubMed]
- Joseph, J.A.; Shukitt-Hale, B.; Denisova, N.A.; Bielinski, D.; Martin, A.; McEwen, J.J.; Bickford, P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999, 19, 8114–8121. [Google Scholar] [PubMed]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.G.; Smith, M.A.; Joseph, J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar]
- Williams, C.M.; El Mohsen, M.A.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar]
- Ramirez, M.R.; Izquierdo, I.; do Carmo Bassols Raseira, M.; Zuanazzi, J.A.; Barros, D.; Henriques, A.T. Effect of lyophilised Vaccinium berries on memory, anxiety and locomotion in adult rats. Pharmacol. Res. 2005, 52, 457–462. [Google Scholar]
- Barros, D.; Amaral, O.B.; Izquierdo, I.; Geracitano, L.; do Carmo Bassols Raseira, M.; Henriques, A.T.; Ramirez, M.R. Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol. Biochem. Behav. 2006, 84, 229–234. [Google Scholar]
- Goyarzu, P.; Malin, D.H.; Lau, F.C.; Taglialatela, G.; Moon, W.D.; Jennings, R.; Moy, E.; Moy, D.; Lippold, S.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplemented diet: Effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr. Neurosci. 2004, 7, 75–83. [Google Scholar]
- Shukitt-Hale, B.; Carey, A.; Simon, L.; Mark, D.A.; Joseph, J.A. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition 2006, 22, 295–302. [Google Scholar]
- Chan, Y.C.; Hosoda, K.; Tsai, C.J.; Yamamoto, S.; Wang, M.F. Favorable effects of tea on reducing the cognitive deficits and brain morphological changes in senescence-accelerated mice. J. Nutr. Sci. Vitaminol. 2006, 52, 266–273. [Google Scholar]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R.N.; Finn, M.B.; Holtzman, D.M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2006, 24, 506–515. [Google Scholar]
- Bhat, N.R.; Zhang, P.; Lee, J.C.; Hogan, E.L. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J. Neurosci. 1998, 18, 1633–1641. [Google Scholar]
- Clostre, F. Gingko Biloba extract (EGb 761). State of knowledge in the dawn of the year 2000. Ann. Pharm. Fr. 1999, 57, 1S8–1S88. [Google Scholar] [PubMed]
- Cohen-Salmon, C.; Venault, P.; Martin, B.; Raffalli-Sebille, M.J.; Barkats, M.; Clostre, F.; Pardon, M.C.; Christen, Y.; Chapouthier, G. Effects of Ginkgo biloba extract (EGb 761) on learning and possible actions on aging. J. Physiol. Paris 1997, 91, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Diamond, B.J.; Shiflett, S.C.; Feiwel, N.; Matheis, R.J.; Noskin, O.; Richards, J.A.; Schoenberger, N.E. Ginkgo biloba extract: Mechanisms and clinical indications. Arch. Phys. Med. Rehabil. 2000, 81, 668–678. [Google Scholar] [PubMed]
- Itil, T.M.; Eralp, E.; Ahmed, I.; Kunitz, A.; Itil, K.Z. The pharmacological effects of Gingko Biloba, a plant extract, on the brain of dementia patients in comparinson with tacrine. Psychopharmacology 1998, 34, 391–396. [Google Scholar]
- Shif, O.; Gillette, K.; Damkaoutis, C.M.; Carrano, C.; Robbins, S.J.; Hoffman, J.R. Effects of Ginkgo biloba administered after spatial learning on water maze and radial arm maze performance in young adult rats. Pharmacol. Biochem. Behav. 2006, 84, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar]
- Vauzour, D.; Vafeiadou, K.; Rice-Evans, C.; Williams, R.J.; Spencer, J.P. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J. Neurochem. 2007, 103, 1355–1367. [Google Scholar]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.C. The effects of an extract of Ginkgo biloba, EGb 761, on cognitive behavior and longevity in the rat. Physiol. Behav. 1998, 63, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Mishima, K.; Irie, K.; Motohashi, K.; Tanaka, Y.; Orito, K.; Egawa, T.; Kitamura, Y.; Egashira, N.; Iwasaki, K.; Fujiwara, M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J. Pharmacol. Sci. 2007, 104, 329–334. [Google Scholar]
- Maher, P.; Akaishi, T.; Abe, K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. USA 2006, 103, 16568–16573. [Google Scholar]
- Stoll, S.; Scheuer, K.; Pohl, O.; Muller, W.E. Ginkgo biloba extract (EGb 761) independently improves changes in passive avoidance learning and brain membrane fluidity in the aging mouse. Pharmacopsychiatry 1996, 29, 144–149. [Google Scholar] [PubMed]
- Topic, B.; Hasenohrl, R.U.; Hacker, R.; Huston, J.P. Enhanced conditioned inhibitory avoidance by a combined extract of Zingiber officinale and Ginkgo biloba. Phytother. Res. 2002, 16, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, K.; Vauzour, D.; Lee, H.Y.; Rodriguez-Mateos, A.; Williams, R.J.; Spencer, J.P. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch. Biochem. Biophys. 2009, 484, 100–109. [Google Scholar]
- Wang, X.; Chen, S.; Ma, G.; Ye, M.; Lu, G. Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport 2005, 16, 267–270. [Google Scholar]
- Bhat, N.R.; Feinstein, D.L.; Shen, Q.; Bhat, A.N. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J. Biol. Chem. 2002, 277, 29584–29592. [Google Scholar] [PubMed]
- Lee, S.-J.; Lee, K.-W. Protective Effect of (−)-Epigallocatechin Gallate against Advanced Glycation Endproducts-Induced Injury in Neuronal Cells. Biol. Pharm. Bull. 2007, 30, 1369–1373. [Google Scholar]
- Vlahos, C.J.; Matter, W.F.; Hui, K.Y.; Brown, R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 1994, 269, 5241–5248. [Google Scholar]
- Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J. Biol. Chem. 2003, 278, 34783–34793. [Google Scholar] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106-1131. https://doi.org/10.3390/nu2111106
Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JPE. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients. 2010; 2(11):1106-1131. https://doi.org/10.3390/nu2111106
Chicago/Turabian StyleVauzour, David, Ana Rodriguez-Mateos, Giulia Corona, Maria Jose Oruna-Concha, and Jeremy P. E. Spencer. 2010. "Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action" Nutrients 2, no. 11: 1106-1131. https://doi.org/10.3390/nu2111106
APA StyleVauzour, D., Rodriguez-Mateos, A., Corona, G., Oruna-Concha, M. J., & Spencer, J. P. E. (2010). Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients, 2(11), 1106-1131. https://doi.org/10.3390/nu2111106




