Yogurt Alleviates Cyclophosphamide-Induced Immunosuppression in Mice through D-Lactate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Yogurt Preparation
2.3. Determination of D-Lactate in Yogurt by High-Performance Liquid Chromatography (HPLC)
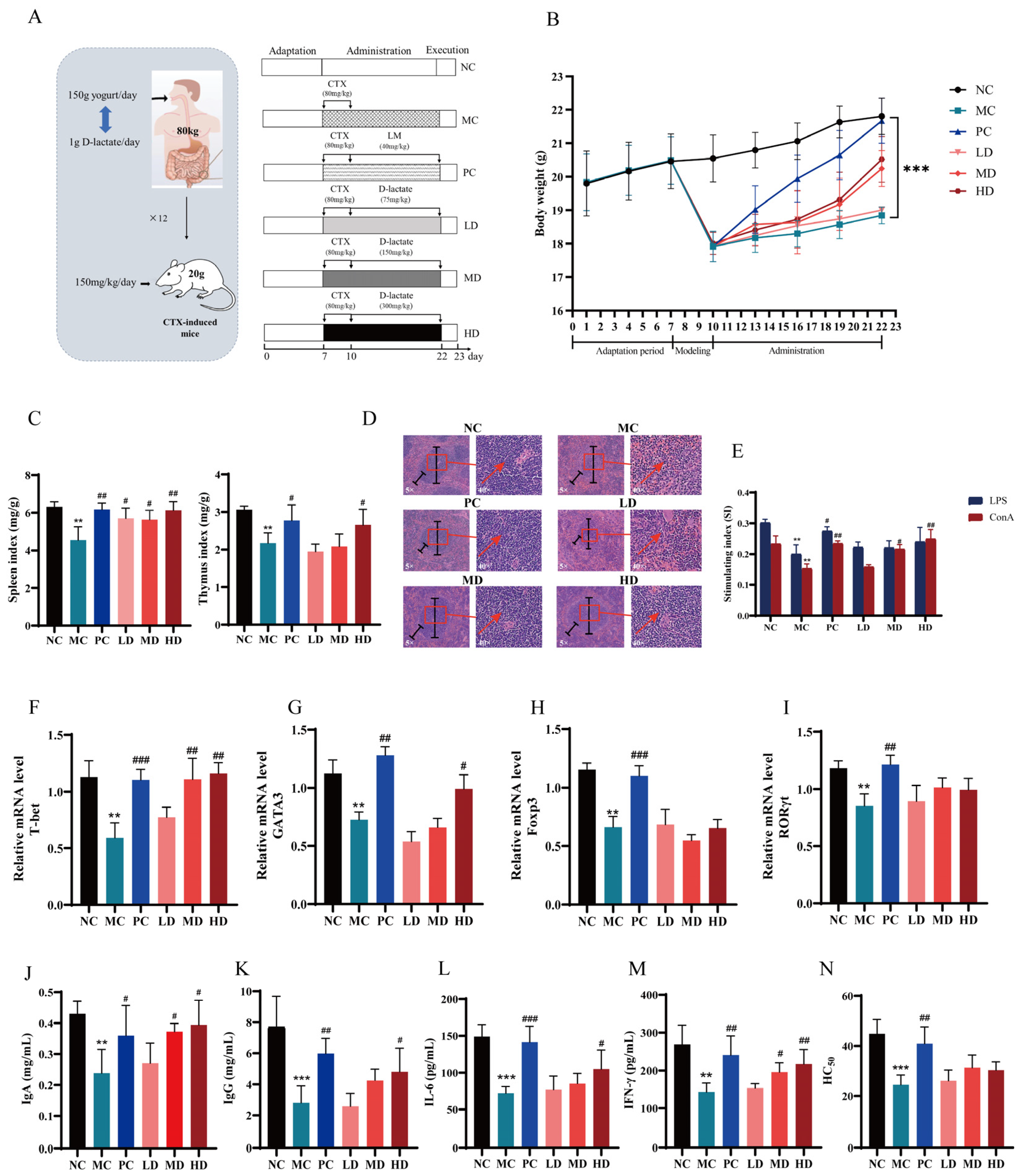
2.4. Animal Experiment
2.4.1. Animal Experiment I
2.4.2. Animal Experiment II
2.4.3. Animal Experiment III
2.5. Methods for Physiological and Pathological Analysis
2.5.1. ELISA Assay
2.5.2. Histopathological Staining
2.5.3. RT-qPCR Analysis
2.5.4. Proliferation Assay of Splenic Lymphocytes
2.5.5. Delayed-Type Hypersensitivity (DTH)
2.5.6. Serum Hemolysin
2.5.7. Gut Microbiota Analysis
2.6. Statistical Analyses
3. Results
3.1. Detection of D-Lactate in Yogurt
3.2. Animal Experiment I: Toxicity Evaluation of Yogurt and D-Lactate in Healthy Mice
3.3. Animal Experiment II: Evaluation of Immune Efficacy of Yogurt and D-Lactate in Immunosuppressive Mice Induced by CTX
3.3.1. Yogurt Supplementation Ameliorated CTX-Induced Immunosuppression in Mice
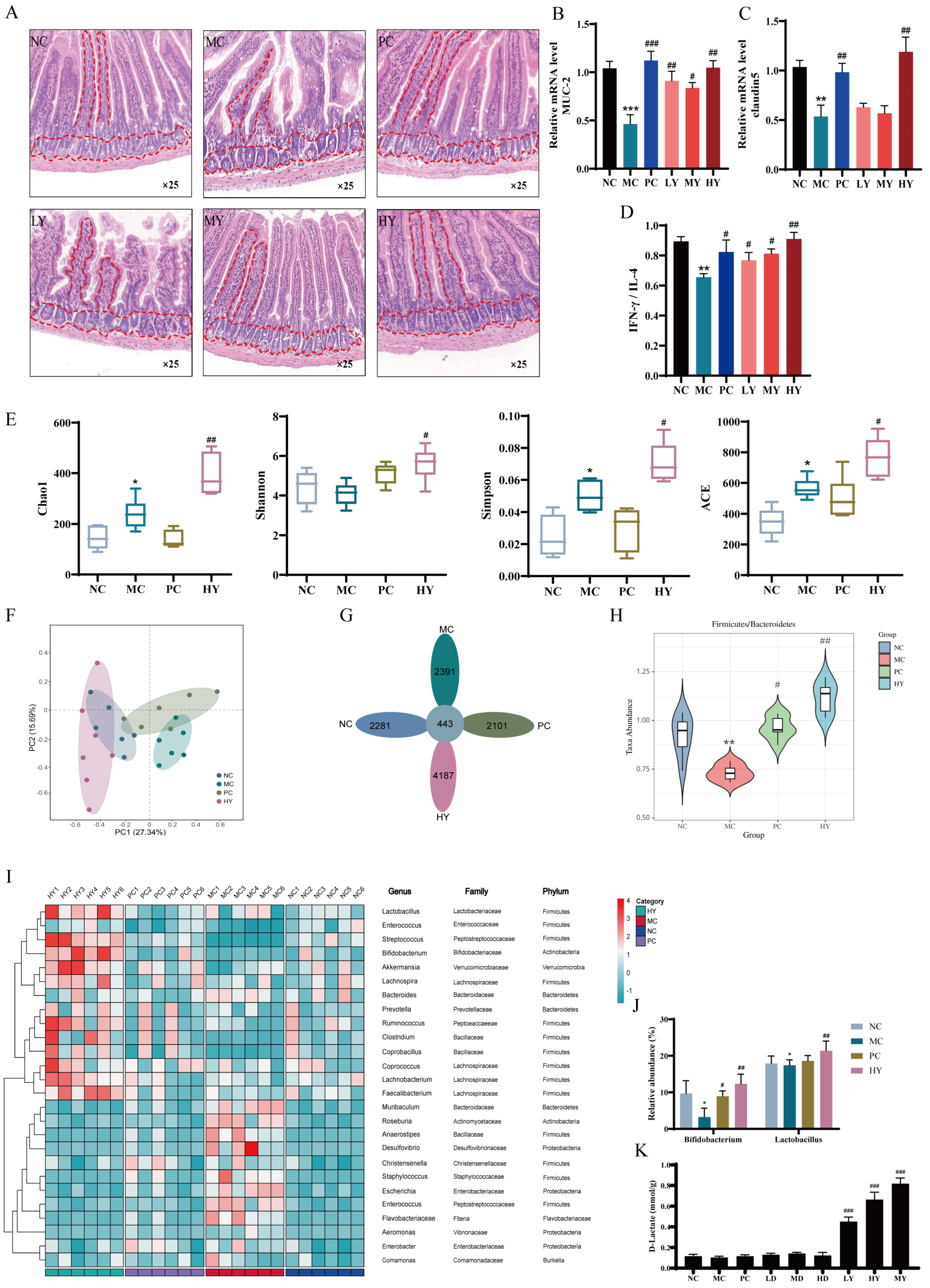
3.3.2. Yogurt Supplementation Regulated Intestinal Immunity and Gut Microbiota in CTX-Induced Mice
3.3.3. D-Lactate Supplementation Ameliorated Immunosuppression in CTX-Induced Mice
3.4. Animal Experiment III: Evaluation of Immune Efficacy of Yogurt and D-Lactate in CT26 Tumor-Bearing Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, K.; Cao, Y.X.; Jiao, S.M.; Du, G.H.; Du, Y.G.; Qin, X.M. Structural Characterization and Immune Activity Screening of Polysaccharides with Different Molecular Weights from Astragali Radix. Front. Pharmacol. 2020, 11, 582091. [Google Scholar] [CrossRef]
- Xie, H.; Fang, J.; Farag, M.A.; Li, Z.; Sun, P.; Shao, P. Dendrobium officinale leaf polysaccharides regulation of immune response and gut microbiota composition in cyclophosphamide-treated mice. Food Chem. X 2022, 13, 100235. [Google Scholar] [CrossRef]
- Douglas, A.; Stevens, B.; Lynch, L. Interleukin-17 as a key player in neuroimmunometabolism. Nat. Metab. 2023, 5, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.D.; Patel, R.S.; Cook, D.R.; Choi, M.Y.; Patil, A.B.; Liang, A.C.; Li, M.Z.; Haigis, K.M.; Elledge, S.J. The adaptive immune system is a major driver of selection for tumor suppressor gene inactivation. Science 2021, 373, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Kempen, J.H.; Newcomb, C.W.; Washington, T.L.; Foster, C.S.; Sobrin, L.; Thorne, J.E.; Jabs, D.A.; Suhler, E.B.; Rosenbaum, J.T.; Sen, H.N.; et al. Use of Immunosuppression and the Risk of Subsequent Overall or Cancer Mortality. Ophthalmology 2023, 130, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Daniel, N.; Nachbar, R.; Tran, T.T.T.; Ouellette, A.; Varin, T.V.; Cotillard, A.; Quinquis, L.; Gagné, A.; St-Pierre, P.; Trottier, J.; et al. Gut microbiota and fermentation-derived branched chain hydroxy acids mediate health benefits of yogurt consumption in obese mice. Nat. Commun. 2022, 13, 1343. [Google Scholar] [CrossRef] [PubMed]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E.; Smith, E.D.; Reutrakul, S.; Tonucci, L.; Anothaisintawee, T. The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials. Nutrients 2019, 11, 671. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D. Consumption of Yogurt and the Incident Risk of Cardiovascular Disease: A Meta-Analysis of Nine Cohort Studies. Nutrients 2017, 9, 315. [Google Scholar] [CrossRef]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef]
- Başpinar, B.; Güldaş, M. Traditional plain yogurt: A therapeutic food for metabolic syndrome? Crit. Rev. Food Sci. Nutr. 2020, 61, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Pu, Z.J.; Tang, K.; Jia, X.; Kim, K.H.; Liu, X.; Jeon, C.O. Catalytic, Computational, and Evolutionary Analysis of the d-Lactate Dehydrogenases Responsible for d-Lactic Acid Production in Lactic Acid Bacteria. J. Agric. Food Chem. 2018, 66, 8371–8381. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.B.; Guard, B.C.; Honneffer, J.B.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. PLoS ONE 2019, 14, e0224454. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.-x.; Yang, Q.; Zhang, H.; Hettinga, K.A.; Li, H.; Chen, W. Dietary D-Lactate Intake Facilitates Inflammatory Resolution by Modulating M1 Macrophage Polarization. Mol. Nutr. Food Res. 2022, 66, e2200196. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Y.; Du, X.; Zhang, H.; Li, H.; Chen, W. Yogurt Prevents Colitis-Associated Colorectal Cancer in Mice. Mol. Nutr. Food Res. 2023, 67, e2300444. [Google Scholar] [CrossRef]
- Cho, W.-Y.; Hong, G.-E.; Lee, H.-J.; Yeon, S.-J.; Paik, H.-D.; Hosaka, Y.Z.; Lee, C.-H. Effect of Yogurt Fermented by Lactobacillus Fermentum TSI and L. Fermentum S2 Derived from a Mongolian Traditional Dairy Product on Rats with High-Fat-Diet-Induced Obesity. Foods 2020, 9, 594. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, H.; Kong, L.; Meng, F.; Zhou, L.; Lu, Z.; Lu, Y. Probiotic Yogurt Alleviates High-Fat Diet-Induced Lipid Accumulation and Insulin Resistance in Mice via the Adiponectin Pathway. J. Agric. Food. Chem. 2023, 71, 1464–1476. [Google Scholar] [CrossRef]
- Fan, X.; Shi, Z.-Q.; Xu, J.; Li, C.; Li, X.; Jiang, X.; Du, L.; Tu, M.; Zeng, X.; Wu, Z.; et al. Characterization of the effects of binary probiotics and wolfberry dietary fiber on the quality of yogurt. Food Chem. 2022, 406, 135020. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, R.; Li, X.-x.; Yao, Z.; Zhang, H.; Li, H.; Chen, W. Unexpected immunoregulation effects of D-lactate, different from L-lactate. Food Agric. Immunol. 2022, 33, 286–301. [Google Scholar] [CrossRef]
- Zhu, Z.; Neirinck, L.G. Chiral separation and determination of R-(-)- and S-(+)-baclofen in human plasma by high-performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 785, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.S.; Yang, G.; Lee, H.-H.; Shim, G.; Lee, J.; Oh, Y.K. Lysyl oxidase-responsive anchoring nanoparticles for modulation of the tumor immune microenvironment. J. Control. Release 2023, 360, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, P.; Yu, F.; Li, Z.; Yao, Z.; Martinez, J.; Li, M.; Xu, H. Combining adoptive NK cell infusion with a dopamine-releasing peptide reduces senescent cells in aged mice. Cell Death Dis. 2022, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, J.-l.; Zhong, Y.; Zhang, Y.; Liu, W.; Nie, S.; Xie, M. Effects of Rosa roxburghii & edible fungus fermentation broth on immune response and gut microbiota in immunosuppressed mice. Food Sci. Hum. Wellness 2023, 13, 154–165. [Google Scholar]
- Zhang, Y.; Tang, Y.; Cai, L.; He, J.; Chen, L.; Ouyang, K.; Wang, W. Chimonanthus nitens Oliv Polysaccharides Modulate Immunity and Gut Microbiota in Immunocompromised Mice. Oxidative Med. Cell. Longev. 2023, 2023, 6208680. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Wang, M.; Zhao, F.; Ge, M.; Liu, L.; Jiang, E.; Feng, S.; Han, M.; Pei, X.; et al. Levamisole Suppresses CD4(+) T-Cell Proliferation and Antigen-Presenting Cell Activation in Aplastic Anemia by Regulating the JAK/STAT and TLR Signaling Pathways. Front. Immunol. 2022, 13, 907808. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, S.; Wang, J.; Liu, X.; Yin, P.-F.; Huang, D.; Li, W.; Gong, D.; Xie, M. Chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced mice. Int. J. Biol. Macromol. 2014, 64, 395–401. [Google Scholar] [CrossRef]
- Yin, H.; Li, R.; Liu, J.; Sun, Y.; Zhao, L.; Mou, J.; Yang, J. Fucosylated chondroitin sulfate from sea cucumber Stichopus chloronotus alleviate the intestinal barrier injury and oxidative stress damage in vitro and in vivo. Carbohydr. Polym. 2024, 328, 121722. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Pei, R.; Raghuvanshi, R.; Liu, Z.; Bolling, B.W. Yogurt Supplementation Attenuates Insulin Resistance in Obese Mice by Reducing Metabolic Endotoxemia and Inflammation. J. Nutr. 2023, 153, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, J.; Salminen, S.; Szajewska, H. Rapid review shows that probiotics and fermented infant formulas do not cause d-lactic acidosis in healthy children. Acta Paediatr. 2018, 107, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.; Abrahamsson, T.R.; Björkstén, B. Safety of D(-)-Lactic Acid Producing Bacteria in the Human Infant. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Haschke-Becher, E.; Brunser, O.; Cruchet, S.; Gotteland, M.; Haschke, F.; Bachmann, C. Urinary D-Lactate Excretion in Infants Receiving Lactobacillus johnsonii with Formula. Ann. Nutr. Metab. 2008, 53, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Papagaroufalis, K.; Fotiou, A.; Egli, D.; Tran, L.-A.; Steenhout, P.G. A Randomized Double Blind Controlled Safety Trial Evaluating d-Lactic Acid Production in Healthy Infants Fed a Lactobacillus reuteri-containing Formula. Nutr. Metab. Insights 2014, 7, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Laura, B.L.; Chen, X.; Wang, M.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Fahey, G.C.; Donovan, S.M. Microbial composition and in vitro fermentation patterns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. FASEB J. 2012, 142, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Umemoto, E.; Fujita, S.; Hayashi, A.; Kikuta, J.; Kimura, I.; Haneda, T.; Imai, T.; Inoue, A.; Mimuro, H.; et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1+ cells by bacterial metabolites. Nature 2019, 566, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Min, F.; Hu, J.-l.; Huang, T.; Huang, Y.; Nie, S.; Xiong, T.; Xie, M. Effects of Lactobacillus casei NCU011054 on immune response and gut microbiota of cyclophosphamide induced immunosuppression mice. Food Chem. Toxicol. 2023, 174, 113662. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; You, S. Dried Ginger Extract Restores the T Helper Type 1/T Helper Type 2 Balance and Antibody Production in Cyclophosphamide-Induced Immunocompromised Mice after Flu Vaccination. Nutrients 2022, 14, 1984. [Google Scholar] [CrossRef]
- Bai, R.-b.; Zhang, Y.; Fan, J.-m.; Jia, X.; Li, D.; Wang, Y.; Zhou, J.; Yan, Q.; Hu, F. Immune-enhancement effects of oligosaccharides from Codonopsis pilosula on cyclophosphamide induced immunosuppression in mice. Food Funct. 2020, 11, 3306–3315. [Google Scholar] [CrossRef]
- Colucci, A.; Tassinari, I.D.Á.; Loss, E.d.S.; Fraga, L.S.d. History and Function of the Lactate Receptor GPR81/HCAR1 in the Brain: A Putative Therapeutic Target for the Treatment of Cerebral Ischemia. Neuroscience 2023, 526, 144–163. [Google Scholar] [CrossRef] [PubMed]


| Genes | Primer Forward | Primer Reverse |
|---|---|---|
| MUC-2 | GATTCGAAGTGAAGAGCAAG | CACTTGGAGGAATAAACTGG |
| Claudin5 | GAGAGGAACTACCCTTATGCC | ATTGAGTAATTAAACGGGACAGG |
| T-bet | CGTTTCTACCCCGACCTTCC | ATGCTCACAGCTCGGAACTC |
| GATA-3 | AAGCTCAGTATCCGCTGACG | GATACCTCTGCACCGTAGCC |
| Bax | ACAGATCATGAAGACAGGGG | CAAAGTAGAAGAGGGCAACC |
| Bcl-2 | ATGTGTGTGGAGAGCGTCAAC | AGACAGCCAGGAGAAATCAAAC |
| β-actin | TGCTCTCCCTCACGCCATC | GAGGAAGAGGATGCGGCAGT |
| Normal Control | Positive Control | Yogurt (200 μL) | Yogurt (600 μL) | D-Lactate (75 mg/kg) | D-Lactate (300 mg/kg) | |
|---|---|---|---|---|---|---|
| Body weight (g) | 28.01 ± 0.8 | 29.11 ± 1.2 | 28.31 ± 0.7 | 29.21 ± 0.5 | 27.26 ± 1.9 | 28.09 ± 1.1 |
| Thymus index (%) | 0.27 ± 0.06 | 0.36 ± 0.09 * | 0.28 ± 0.12 | 0.32 ± 0.03 * | 0.28 ± 0.04 | 0.38 ± 0.02 * |
| Spleen index (%) | 0.32 ± 0.05 | 0.42 ± 0.12 * | 0.38 ± 0.17 * | 0.40 ± 0.19 * | 0.32 ± 0.09 | 0.40 ± 0.10 * |
| Liver index (%) | 4.31 ± 0.21 | 4.51 ± 0.09 | 4.33 ± 0.19 | 4.68 ± 0.16 | 4.53 ± 0.14 | 4.61 ± 0.08 |
| BUN (mmol/L) | 10.09 ± 0.19 | 10.13 ± 0.31 | 10.01 ± 0.46 | 10.32 ± 0.36 | 10.16 ± 0.21 | 10.11 ± 0.31 |
| CRE (μmol/L) | 44.03 ± 1.91 | 45.21 ± 2.31 | 42.91 ± 3.33 | 44.19 ± 2.98 | 42.98 ± 1.08 | 43.26 ± 0.09 |
| ALT (IU/L) | 38.21 ± 3.41 | 42.87 ± 2.12 | 40.23 ± 3.61 | 41.87 ± 1.97 | 42.09 ± 2.71 | 40.98 ± 3.01 |
| AST (IU/L) | 129.23 ± 23.12 | 136.21 ± 11.21 | 132.98 ± 31.21 | 138.12 ± 29.12 | 119.21 ± 38.24 | 129.12 ± 31.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Yan, Y.; Dai, Y.; Xu, R. Yogurt Alleviates Cyclophosphamide-Induced Immunosuppression in Mice through D-Lactate. Nutrients 2024, 16, 1395. https://doi.org/10.3390/nu16091395
Du X, Yan Y, Dai Y, Xu R. Yogurt Alleviates Cyclophosphamide-Induced Immunosuppression in Mice through D-Lactate. Nutrients. 2024; 16(9):1395. https://doi.org/10.3390/nu16091395
Chicago/Turabian StyleDu, Xinru, Yongheng Yan, Yufeng Dai, and Ruijie Xu. 2024. "Yogurt Alleviates Cyclophosphamide-Induced Immunosuppression in Mice through D-Lactate" Nutrients 16, no. 9: 1395. https://doi.org/10.3390/nu16091395





