Rice Bran Oil Improves Emphysema in Cigarette Smoke Extract-Induced Mice through Anti-Inflammatory and Antioxidative Effects
Abstract
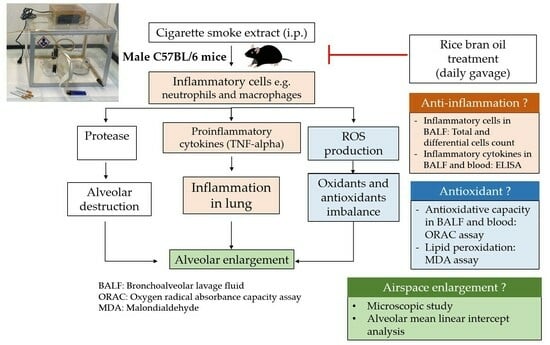
:1. Introduction
2. Materials and Methods
2.1. Cigarette Smoke Extract (CSE) Preparation
2.2. Animal Experiment
2.3. Sample Collection
2.4. Total and Differential Cell Count in Bronchoalveolar Lavage Fluid (BALF)
2.5. Lung Histopathological Examination
2.6. Cytokines Analysis in BALF
2.7. Lipid Peroxidation Estimation
2.8. Oxygen Radical Absorbance Capacity (ORAC) Analysis
2.9. Statistical Analysis
3. Results
3.1. Body Weight and Weight Gain in CSE-Induced Emphysema in Mice
3.2. Effect of RBO on Lung Morphology and Airspace Size in CSE-Induced Emphysema in Mice
3.3. Effect of RBO on Inflammatory Cells in CSE-Induced Emphysema in Mice
3.4. Effect of RBO on the Levels of TNF-Alpha in CSE-Induced Emphysema in Mice
3.5. Effect of RBO on the Levels of MDA in CSE-Induced Emphysema in Mice
3.6. Effect of RBO on the Total Antioxidant Capacity in BALF and Serum of CSE-Induced Emphysema in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillien, A.; Soumagne, T.; Dalphin, J.C.; Degano, B. COPD, airflow limitation and chronic bronchitis in farmers: A systematic review and meta-analysis. Occup. Environ. Med. 2019, 76, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Durawa, A.; Dziadziuszko, K.; Jelitto-Górska, M.; Szurowska, E. Emphysema—The review of radiological presentation and its clinical impact in the LDCT screening era. Clin. Imaging 2020, 64, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef] [PubMed]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef] [PubMed]
- Global Health Estimates: Life expectancy and Leading Causes of Death and Disability. World Health Organization. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 5 January 2024).
- Echevarria, C.; Steer, J.; Hartley, T.; Lane, N.; Bourke, S.C. Predictors of NIV Treatment in Patients with COPD Exacerbation Complicated by Respiratory Acidaemia. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Han, M.K.; Allinson, J.P.; Barr, R.G.; Boucher, R.C.; Calverley, P.M.A.; Celli, B.R.; Christenson, S.A.; Crystal, R.G.; Fagerås, M.; et al. At the Root: Defining and Halting Progression of Early Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 197, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Christopoulou, M.E.; Papakonstantinou, E.; Stolz, D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 3786. [Google Scholar] [CrossRef]
- McGuinness, A.J.; Sapey, E. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. J. Clin. Med. 2017, 6, 21. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: A meta-analysis. Eur. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.M.; Verbraecken, J.; Darquennes, K.; De Backer, W.A. Role of N-acetylcysteine in the management of COPD. Int. J. Chron. Obs. Pulmon Dis. 2006, 1, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Celli, B. Challenges in the Pharmacotherapy of COPD Subtypes. Arch. Bronconeumol. 2023, 59, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Nagasaka, R.; Ohara, K.; Hosoya, T.; Ozaki, H.; Ushio, H.; Hori, M. Biological abilities of rice bran-derived antioxidant phytochemicals for medical therapy. Curr. Top. Med. Chem. 2011, 11, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Saenjum, C.; Chaiyasut, C.; Chansakaow, S.; Suttajit, M.; Sirithunyalug, B. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J. Med. Plants Res. 2012, 6, 1070–1077. [Google Scholar]
- Mingyai, S.; Kettawan, A.; Srikaeo, K.; Singanusong, R. Physicochemical and Antioxidant Properties of Rice Bran Oils Produced from Colored Rice Using Different Extraction Methods. J. Oleo Sci. 2017, 66, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.P.C.; Sugasini, D.; Lokesh, B.R. Dietary gamma oryzanol plays a significant role in the anti-inflammatory activity of rice bran oil by decreasing pro-inflammatory mediators secreted by peritoneal macrophages of rats. Biochem. Biophys. Res. Commun. 2016, 479, 747–752. [Google Scholar] [CrossRef]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A comprehensive review on anti-diabetic property of rice bran. Asian Pac. J. Trop. Biomed. 2018, 8, 79. [Google Scholar] [CrossRef]
- Lee, S.; Yu, S.; Park, H.J.; Jung, J.; Go, G.W.; Kim, W. Rice bran oil ameliorates inflammatory responses by enhancing mitochondrial respiration in murine macrophages. PLoS ONE 2019, 14, e0222857. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.; Al-Siedy, E.S.K. Rice bran as source of nutraceuticals for management of cardiovascular diseases, cardio-renal syndrome and hepatic cancer. J. Herbmed. Pharmacol. 2020, 9, 68–74. [Google Scholar] [CrossRef]
- Cicero, A.F.; Gaddi, A. Rice bran oil and gamma-oryzanol in the treatment of hyperlipoproteinaemias and other conditions. Phytother. Res. 2001, 15, 277–289. [Google Scholar] [CrossRef]
- de Oliveira, M.V.; Silva, P.L.; Rocco, P.R.M.; Oliveira, M.V.d.; Silva, P.L.; Rocco, P.R.M. Animal Models of Chronic Obstructive Pulmonary Disease Exacerbations: A Review of the Current Status. J. Biomed. Sci. 2016, 5, 8. [Google Scholar] [CrossRef]
- He, Z.H.; Chen, P.; Chen, Y.; He, S.D.; Ye, J.R.; Zhang, H.L.; Cao, J. Comparison between cigarette smoke-induced emphysema and cigarette smoke extract-induced emphysema. Tob. Induc. Dis. 2015, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, M.; Droma, Y.; Chen, Y.; Agatsuma, T.; Kitaguchi, Y.; Voelkel, N.F.; Kubo, K. Carbocisteine protects against emphysema induced by cigarette smoke extract in rats. Chest 2011, 139, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, X.; Luo, L.; Li, H.; Zeng, Z.; Chen, Y. Effect of pirfenidone protecting against cigarette smoke extract induced apoptosis. Tob. Induc. Dis. 2022, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, A.; Feng, X.; Sun, X.; Zhu, X.; Zhao, Z. Pharmacological Investigation of the Anti-Inflammation and Anti-Oxidation Activities of Diallyl Disulfide in a Rat Emphysema Model Induced by Cigarette Smoke Extract. Nutrients 2018, 10, 79. [Google Scholar] [CrossRef]
- Thongchai, W.; Liawruangrath, B. Determination of gamma oryzanol in rice bran oil by HPLC with molecularly imprinted solid-phase extraction. Int. Food Res. J. 2016, 23, 1389–1395. [Google Scholar]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Crowley, G.; Kwon, S.; Caraher, E.J.; Haider, S.H.; Lam, R.; Batra, P.; Melles, D.; Liu, M.; Nolan, A.J. Quantitative lung morphology: Semi-automated measurement of mean linear intercept. BMC Pulm. Med. 2019, 19, 206. [Google Scholar] [CrossRef]
- Henderson, A.J.; Kumar, A.; Barnett, B.; Dow, S.W.; Ryan, E.P. Consumption of rice bran increases mucosal immunoglobulin A concentrations and numbers of intestinal Lactobacillus spp. J. Med. Food 2012, 15, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Faner, R.; Cruz, T.; Agusti, A. Immune response in chronic obstructive pulmonary disease. Expert. Rev. Clin. Immunol. 2013, 9, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, G.; Yuan, S.; Tan, C.; Lian, P.; Fu, L.; Hou, Q.; Xu, B.; Wang, H. Cigarette Smoke-Induced Pulmonary Inflammation and Autophagy Are Attenuated in Ephx2-Deficient Mice. Inflammation 2017, 40, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, P.; Closa, D.; Piñer, R.; Bulbena, O.; Menéndez, R.; Torres, A. Macrophage activation in exacerbated COPD with and without community-acquired pneumonia. Eur. Respir. J. 2010, 36, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Quint, J.K.; Wedzicha, J.A. The neutrophil in chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2007, 119, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yu, S.; Kim, W. Rice Bran Oil Attenuates Chronic Inflammation by Inducing M2 Macrophage Switching in High-Fat Diet-Fed Obese Mice. Foods 2021, 10, 359. [Google Scholar] [CrossRef]
- Kobayashi, E.; Ito, J.; Kato, S.; Sawada, K.; Matsuki, M.; Hashimoto, H.; Miyazawa, T.; Nakagawa, K. Presence of orally administered rice bran oil γ-oryzanol in its intact form in mouse plasma. Food Funct. 2016, 7, 4816–4822. [Google Scholar] [CrossRef]
- Islam, M.S.; Murata, T.; Fujisawa, M.; Nagasaka, R.; Ushio, H.; Bari, A.M.; Hori, M.; Ozaki, H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2008, 154, 812–824. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; de Sotomayor, M.A.; Herrera, M.D. Contribution of ferulic acid, γ-oryzanol and tocotrienols to the cardiometabolic protective effects of rice bran. J. Funct. Foods 2017, 32, 58–71. [Google Scholar] [CrossRef]
- Park, H.Y.; Lee, K.W.; Choi, H.D. Rice bran constituents: Immunomodulatory and therapeutic activities. Food Funct. 2017, 8, 935–943. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, C.H. Effects of dietary supplementation with rice bran oil on the growth performance, blood parameters, and immune response of broiler chickens. J. Anim. Sci. Technol. 2016, 58, 12. [Google Scholar] [CrossRef]
- Ministry of Public Health. Notification of the Ministry of Public Health (No. 182): Thai Recommended Daily in Takes-Thai RDI. 1998. Available online: http://food.fda.moph.go.th/Rules/dataRules/4-4-2ThaiRDI.pdf (accessed on 1 July 2023).
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.A.; Spooner, G.; Ffoulkes-Jones, C.; Calvez, R. Cigarette smoke triggers macrophage adhesion and activation: Role of lipid peroxidation products and scavenger receptor. Free Radic. Biol. Med. 2003, 35, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D.; Cuccurullo, F. TBA test and “free” MDA assay in evaluation of lipid peroxidation and oxidative stress in tissue systems. Am. J. Physiol. 1993, 265, H1030–H1032. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, I.; Dane, S.; Gümüştekin, K. Effects of cigarette smoking on lipid peroxidation. J. Basic. Clin. Physiol. Pharmacol. 2002, 13, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Agarwal, R.; Anand, C.V. Pulmonary lipid peroxidation in cigarette smokers and lung cancer patients. Chest 1992, 101, 290. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Abd El-Aty, A.M.; Ma, A.; Jia, Y. An overview on antioxidant peptides from rice bran proteins: Extraction, identification, and applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Zigoneanu, I.G.; Williams, L.; Xu, Z.; Sabliov, C.M. Determination of antioxidant components in rice bran oil extracted by microwave-assisted method. Bioresour. Technol. 2008, 99, 4910–4918. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Haleem, D.J. Antioxidant effects of rice bran oil mitigate repeated haloperidol-induced tardive dyskinesia in male rats. Metab. Brain Dis. 2017, 32, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Ochs, M. Stereological analysis of acute lung injury. Eur. Respir. Rev. 2006, 15, 115–121. [Google Scholar] [CrossRef]
- Solak, Z.A.; Kabaroğlu, C.; Cok, G.; Parildar, Z.; Bayindir, U.; Ozmen, D.; Bayindir, O. Effect of different levels of cigarette smoking on lipid peroxidation, glutathione enzymes and paraoxonase 1 activity in healthy people. Clin. Exp. Med. 2005, 5, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J. Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: Impact of dietary intake. Nutr. J. 2007, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Thirumalai, T.; Therasa, S.V.; Elumalai, E.; David, E. Effect in-vivo of cigarette smoke on lipid peroxidation and antioxidant status in male albino mice. J. Pharm. Sci. Res. 2010, 2, 579–582. [Google Scholar]
- Arab, F.; Alemzadeh, I.; Maghsoudi, V. Determination of antioxidant component and activity of rice bran extract. Sci. Iran. 2011, 18, 1402–1406. [Google Scholar] [CrossRef]
- Sengupta, A.; Ghosh, M.; Bhattacharyya, D.K. Antioxidative effect of rice bran oil and medium chain fatty acid rich rice bran oil in arsenite induced oxidative stress in rats. J. Oleo Sci. 2014, 63, 1117–1124. [Google Scholar] [CrossRef]
- Biselli, P.J.; Lopes, F.D.; Moriya, H.T.; Rivero, D.H.; Toledo, A.C.; Saldiva, P.H.; Mauad, T.; Martins, M.A. Short-term exposure of mice to cigarette smoke and/or residual oil fly ash produces proximal airspace enlargements and airway epithelium remodeling. Braz. J. Med. Biol. Res. 2011, 44, 460–468. [Google Scholar] [CrossRef]
- Chow, L.; Smith, D.; Chokshi, K.; Ezegbunam, W.; Charoenpong, P.; Foley, K.; Cargill, A.; Geraghty, P. Animal Models of Chronic Obstructive Pulmonary Disease. In COPD—An Update in Pathogenesis and Clinical Management Edited by Cormac McCarthy; BoD—Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- Plantier, L.; Boczkowski, J.; Crestani, B. Defect of alveolar regeneration in pulmonary emphysema: Role of lung fibroblasts. Int. J. Chron. Obs. Pulmon Dis. 2007, 2, 463–469. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kettawan, A.; Ruangklai, S.; Rungruang, T.; Thongam, J.; Kettawan, A.K.; Nirmal, N.; Srisuma, S. Rice Bran Oil Improves Emphysema in Cigarette Smoke Extract-Induced Mice through Anti-Inflammatory and Antioxidative Effects. Nutrients 2024, 16, 433. https://doi.org/10.3390/nu16030433
Kettawan A, Ruangklai S, Rungruang T, Thongam J, Kettawan AK, Nirmal N, Srisuma S. Rice Bran Oil Improves Emphysema in Cigarette Smoke Extract-Induced Mice through Anti-Inflammatory and Antioxidative Effects. Nutrients. 2024; 16(3):433. https://doi.org/10.3390/nu16030433
Chicago/Turabian StyleKettawan, Aikkarach, Sukpattaraporn Ruangklai, Thanaporn Rungruang, Julalux Thongam, Aurawan Kringkasemsee Kettawan, Nilesh Nirmal, and Sorachai Srisuma. 2024. "Rice Bran Oil Improves Emphysema in Cigarette Smoke Extract-Induced Mice through Anti-Inflammatory and Antioxidative Effects" Nutrients 16, no. 3: 433. https://doi.org/10.3390/nu16030433