Moderate-Intensity and High-Intensity Interval Exercise Training Offer Equal Cardioprotection, with Different Mechanisms, during the Development of Type 2 Diabetes in Rats
Abstract
:1. Introduction
2. Methods
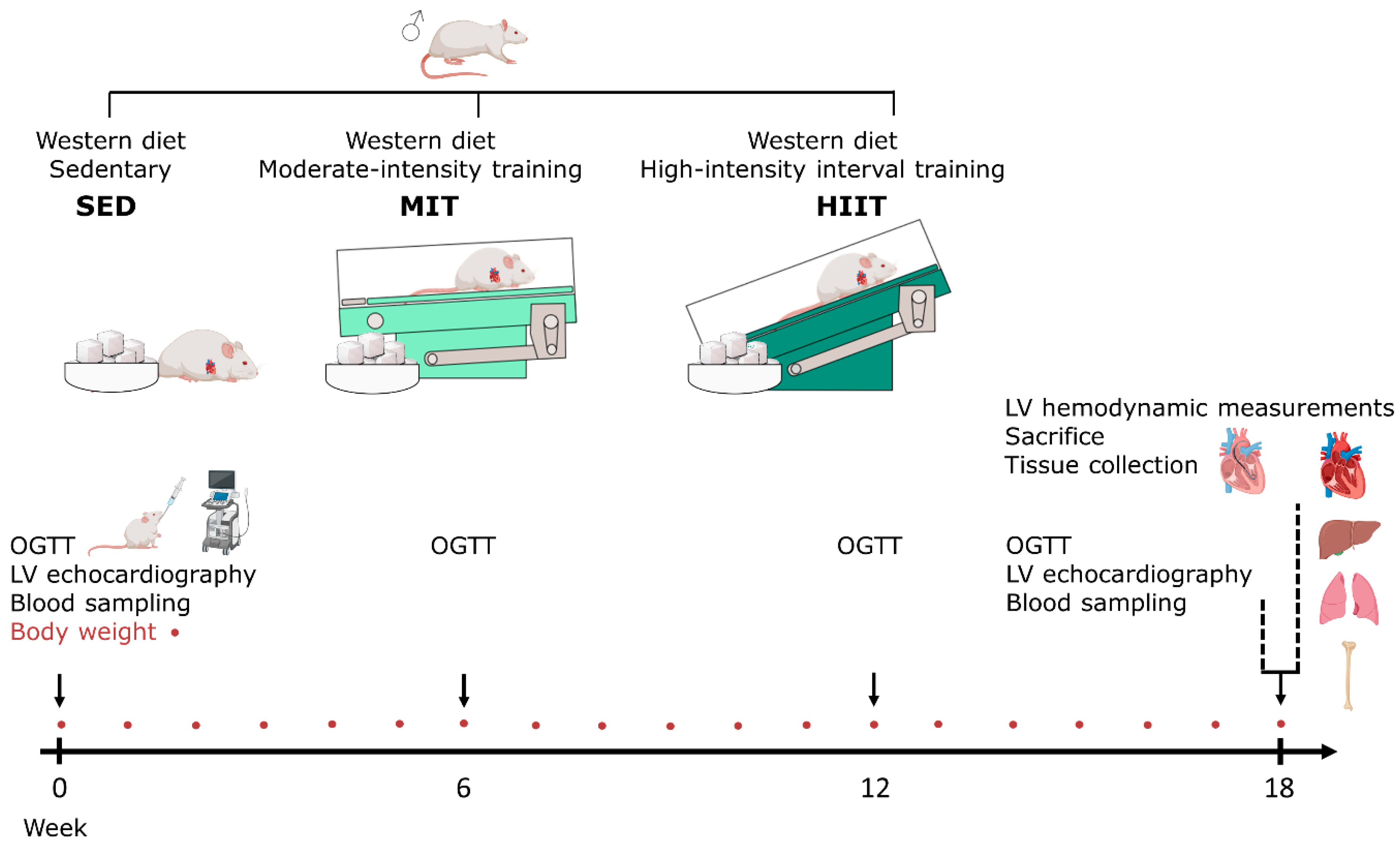
2.1. Animal Experiments and Study Design
2.2. Exercise Training Protocol
2.3. Conventional Echocardiographic Measurements
2.4. Hemodynamic Measurements
2.5. Oral Glucose Tolerance Test and Insulin Resistance Assessment
2.6. Histology
2.7. Immunohistochemistry
2.8. RT-qPCR
2.9. Quantification of Circulating Cardiac Injury Biomarkers
2.10. Statistical Analysis
3. Results
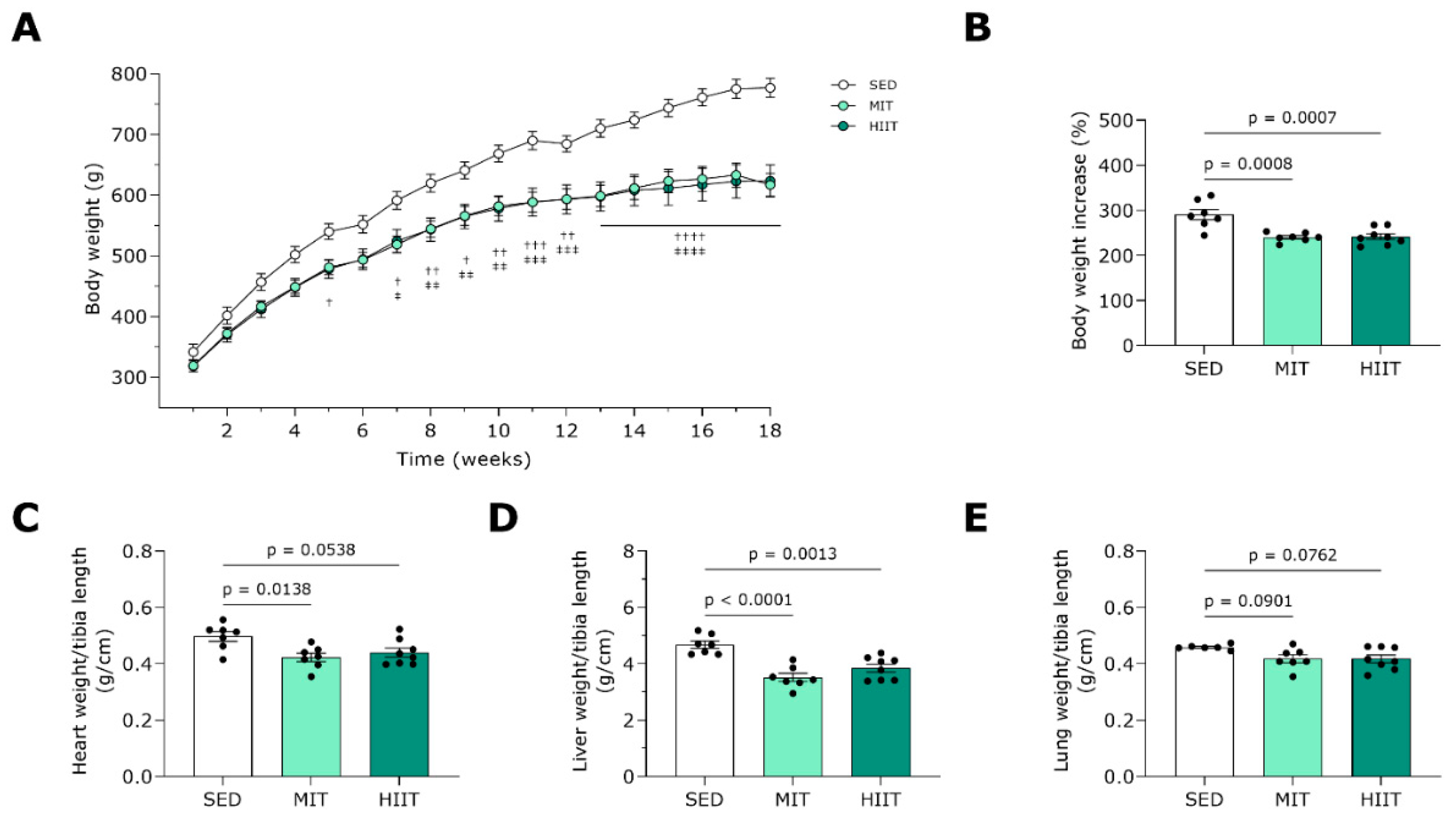
3.1. Exercise Training Prevents Body and Heart Weight Gain
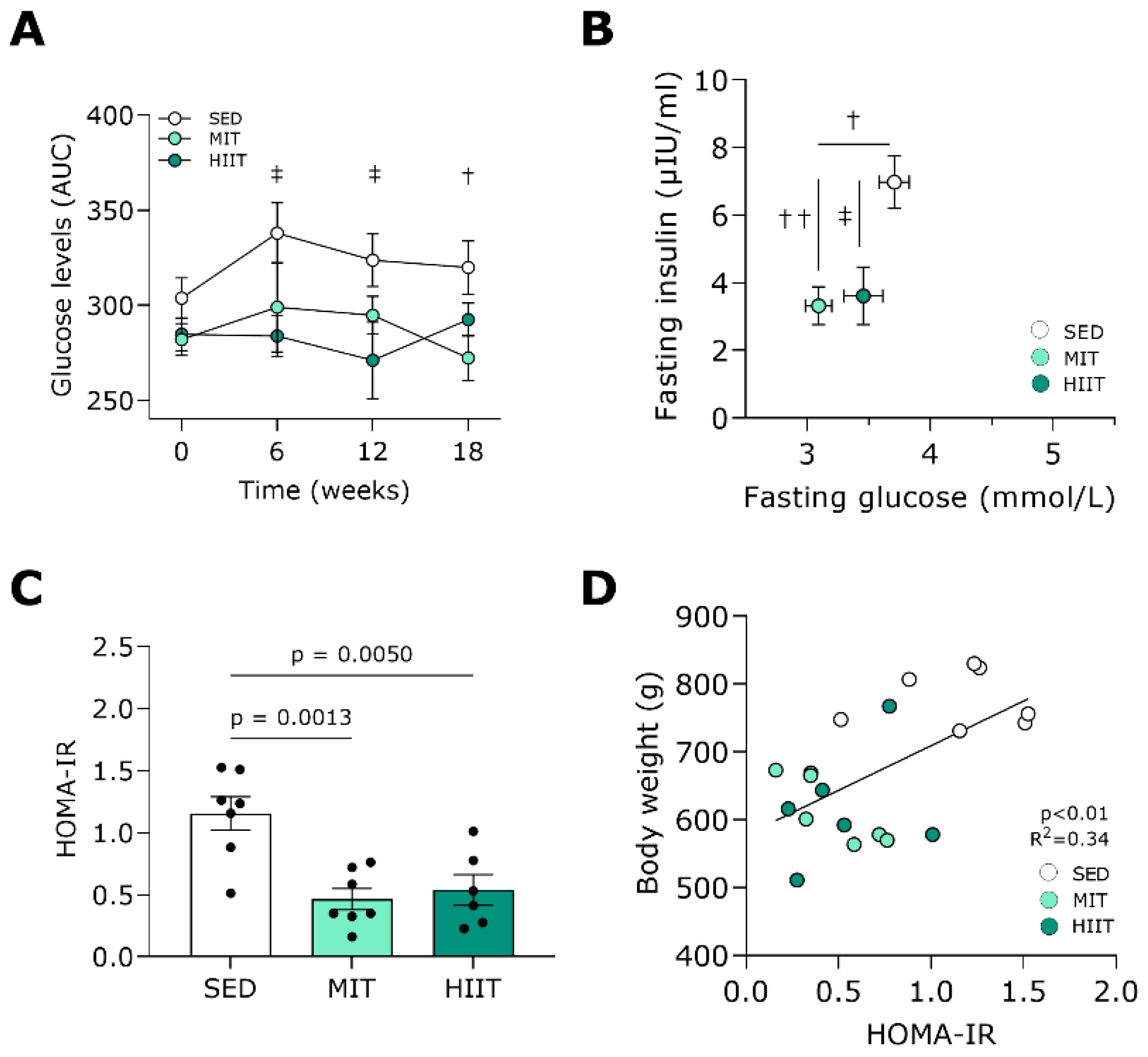
3.2. Exercise Training Improves Glucose Tolerance and Insulin Sensitivity
3.3. Exercise Training Ameliorates Diastolic Function and Prevents Adverse LV Cardiac Remodeling
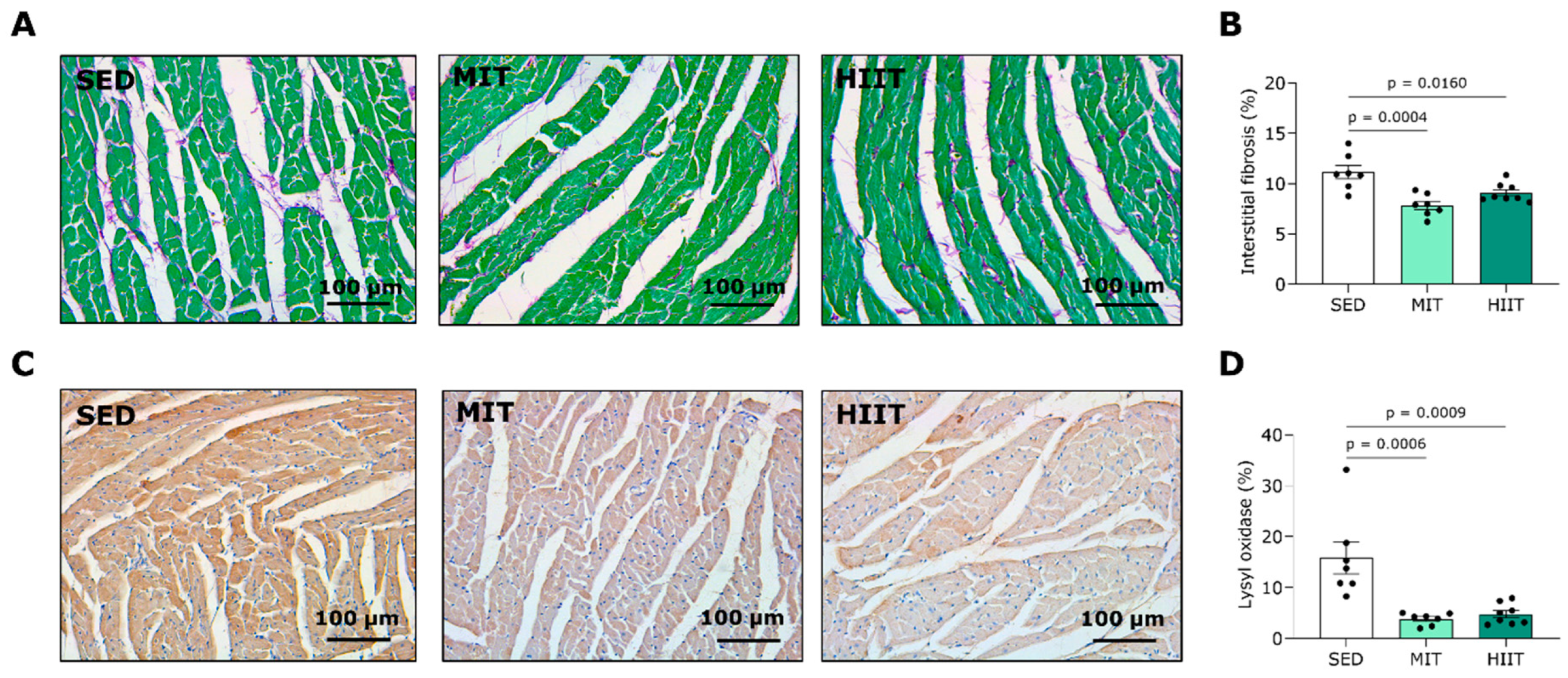
3.4. Exercise Training Limits LV Fibrosis
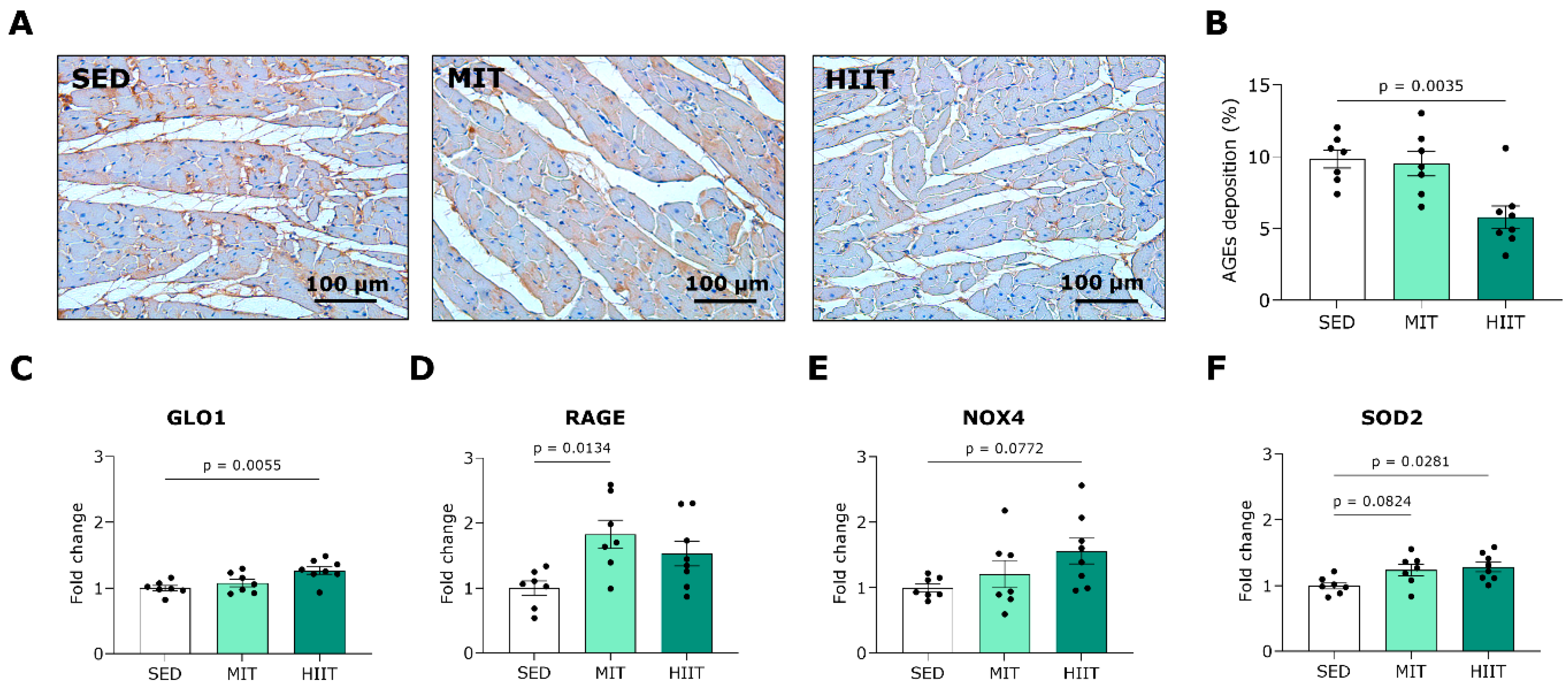
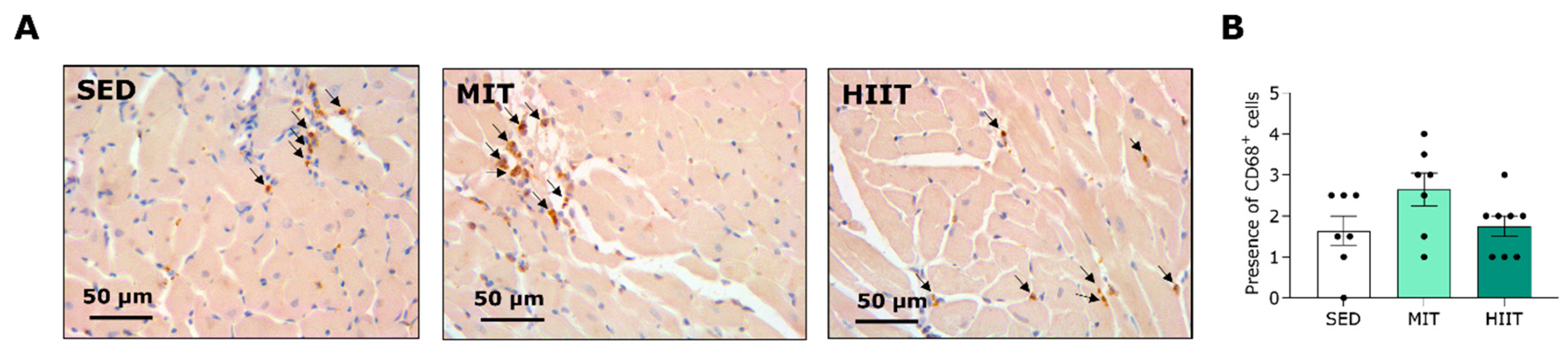
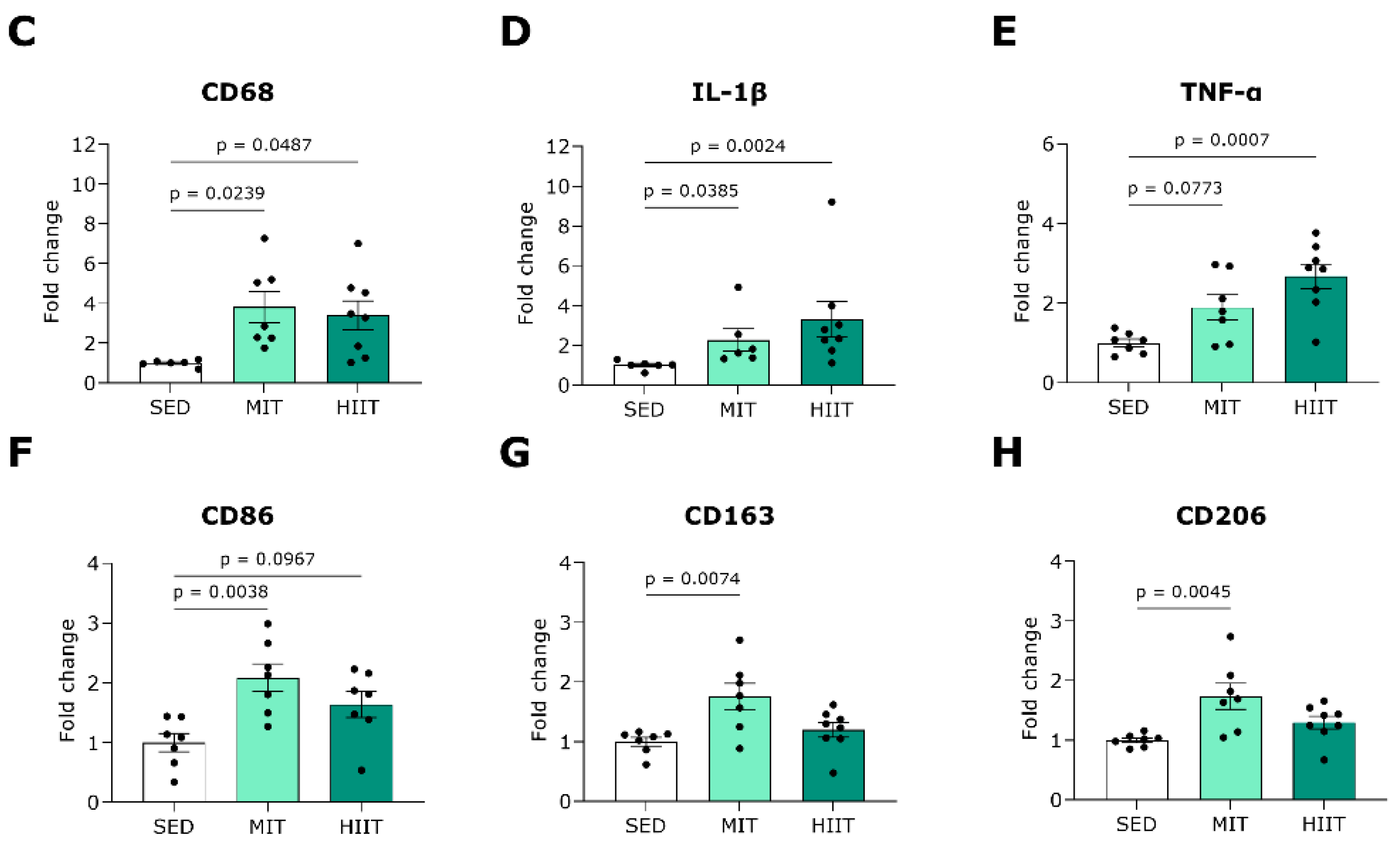
3.5. Exercise Training Triggers LV Oxidative Stress and Inflammation but Also Upregulates Protective Mechanisms
4. Discussion
4.1. MIT and HIIT as Cardioprotective Strategies in the Development of T2DM
4.2. Mechanisms of Exercise-Training-Induced Cardioprotection
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| AUC | Area under the curve |
| AWTd | Anterior wall thickness in diastole |
| AWTs | Anterior wall thickness in systole |
| BSA | Body surface area |
| CD163 | Cluster of differentiation 163 |
| CD206 | Cluster of differentiation 206 |
| CD68 | Cluster of differentiation 68 |
| CD86 | Cluster of differentiation 86 |
| CO | Cardiac output |
| CVD | Cardiovascular disease |
| dP/dtmax | Maximum peak time derivative |
| dP/dtmin | Minimum peak time derivative |
| E/A | Ratio of peak mitral flow velocity in early versus late filling |
| E′ | Peak septal mitral annulus velocity in early filling phase |
| E/E′ | Ratio of peak mitral flow velocity versus peak mitral annular velocity |
| ECM | Extracellular matrix |
| EDP | End-diastolic pressure |
| EDV | End-diastolic volume |
| EF | Ejection fraction |
| ESP | End-systolic pressure |
| ESV | End-diastolic volume |
| FABP | Fatty acid-binding protein |
| FS | Fractional shortening |
| GLO1 | Glyoxalase 1 |
| HFD | High-fat diet |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| HIIT | High-intensity interval exercise training |
| HMBS | Hydroxymethylbilane synthase |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| HR | Heart rate |
| IL-1β | Interleukin 1 beta |
| i.p. | Intraperitoneally |
| LOX | Lysyl oxidase |
| LV | Left ventricular |
| MGO | Methylglyoxal |
| MIT | Moderate-intensity exercise training |
| Myl3 | Myosin light chain 3 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NOX4 | Nicotinamide adenine dinucleotide phosphate oxidase 4 |
| OGTT | Oral glucose tolerance test |
| PWTd | Posterior wall thickness in diastole |
| PWTs | Posterior wall thickness in systole |
| RAGE | Receptor for advanced glycation end products |
| ROS | Reactive oxygen species |
| RPL13a | Ribosomal protein L13a |
| RT | Room temperature |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| SED | Sedentary lifestyle |
| SEM | Standard error of the mean |
| SOD2 | Superoxide dismutase |
| SPTI | Systolic pressure–time index |
| T2DM | Type 2 diabetes mellitus |
| Tau | Time constant for isovolumetric relaxation |
| TNF-α | Tumor necrosis factor alpha |
| TnI | Troponin I |
| WD | Western diet |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Paulus, W.J. Clinical diabetic cardiomyopathy: A two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 2015, 36, 1718–1727. [Google Scholar] [CrossRef]
- Phang, R.J.; Ritchie, R.H.; Hausenloy, D.J.; Lees, J.G.; Lim, S.Y. Cellular interplay between cardiomyocytes and non-myocytes in diabetic cardiomyopathy. Cardiovasc. Res. 2023, 119, 668–690. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: Developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Palo, K.E.D.; Golden, S.H.; Sperling, L.S. Comprehensive Management of Cardiovascular Risk Factors for Adults with Type 2 Diabetes: A Scientific Statement from the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef]
- Kemps, H.; Kränkel, N.; Dörr, M.; Moholdt, T.; Wilhelm, M.; Paneni, F.; Serratosa, L.; Ekker Solberg, E.; Hansen, D.; Halle, M.; et al. Exercise training for patients with type 2 diabetes and cardiovascular disease: What to pursue and how to do it. A Position Paper of the European Association of Preventive Cardiology (EAPC). Eur. J. Prev. Cardiol. 2020, 26, 709–727. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Swarbrick, D.J.; Athithan, L.; Brady, E.M.; Henson, J.; Baldry, E.; Argyridou, S.; Jaicim, N.B.; Squire, G.; Walters, Y.; et al. Effects of Low-Energy Diet or Exercise on Cardiovascular Function in Working-Age Adults with Type 2 Diabetes: A Prospective, Randomized, Open-Label, Blinded End Point Trial. Diabetes Care 2020, 43, 1300–1310. [Google Scholar] [CrossRef]
- Hollekim-Strand Siri, M.; Bjørgaas Marit, R.; Albrektsen, G.; Tjønna Arnt, E.; Wisløff, U.; Ingul Charlotte, B. High-Intensity Interval Exercise Effectively Improves Cardiac Function in Patients with Type 2 Diabetes Mellitus and Diastolic Dysfunction. J. Am. Coll. Cardiol. 2014, 64, 1758–1760. [Google Scholar] [CrossRef]
- Van Ryckeghem, L.; Keytsman, C.; De Brandt, J.; Verboven, K.; Verbaanderd, E.; Marinus, N.; Franssen, W.M.A.; Frederix, I.; Bakelants, E.; Petit, T.; et al. Impact of continuous vs. interval training on oxygen extraction and cardiac function during exercise in type 2 diabetes mellitus. Eur. J. Appl. Physiol. 2022, 122, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.B.; Gentil, P.; Seguro, C.S.; de Oliveira, J.C.M.; Silva, M.S.; Marques, V.A.; Beltrame, T.; Rebelo, A.C.S. High-Intensity Interval Training Improves Cardiac Autonomic Function in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Biology 2022, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, J.; Zheng, S.; Zhang, L.; Guo, X.; Zhang, J.; Xiao, X. Physical Exercise and Its Protective Effects on Diabetic Cardiomyopathy: What Is. the Evidence? Front. Endocrinol. 2018, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Verboven, M.; Van Ryckeghem, L.; Belkhouribchia, J.; Dendale, P.; Eijnde, B.O.; Hansen, D.; Bito, V. Effect of Exercise Intervention on Cardiac Function in Type 2 Diabetes Mellitus: A Systematic Review. Sports Med. 2019, 49, 255–268. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, S.; Verboven, M.; Evens, L.; Deluyker, D.; Lambrichts, I.; Eijnde, B.O.; Hansen, D.; Bito, V. Moderate- and High-Intensity Endurance Training Alleviate Diabetes-Induced Cardiac Dysfunction in Rats. Nutrients 2023, 15, 3950. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Sattar, N.; Ali, M.K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016, 4, 537–547. [Google Scholar] [CrossRef]
- Verboven, M.; Deluyker, D.; Ferferieva, V.; Lambrichts, I.; Hansen, D.; Eijnde, B.O.; Bito, V. Western diet given to healthy rats mimics the human phenotype of diabetic cardiomyopathy. J. Nutr. Biochem. 2018, 61, 140–146. [Google Scholar] [CrossRef]
- Vaisy, M.; Szlufcik, K.; De Bock, K.; Eijnde, B.O.; Van Proeyen, K.; Verbeke, K.; Van Veldhoven, P.; Hespel, P. Exercise-induced, but not creatine-induced, decrease in intramyocellular lipid content improves insulin sensitivity in rats. J. Nutr. Biochem. 2011, 22, 1178–1185. [Google Scholar] [CrossRef]
- Verboven, M.; Cuypers, A.; Deluyker, D.; Lambrichts, I.; Eijnde, B.O.; Hansen, D.; Bito, V. High intensity training improves cardiac function in healthy rats. Sci. Rep. 2019, 9, 5612. [Google Scholar] [CrossRef]
- Evens, L.; Beliën, H.; D’Haese, S.; Haesen, S.; Verboven, M.; Rummens, J.L.; Bronckaers, A.; Hendrikx, M.; Deluyker, D.; Bito, V. Combinational Therapy of Cardiac Atrial Appendage Stem Cells and Pyridoxamine: The Road to Cardiac Repair? Int. J. Mol. Sci. 2021, 22, 9266. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- de Koning, L.; Malik, V.S.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am. J. Clin. Nutr. 2011, 93, 1321–1327. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Croteau, D.; Luptak, I.; Chambers, J.M.; Hobai, I.; Panagia, M.; Pimentel, D.R.; Siwik, D.A.; Qin, F.; Colucci, W.S. Effects of Sodium-Glucose Linked Transporter 2 Inhibition with Ertugliflozin on Mitochondrial Function, Energetics, and Metabolic Gene Expression in the Presence and Absence of Diabetes Mellitus in Mice. J. Am. Heart Assoc. 2021, 10, e019995. [Google Scholar] [CrossRef]
- Dia, M.; Leon, C.; Chanon, S.; Bendridi, N.; Gomez, L.; Rieusset, J.; Thibault, H.; Paillard, M. Effect of Metformin on T2D-Induced MAM Ca(2+) Uncoupling and Contractile Dysfunction in an Early Mouse Model of Diabetic HFpEF. Int. J. Mol. Sci. 2022, 23, 3569. [Google Scholar] [CrossRef] [PubMed]
- Velagic, A.; Li, M.Y.; Deo, M.; Li, J.C.; Kiriazis, H.; Donner, D.G.; Anderson, D.; Blasio, M.J.D.; Woodman, O.L.; Kemp-Harper, B.K.; et al. A high-sucrose diet exacerbates the left ventricular phenotype in a high fat-fed streptozotocin rat model of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H241–H257. [Google Scholar] [CrossRef]
- Hou, L.Y.; Wang, Q.; Pan, B.; Li, R.; Li, Y.F.; He, J.J.; Qin, T.Z.; Cao, L.J.; Zhang, N.; Cao, C.H.; et al. Exercise modalities for type 2 diabetes: A systematic review and network meta-analysis of randomized trials. Diabetes Metab. Res. Rev. 2023, 39, e3591. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Hieda, M.; Sarma, S.; Hearon, C.M., Jr.; MacNamara, J.P.; Dias, K.A.; Samels, M.; Palmer, D.; Livingston, S.; Morris, M.; Levine, B.D. One-Year Committed Exercise Training Reverses Abnormal Left Ventricular Myocardial Stiffness in Patients with Stage B Heart Failure with Preserved Ejection Fraction. Circulation 2021, 144, 934–946. [Google Scholar] [CrossRef]
- Howden, E.J.; Sarma, S.; Lawley, J.S.; Opondo, M.; Cornwell, W.; Stoller, D.; Urey, M.A.; Adams-Huet, B.; Levine, B.D. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications for Heart Failure Prevention. Circulation 2018, 137, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Boardman, N.T.; Hafstad, A.D.; Lund, J.; Rossvoll, L.; Aasum, E. Exercise of obese mice induces cardioprotection and oxygen sparing in hearts exposed to high-fat load. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1054–H1062. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Shahshahan, H.R.; Hackfort, B.T.; Yadav, S.K.; Yadav, R.; Kambis, T.N.; Lefer, D.J.; Mishra, P.K. Exercise Training Promotes Cardiac Hydrogen Sulfide Biosynthesis and Mitigates Pyroptosis to Prevent High-Fat Diet-Induced Diabetic Cardiomyopathy. Antioxidants 2019, 8, 638. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, M.; Gao, D.; Su, Q.; Laher, I. Changes in Titin and Collagen Modulate Effects of Aerobic and Resistance Exercise on Diabetic Cardiac Function. J. Cardiovasc. Transl. Res. 2019, 12, 404–414. [Google Scholar] [CrossRef]
- Khakdan, S.; Delfan, M.; Heydarpour Meymeh, M.; Kazerouni, F.; Ghaedi, H.; Shanaki, M.; Kalaki-Jouybari, F.; Gorgani-Firuzjaee, S.; Rahimipour, A. High-intensity interval training (HIIT) effectively enhances heart function via miR-195 dependent cardiomyopathy reduction in high-fat high-fructose diet-induced diabetic rats. Arch. Physiol. Biochem. 2020, 126, 250–257. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Li, D.; Yuan, Y. Long-term moderate intensity exercise alleviates myocardial fibrosis in type 2 diabetic rats via inhibitions of oxidative stress and TGF-β1/Smad pathway. J. Physiol. Sci. 2019, 69, 861–873. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Li, H.; Zhong, X.; Wang, L.; Zhao, S.; Liu, X.; Huang, Z.; Wang, Y. Aerobic Exercise Inhibited P2X7 Purinergic Receptors to Improve Cardiac Remodeling in Mice with Type 2 Diabetes. Front. Physiol. 2022, 13, 828020. [Google Scholar] [CrossRef]
- Cassidy, S.; Thoma, C.; Hallsworth, K.; Parikh, J.; Hollingsworth, K.G.; Taylor, R.; Jakovljevic, D.G.; Trenell, M.I. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: A randomised controlled trial. Diabetologia 2016, 59, 56–66. [Google Scholar] [CrossRef]
- De Bosscher, R.; Janssens, K.; Dausin, C.; Goetschalckx, K.; Bogaert, J.; Ghekiere, O.; Van De Heyning, C.; Elliott, A.; Sanders, P.; Kalman, J.; et al. The prevalence and clinical significance of a reduced ventricular ejection fraction in asymptomatic young elite endurance athletes. Eur. J. Prev. Cardiol. 2022, 29, zwac056.263. [Google Scholar] [CrossRef]
- Luti, S.; Modesti, A.; Modesti, P.A. Inflammation, Peripheral Signals and Redox Homeostasis in Athletes Who Practice Different Sports. Antioxidants 2020, 9, 1065. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Hafstad, A.D.; Boardman, N.T.; Rossvoll, L.; Rolim, N.P.; Ahmed, M.S.; Florholmen, G.; Attramadal, H.; Wisløff, U.; Larsen, T.S.; et al. Exercise training promotes cardioprotection through oxygen-sparing action in high fat-fed mice. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H823–H829. [Google Scholar] [CrossRef] [PubMed]
- Al-U’datt, D.; Allen, B.G.; Nattel, S. Role of the lysyl oxidase enzyme family in cardiac function and disease. Cardiovasc. Res. 2019, 115, 1820–1837. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yang, A.L.; Wong, J.K.S.; Masodsai, K.; Lee, S.D.; Lin, Y.Y. Exercise intervention prevents early aged hypertension-caused cardiac dysfunction through inhibition of cardiac fibrosis. Aging 2022, 14, 4390–4401. [Google Scholar] [CrossRef] [PubMed]
- Rubies, C.; Batlle, M.; Sanz-de la Garza, M.; Dantas, A.P.; Jorba, I.; Fernandez, G.; Sangüesa, G.; Abuli, M.; Brugada, J.; Sitges, M.; et al. Long-Term Strenuous Exercise Promotes Vascular Injury by Selectively Damaging the Tunica Media: Experimental Evidence. JACC Basic Transl. Sci. 2022, 7, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Bodiga, V.L.; Eda, S.R.; Bodiga, S. Advanced glycation end products: Role in pathology of diabetic cardiomyopathy. Heart Fail. Rev. 2014, 19, 49–63. [Google Scholar] [CrossRef]
- Deluyker, D.; Evens, L.; Bito, V. Advanced glycation end products (AGEs) and cardiovascular dysfunction: Focus on high molecular weight AGEs. Amino Acids 2017, 49, 1535–1541. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and other Age-Related Diseases. Physiol. Rev. 2019, 100, 407–461. [Google Scholar] [CrossRef]
- Maessen, M.F.H.; Schalkwijk, C.G.; Verheggen, R.; Aengevaeren, V.L.; Hopman, M.T.E.; Eijsvogels, T.M.H. A comparison of dicarbonyl stress and advanced glycation endproducts in lifelong endurance athletes vs. sedentary controls. J. Sci. Med. Sport 2017, 20, 921–926. [Google Scholar] [CrossRef]
- Wright, K.J.; Thomas, M.M.; Betik, A.C.; Belke, D.; Hepple, R.T. Exercise training initiated in late middle age attenuates cardiac fibrosis and advanced glycation end-product accumulation in senescent rats. Exp. Gerontol. 2014, 50, 9–18. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Schalkwijk, C.G. Quantification of glyoxal, methylglyoxal and 3-deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: Evaluation of blood specimen. Clin. Chem. Lab. Med. 2014, 52, 85–91. [Google Scholar] [CrossRef]
- Al-Robaiy, S.; Kindermann, A.; Wodischeck, S.; Simm, A.; Treede, H.; Bartling, B. Long-term endurance running activity causes pulmonary changes depending on the receptor for advanced glycation end-products. Pflügers Arch. 2018, 470, 1543–1553. [Google Scholar] [CrossRef]
- Hansen, S.S.; Aasum, E.; Hafstad, A.D. The role of NADPH oxidases in diabetic cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1908–1913. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Sarkar, S.; Debnath, M.; Das, M.; Bandyopadhyay, A.; Dey, S.K.; Datta, G. Effect of high intensity interval training on antioxidant status, inflammatory response and muscle damage indices in endurance team male players. Apunt. Sports Med. 2021, 56, 100352. [Google Scholar] [CrossRef]
- Hancock, M.; Hafstad, A.D.; Nabeebaccus, A.A.; Catibog, N.; Logan, A.; Smyrnias, I.; Hansen, S.S.; Lanner, J.; Schröder, K.; Murphy, M.P.; et al. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. eLife 2018, 7, e41044. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.R.; Kannan, S.; Sadhaasivam, K.; Gounder, S.S.; Davidson, C.J.; Boeheme, C.; Hoidal, J.R.; Wang, L.; Rajasekaran, N.S. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic. Biol. Med. 2012, 52, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Ngcobo, S.R.; Nkambule, B.B.; Nyambuya, T.M.; Mokgalaboni, K.; Ntsethe, A.; Mxinwa, V.; Ziqubu, K.; Ntamo, Y.; Nyawo, T.A.; Dludla, P.V. Activated monocytes as a therapeutic target to attenuate vascular inflammation and lower cardiovascular disease-risk in patients with type 2 diabetes: A systematic review of preclinical and clinical studies. Biomed. Pharmacother. 2022, 146, 112579. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Jiménez, A.; Peón, A.N.; Terrazas, L.I. Alternatively activated macrophages in types 1 and 2 diabetes. Mediat. Inflamm. 2012, 2012, 815953. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.B.; Slentz, C.A.; Willis, L.H.; Hoselton, A.; Huebner, J.L.; Kraus, V.B.; Moss, J.; Muehlbauer, M.J.; Spielmann, G.; Muoio, D.M.; et al. Rejuvenation of Neutrophil Functions in Association with Reduced Diabetes Risk Following Ten Weeks of Low-Volume High Intensity Interval Walking in Older Adults with Prediabetes—A Pilot Study. Front. Immunol. 2020, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Jang, Y.C.; Quindry, J.; Guttmann, R.; Cosio-Lima, L.; Powers, S.K.; Lee, Y. Exercise-Induced Antisenescence and Autophagy Restoration Mitigate Metabolic Disorder–Induced Cardiac Disruption in Mice. Med. Sci. Sports Exerc. 2023, 55, 376–388. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Inder, W.J.; Roberts, T.J.; Brosnan, M.J.; Heidbuchel, H.; Prior, D.L. Relationship between Inflammatory Cytokines and Indices of Cardiac Dysfunction following Intense Endurance Exercise. PLoS ONE 2015, 10, e0130031. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.; Xu, Y.; Wang, W.; Zhuang, S.; Wang, R.; Xiao, W. HIIT Ameliorates Inflammation and Lipid Metabolism by Regulating Macrophage Polarization and Mitochondrial Dynamics in the Liver of Type 2 Diabetes Mellitus Mice. Metabolites 2022, 13, 14. [Google Scholar] [CrossRef]
- Fourny, N.; Beauloye, C.; Bernard, M.; Horman, S.; Desrois, M.; Bertrand, L. Sex Differences of the Diabetic Heart. Front. Physiol. 2021, 12, 661297. [Google Scholar] [CrossRef]
- Islam, R.A.; Khalsa, S.S.S.; Vyas, A.K.; Rahimian, R. Sex-Specific Impacts of Exercise on Cardiovascular Remodeling. J. Clin. Med. 2021, 10, 3833. [Google Scholar] [CrossRef]
| SED | MIT | HIIT | |
|---|---|---|---|
| PWTd (mm) | 2.78 ± 0.23 | 2.08 ± 0.08 ** | 1.98 ± 0.06 ** |
| PWTs (mm) | 3.93 ± 0.18 | 2.89 ± 0.06 ** | 3.08 ± 0.15 * |
| AWTd (mm) | 2.39 ± 0.16 | 2.16 ± 0.15 | 2.16 ± 0.08 |
| AWTs (mm) | 3.96 ± 0.19 | 3.66 ± 0.22 | 3.31 ± 0.11 * |
| EDV/BSA (µL/cm2) | 0.827 ± 0.048 | 0.944 ± 0.052 | 0.866 ± 0.064 |
| ESV/BSA (µL/cm2) | 0.212 ± 0.020 | 0.280 ± 0.030 | 0.296 ± 0.037 |
| Cardiac Index (mL/min/cm2) | 0.201 ± 0.018 | 0.201 ± 0.009 | 0.184 ± 0.017 |
| Radial FS (%) | 50 ± 3 | 41 ± 1 * | 40 ± 1 ** |
| EF (%) | 75 ± 1 | 70 ± 3 | 68 ± 2 |
| HR (bpm) | 342 ± 12 | 331 ± 10 | 321 ± 14 |
| BSA (cm2) | 828 ± 11 | 710 ± 14 *** | 715 ± 20 *** |
| SED | MIT | HIIT | |
|---|---|---|---|
| EDP (mmHg) | 8.6 ± 0.6 | 4.8 ± 1.3 | 6.5 ± 0.8 |
| ESP (mmHg) | 109 ± 3 | 99 ± 2 * | 93 ± 2 *** |
| Tau (s) | 0.0163 ± 0.0021 | 0.0108 ± 0.0007 ** | 0.0105 ± 0.0004 ** |
| SPTI (mmHg*s) | 7.983 ± 0.264 | 6.734 ± 0.263 * | 6.137 ± 0.336 ** |
| Diastolic Duration (s) | 0.0848 ± 0.0015 | 0.0917 ± 0.0044 | 0.0868 ± 0.0030 |
| Systolic Duration (s) | 0.0880 ± 0.0022 | 0.0799 ± 0.0017 * | 0.0851 ± 0.0017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Haese, S.; Claes, L.; de Laat, I.; Van Campenhout, S.; Deluyker, D.; Heeren, E.; Haesen, S.; Lambrichts, I.; Wouters, K.; Schalkwijk, C.G.; et al. Moderate-Intensity and High-Intensity Interval Exercise Training Offer Equal Cardioprotection, with Different Mechanisms, during the Development of Type 2 Diabetes in Rats. Nutrients 2024, 16, 431. https://doi.org/10.3390/nu16030431
D’Haese S, Claes L, de Laat I, Van Campenhout S, Deluyker D, Heeren E, Haesen S, Lambrichts I, Wouters K, Schalkwijk CG, et al. Moderate-Intensity and High-Intensity Interval Exercise Training Offer Equal Cardioprotection, with Different Mechanisms, during the Development of Type 2 Diabetes in Rats. Nutrients. 2024; 16(3):431. https://doi.org/10.3390/nu16030431
Chicago/Turabian StyleD’Haese, Sarah, Lisa Claes, Iris de Laat, Sven Van Campenhout, Dorien Deluyker, Ellen Heeren, Sibren Haesen, Ivo Lambrichts, Kristiaan Wouters, Casper G. Schalkwijk, and et al. 2024. "Moderate-Intensity and High-Intensity Interval Exercise Training Offer Equal Cardioprotection, with Different Mechanisms, during the Development of Type 2 Diabetes in Rats" Nutrients 16, no. 3: 431. https://doi.org/10.3390/nu16030431





