Akkermansia muciniphila Cell-Free Supernatant Improves Glucose and Lipid Metabolisms in Caenorhabditis elegans
Abstract
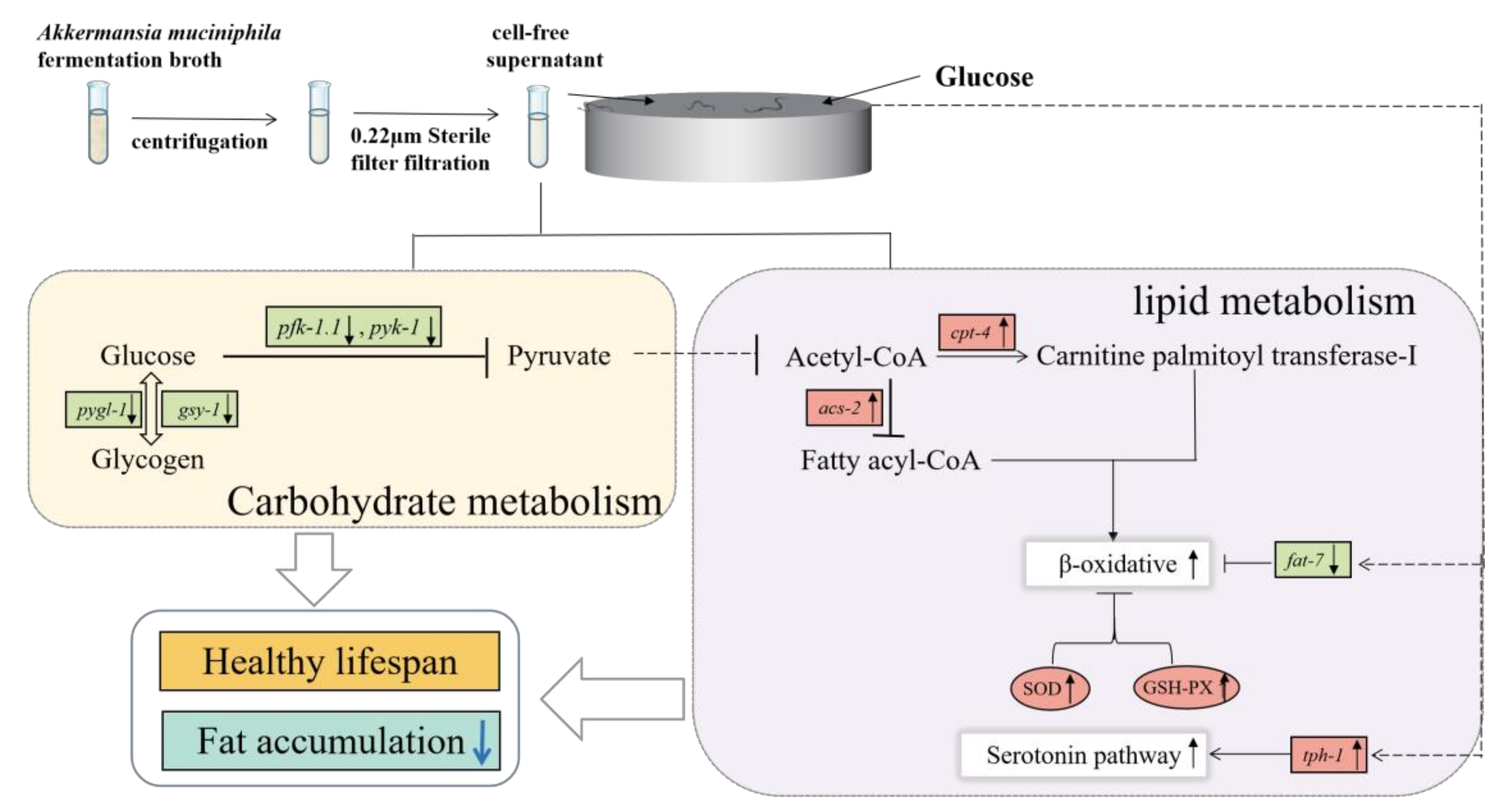
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Culture of Caenorhabditis elegans
2.4. Lifespan Analysis
2.5. Healthy Lifespan: Head Swings and Pharyngeal Pump Assay
2.6. Determination of Glycogen and Glucose Content in C. elegans
2.7. Determination of Triglycerides (TG) Content in C. elegans
2.8. Oil Red O Staining
2.9. Measurement of Reactive Oxygen Species (ROS)
2.10. Antioxidant Enzyme Assay
2.11. RNA Extraction and qRT-PCR
2.12. Statistical Analysis
3. Results
3.1. Effect of A. muciniphila Cell-Free Supernatant on Healthy Lifespan of C. elegans
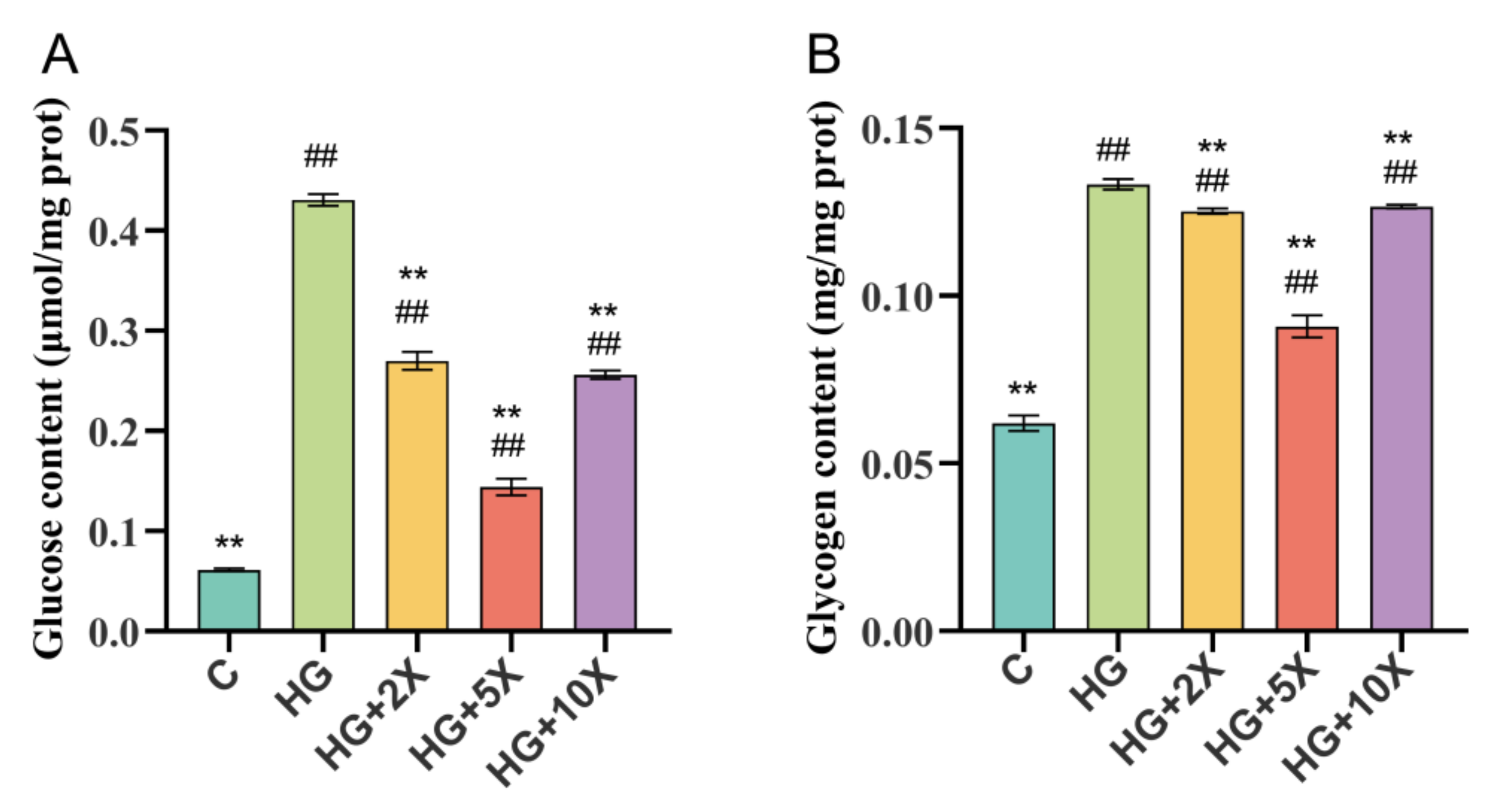
3.2. Effects of A. muciniphila Cell-Free Supernatant on Glycogen and Glucose Levels of C. elegans
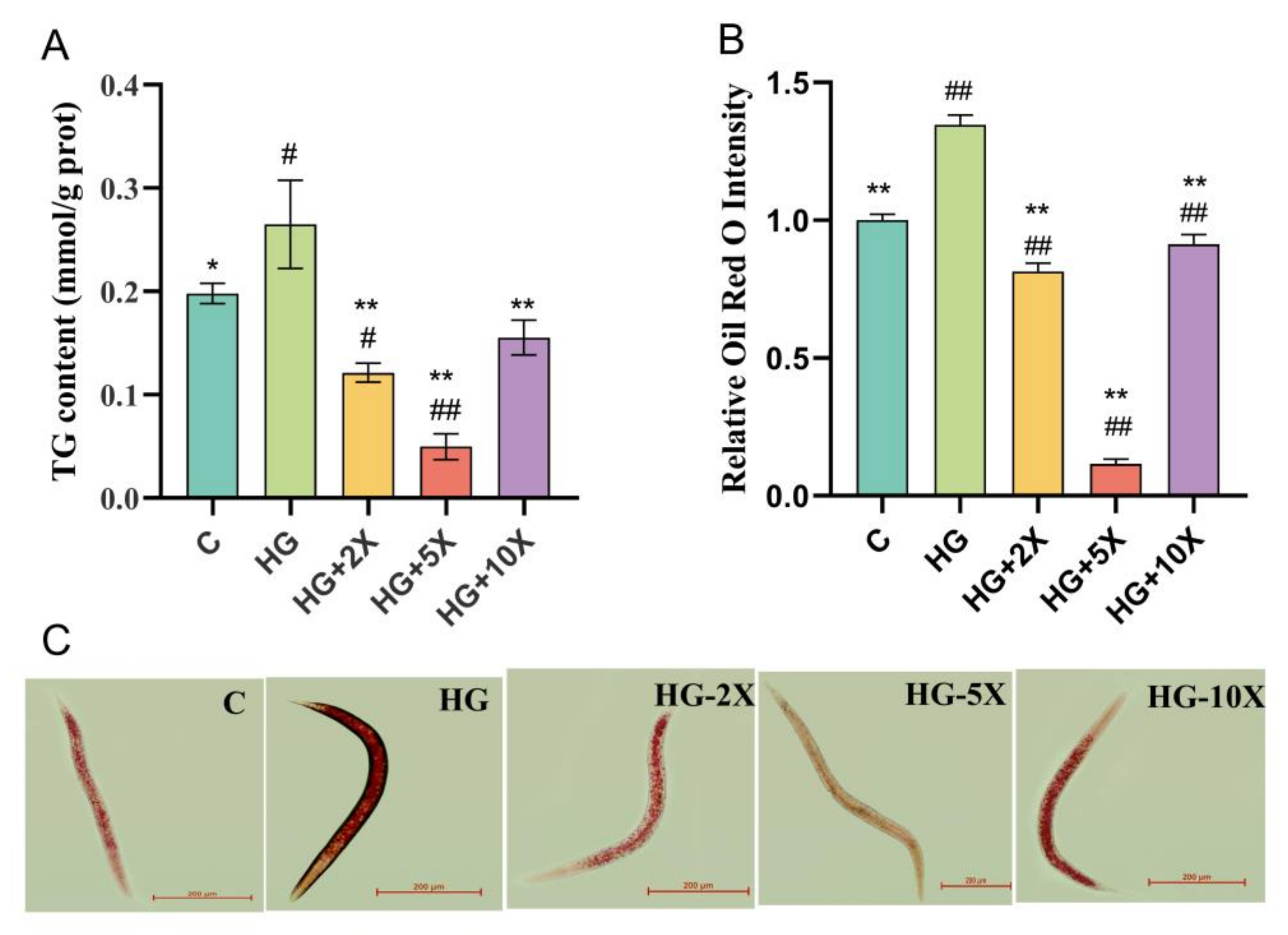
3.3. Effect of A. muciniphila Cell-Free Supernatant on the Level of TG in C. elegans
3.4. Effects of A. muciniphila Cell-Free Supernatant on Levels of Free Radicals and Antioxidant Enzymes in C. elegans
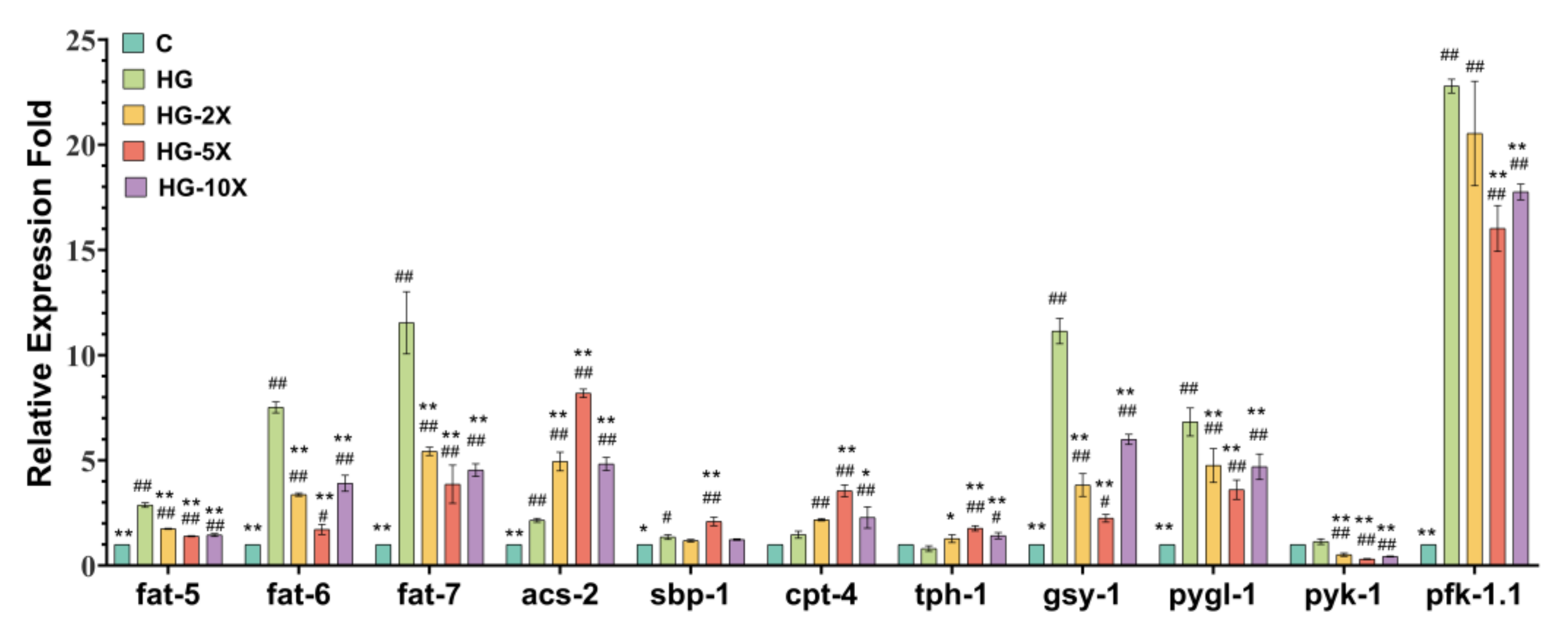
3.5. Effects of A. muciniphila Cell-Free Supernatant on Glucose and Lipid Key Gene Expression in C. elegans
4. Discussion
5. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez, C.; Romero, E.; Garrido-Sanchez, L.; Alcain-Martinez, G.; Andrade, R.J.; Taminiau, B.; Daube, G.; Garcia-Fuentes, E. Microbiota Insights in Clostridium Difficile Infection and Inflammatory Bowel Disease. Gut Microbes 2020, 12, 1725220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Passel, M.W.; Kant, R.; Zoetendal, E.G.; Plugge, C.M.; Derrien, M.; Malfatti, S.A.; Chain, P.S.; Woyke, T.; Palva, A.; de Vos, W.M.; et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS ONE 2011, 6, e16876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzeskowiak, L.; Gronlund, M.M.; Beckmann, C.; Salminen, S.; von Berg, A.; Isolauri, E. The impact of perinatal probiotic intervention on gut microbiota: Double-blind placebo-controlled trials in Finland and Germany. Anaerobe 2012, 18, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; De Vos, W.M. The Mucin Degrader Akkermansia muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [Green Version]
- Schneeberger, M.; Everard, A.; Gomez-Valades, A.G.; Matamoros, S.; Ramirez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F.; Wang, G. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog. Dis. 2018, 76, fty028. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, M.; Ohtake, N.; Kaneko, A.; Tsuchiya, N.; Imamura, S.; Iizuka, S.; Ishizawa, S.; Nishi, A.; Yamamoto, M.; Taketomi, A.; et al. Increase of Akkermansia muciniphila by a Diet Containing Japanese Traditional Medicine Bofutsushosan in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Nutrients 2020, 12, 839. [Google Scholar] [CrossRef] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-h.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Ottman, N.; Davids, M.; Suarez-Diez, M.; Boeren, S.; Schaap, P.J.; Martins Dos Santos, V.A.P.; Smidt, H.; Belzer, C.; de Vos, W.M. Genome-Scale Model and Omics Analysis of Metabolic Capacities of Akkermansia muciniphila Reveal a Preferential Mucin-Degrading Lifestyle. Appl. Environ. Microbiol. 2017, 83, e01014. [Google Scholar] [CrossRef] [Green Version]
- Davey, L.; Malkus, P.; Villa, M.; Dolat, L.; Holmes, Z.; Letourneau, J.; Ansaldo, E.; David, L.; Barton, G.; Valdivia, R. Mucin foraging enables Akkermansia muciniphila to compete against other microbes in the gut and to modulate host sterol biosynthesis. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio 2014, 5, e01438-14. [Google Scholar] [CrossRef] [Green Version]
- Gasmi, A.; Mujawdiya, P.K.; Pivina, L.; Dosa, A.; Semenova, Y.; Benahmed, A.G.; Bjorklund, G. Relationship between Gut Microbiota, Gut Hyperpermeability and Obesity. Curr. Med. Chem. 2021, 28, 827–839. [Google Scholar] [CrossRef]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Raftar, S.K.A.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Badi, S.A.; Khatami, S.; et al. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef]
- Martorell, P.; Llopis, S.; Gonzalez, N.; Chenoll, E.; Lopez-Carreras, N.; Aleixandre, A.; Chen, Y.; Karoly, E.D.; Ramon, D.; Genoves, S. Probiotic Strain Bifidobacterium animalis subsp. lactis CECT 8145 Reduces Fat Content and Modulates Lipid Metabolism and Antioxidant Response in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 3462–3472. [Google Scholar] [CrossRef] [PubMed]
- Nigon, V.M.; Felix, M.-A. History of research on C. elegans and other free-living nematodes as model organisms. WormBook Online Rev. C. Elegans Biol. 2017, 2017, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Watts, J.L.; Ristow, M. Lipid and Carbohydrate Metabolism in Caenorhabditis elegans. Genetics 2017, 207, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef]
- Escorcia, W.; Ruter, D.L.; Nhan, J.; Curran, S.P. Quantification of Lipid Abundance and Evaluation of Lipid Distribution in Caenorhabditis elegans by Nile Red and Oil Red O Staining. J. Vis. Exp. 2018, 133, e57352. [Google Scholar] [CrossRef]
- Ishita, Y.; Chihara, T.; Okumura, M. Serotonergic modulation of feeding behavior in Caenorhabditis elegans and other related nematodes. Neurosci. Res. 2020, 154, 9–19. [Google Scholar] [CrossRef]
- Solis, G.M.; Petrascheck, M. Measuring Caenorhabditis elegans life span in 96 well microtiter plates. J. Vis. Exp. 2011, 49, e2496. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-Q.; Li, K.; Ma, J.-K.; Li, Z.-J. Effects of ethanol intake on anti-oxidant responses and the lifespan of Caenorhabditis elegans. Cyta-J. Food 2019, 17, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Schifano, E.; Conta, G.; Preziosi, A.; Ferrante, C.; Batignani, G.; Mancini, P.; Tomassini, A.; Sciubba, F.; Scopigno, T.; Uccelletti, D.; et al. 2-hydroxyisobutyric acid (2-HIBA) modulates ageing and fat deposition in Caenorhabditis elegans. Front. Mol. Biosci. 2022, 9, 986022. [Google Scholar] [CrossRef] [PubMed]
- Almotayri, A.; Thomas, J.; Munasinghe, M.; Weerasinghe, M.; Heydarian, D.; Jois, M. Metabolic and behavioral effects of olanzapine and fluoxetine on the model organism Caenorhabditis elegans. Saudi Pharm. J. 2021, 29, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Del Valle Carranza, A.; Saragusti, A.; Chiabrando, G.A.; Carrari, F.; Asis, R. Corrigendum to ‘Effects of chlorogenic acid on thermal stress tolerance in C. elegans via HIF-1, HSF-1 and autophagy’. Phytomedicine 2020, 66, 153132. [Google Scholar] [CrossRef] [PubMed]
- Schifano, E.; Ficociello, G.; Vespa, S.; Ghosh, S.; Cipollo, J.F.; Talora, C.; Lotti, L.V.; Mancini, P.; Uccelletti, D. Pmr-1 gene affects susceptibility of Caenorhabditis elegans to Staphylococcus aureus infection through glycosylation and stress response pathways’ alterations. Virulence 2019, 10, 1013–1025. [Google Scholar] [CrossRef] [Green Version]
- Gusarov, I.; Pani, B.; Gautier, L.; Smolentseva, O.; Eremina, S.; Shamovsky, I.; Katkova-Zhukotskaya, O.; Mironov, A.; Nudler, E. Glycogen controls Caenorhabditis elegans lifespan and resistance to oxidative stress. Nat. Commun. 2017, 8, 15868. [Google Scholar] [CrossRef] [Green Version]
- Stuhr, N.L.; Nhan, J.D.; Hammerquist, A.M.; Van Camp, B.; Reoyo, D.; Curran, S.P. Rapid Lipid Quantification in Caenorhabditis elegans by Oil Red O and Nile Red Staining. Bio Protoc. 2022, 12, e4340. [Google Scholar] [CrossRef]
- Wang, K.; Chen, S.; Zhang, C.; Huang, J.; Wu, J.; Zhou, H.; Jin, L.; Qian, X.; Jin, J.; Lyu, J. Enhanced ROS production leads to excessive fat accumulation through DAF-16 in Caenorhabditis elegans. Exp. Gerontol. 2018, 112, 20–29. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Guo, X.; Zhang, M.; Ou, Z.; Wu, D.; Deng, L.; Lu, Z.; Zhang, J.; Deng, G.; Chen, S.; et al. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe 2020, 61, 102138. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P.; Kunze, W.; Bienenstock, J. Moody microbes or fecal phrenology: What do we know about the microbiota-gut-brain axis? BMC Med. 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimann, E.; Nyman, M.; Palbrink, A.K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraca, M.; Putignani, L.; Fierabracci, A.; Teti, A.; Perilongo, G. Gut Microbiota-derived Outer Membrane Vesicles: Under Recognized Major Players in Health and Disease? Discov. Med. 2015, 19, 343–348. [Google Scholar] [PubMed]
- Wu, Z.; Xiao, Y.; Zhou, F.; Chen, J.; Chen, X.; Hou, A.; Wang, Y.; Li, Z. Pasteurized Akkermansia muciniphila Reduces Fat Accumulation via nhr-49-Mediated Nuclear Hormone Signaling Pathway in Caenorhabditis elegans. Molecules 2022, 27, 6159. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef] [Green Version]
- Perez-Torres, I.; Castrejon-Tellez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zou, X.; Ding, Y.; Wang, H.; Wu, X.; Liang, B. Comparative genomics and functional study of lipid metabolic genes in Caenorhabditis elegans. BMC Genom. 2013, 14, 164. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Li, J.; Zhang, X.; Zhang, C.; Bai, J.; Zhao, Y.; Zhu, Y.; Zhang, J.; Xiao, X. Bisphenol S promotes fat storage in multiple generations of Caenorhabditis elegans in a daf-16/nhr-49 dependent manner. Comp. Biochem. Physiol. C Toxicol. Pharm. 2021, 250, 109175. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arriola, E.; El Hafidi, M.; Ortega-Cuellar, D.; Carvajal, K. AMP-Activated Protein Kinase Regulates Oxidative Metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 Transcriptional Regulators. PLoS ONE 2016, 11, e0148089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol 2017, 13, 710–730. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Fu, X.; Chen, J.; Ma, J. Application of Caenorhabditis elegans in Lipid Metabolism Research. Int. J. Mol. Sci. 2023, 24, 1173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Xiao, X.; Dong, Y.; Zhou, X.H. Fermented Barley Extracts with Lactobacillus plantarum dy-1 Rich in Vanillic Acid Modulate Glucose Consumption in Human HepG2 Cells. Biomed. Environ. Sci. 2018, 31, 667–676. [Google Scholar] [CrossRef]
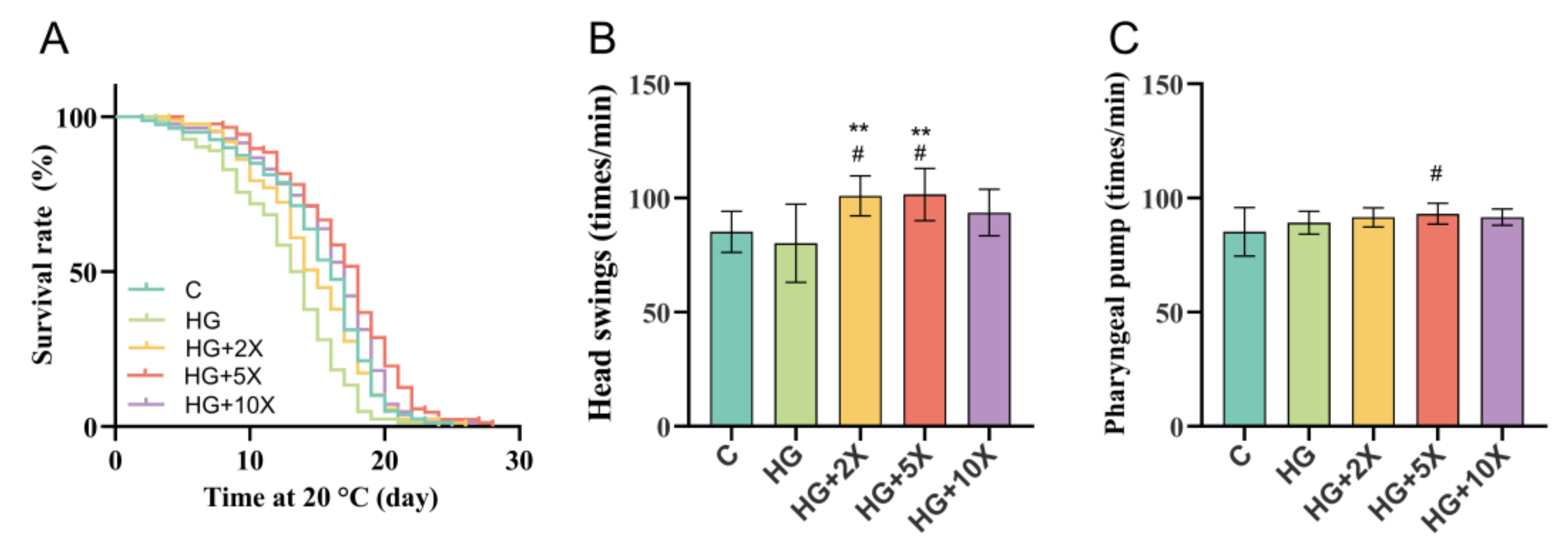

| Group | Number of Worms | Mean Lifespan (Days) | Maximum Lifespan (Days) | Median (Days) |
|---|---|---|---|---|
| C | 79 | 15.10 ± 0.50 | 25.00 | 16.00 |
| HG | 82 | 12.85 ± 0.47 | 24.00 | 13.50 |
| HG + 2× | 87 | 14.61 ± 0.46 | 26.00 | 15.00 |
| HG + 5× | 87 | 16.86 ± 0.48 | 28.00 | 18.00 |
| HG + 10× | 83 | 15.92 ± 0.50 | 28.00 | 17.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-Q.; Chen, X.-M.; Ma, H.-Q.; Li, K.; Wang, Y.-L.; Li, Z.-J. Akkermansia muciniphila Cell-Free Supernatant Improves Glucose and Lipid Metabolisms in Caenorhabditis elegans. Nutrients 2023, 15, 1725. https://doi.org/10.3390/nu15071725
Wu Z-Q, Chen X-M, Ma H-Q, Li K, Wang Y-L, Li Z-J. Akkermansia muciniphila Cell-Free Supernatant Improves Glucose and Lipid Metabolisms in Caenorhabditis elegans. Nutrients. 2023; 15(7):1725. https://doi.org/10.3390/nu15071725
Chicago/Turabian StyleWu, Zhong-Qin, Xin-Ming Chen, Hui-Qin Ma, Ke Li, Yuan-Liang Wang, and Zong-Jun Li. 2023. "Akkermansia muciniphila Cell-Free Supernatant Improves Glucose and Lipid Metabolisms in Caenorhabditis elegans" Nutrients 15, no. 7: 1725. https://doi.org/10.3390/nu15071725
APA StyleWu, Z.-Q., Chen, X.-M., Ma, H.-Q., Li, K., Wang, Y.-L., & Li, Z.-J. (2023). Akkermansia muciniphila Cell-Free Supernatant Improves Glucose and Lipid Metabolisms in Caenorhabditis elegans. Nutrients, 15(7), 1725. https://doi.org/10.3390/nu15071725






