Modulation of Dietary Fatty Acids in an Open-Label Study Improves Psoriasis and Dampens the Inflammatory Activation Status
Abstract
1. Introduction
2. Methods
2.1. Human Study
2.2. Serum Biomarkers
2.3. Lipid Extraction and Analysis of Free Fatty Acids
2.4. Statistics
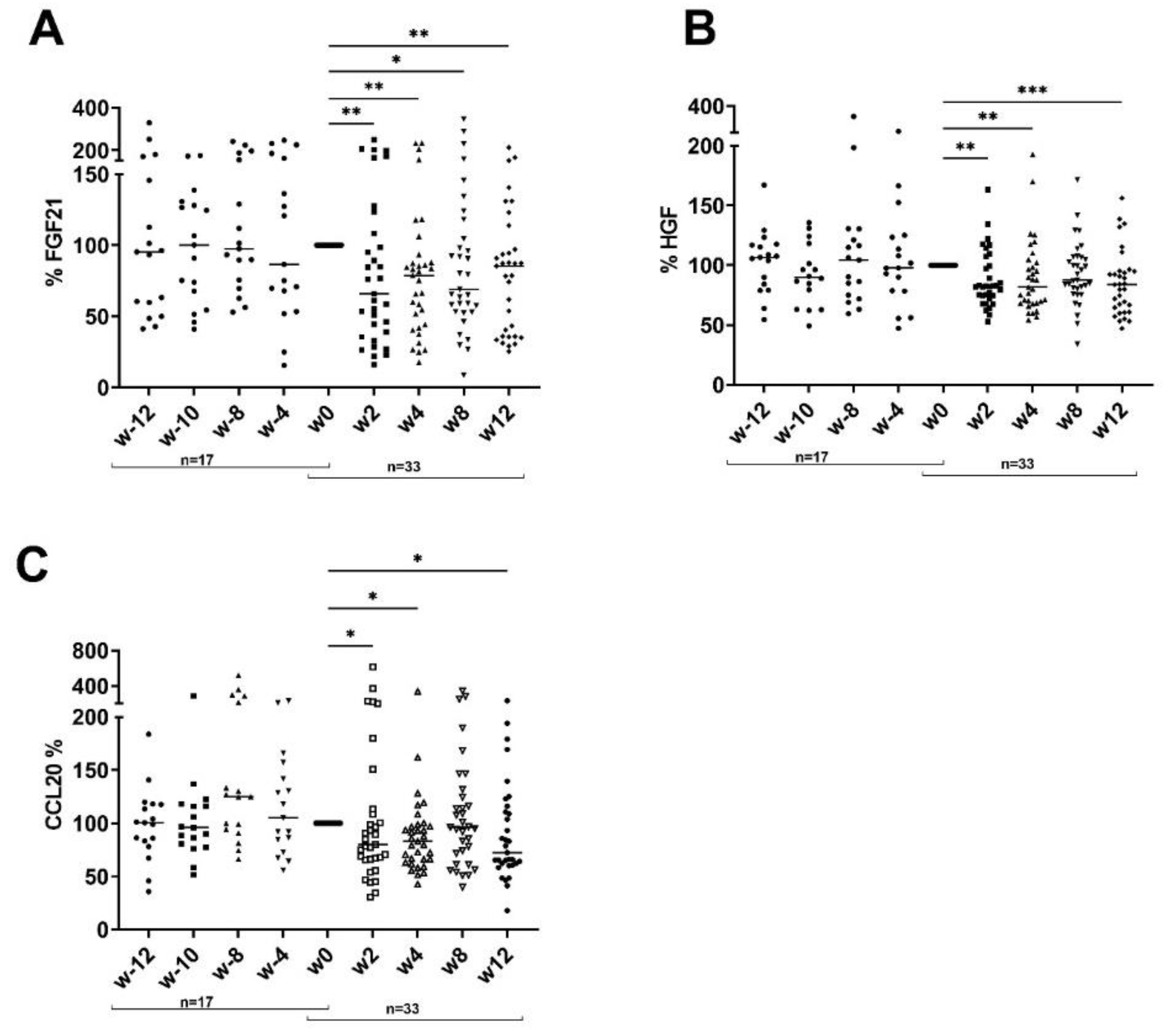
3. Results
3.1. Dietary Modulation of FFA Reduces Disease Severity in Patients with Psoriasis
3.2. Impact of Dietary Intervention on Inflammatory Serum Cytokine Pattern
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kivimäki, M.; Strandberg, T.; Pentti, J.; Nyberg, S.T.; Frank, P.; Jokela, M.; Ervasti, J.; Suominen, S.B.; Vahtera, J.; Sipilä, P.N.; et al. Body-Mass Index and Risk of Obesity-Related Complex Multimorbidity: An Observational Multicohort Study. Lancet Diabetes Endocrinol. 2022, 10, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M. Weighing in on Asthma. Sci. Transl. Med. 2020, 12, eaba2907. [Google Scholar] [CrossRef]
- Duan, L.; Wu, R.; Zhang, X.; Wang, D.; You, Y.; Zhang, Y.; Zhou, L.; Chen, W. HBx-Induced S100A9 in NF-ΚB Dependent Manner Promotes Growth and Metastasis of Hepatocellular Carcinoma Cells. Cell Death Dis. 2018, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Yokote, K.; Nakayama, T. The Obesity-Related Pathology and Th17 Cells. Cell Mol. Life Sci. 2017, 74, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Gremese, E.; Tolusso, B.; Gigante, M.R.; Ferraccioli, G. Obesity as a Risk and Severity Factor in Rheumatic Diseases (Autoimmune Chronic Inflammatory Diseases). Front. Immunol. 2014, 5, 576. [Google Scholar] [CrossRef]
- Schiopu, A.; Cotoi, O.S. S100A8 and S100A9: DAMPs at the Crossroads between Innate Immunity, Traditional Risk Factors, and Cardiovascular Disease. Mediat. Inflamm. 2013, 2013, 828354. [Google Scholar] [CrossRef]
- Zeki, A.A. Twin Towers, Twin Epidemics: Obesity and Asthma. Sci. Transl. Med. 2012, 4, 163ec223. [Google Scholar] [CrossRef]
- Nakamizo, S.; Honda, T.; Adachi, A.; Nagatake, T.; Kunisawa, J.; Kitoh, A.; Otsuka, A.; Dainichi, T.; Nomura, T.; Ginhoux, F.; et al. High Fat Diet Exacerbates Murine Psoriatic Dermatitis by Increasing the Number of IL-17-Producing Γδ T Cells. Sci. Rep. 2017, 7, 14076. [Google Scholar] [CrossRef]
- Paik, J.; Fierce, Y.; Treuting, P.M.; Brabb, T.; Maggio-Price, L. High-Fat Diet-Induced Obesity Exacerbates Inflammatory Bowel Disease in Genetically Susceptible Mdr1a-/- Male Mice. J. Nutr. 2013, 143, 1240–1247. [Google Scholar] [CrossRef]
- Petronilho, F.; Giustina, A.D.; Nascimento, D.Z.; Zarbato, G.F.; Vieira, A.A.; Florentino, D.; Danielski, L.G.; Goldim, M.P.; Rezin, G.T.; Barichello, T. Obesity Exacerbates Sepsis-Induced Oxidativedamage in Organs. Inflammation 2016, 39, 2062–2071. [Google Scholar] [CrossRef]
- Shah, D.; Romero, F.; Duong, M.; Wang, N.; Paudyal, B.; Suratt, B.T.; Kallen, C.B.; Sun, J.; Zhu, Y.; Walsh, K.; et al. Obesity-Induced Adipokine Imbalance Impairs Mouse Pulmonary Vascular Endothelial Function and Primes the Lung for Injury. Sci. Rep. 2015, 5, 11362. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Yamakuchi, M.; Fukushige, T.; Ibusuki, A.; Hashiguchi, T.; Kanekura, T. High-Fat Diet Exacerbates Imiquimod-Induced Psoriasis-like Dermatitis in Mice. Exp. Dermatol. 2018, 27, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Setty, A.R.; Curhan, G.; Choi, H.K. Obesity, Waist Circumference, Weight Change, and the Risk of Psoriasis in Women: Nurses’ Health Study II. Arch. Intern. Med. 2007, 167, 1670–1675. [Google Scholar] [CrossRef]
- Snekvik, I.; Nilsen, T.I.L.; Romundstad, P.R.; Saunes, M. Metabolic Syndrome and Risk of Incident Psoriasis: Prospective Data from the HUNT Study, Norway. Br. J. Dermatol. 2019, 180, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm. Res. 2020, 13, S229691. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Kunz, M.; Simon, J.C.; Saalbach, A. Psoriasis: Obesity and Fatty Acids. Front. Immunol. 2019, 10, 1807. [Google Scholar] [CrossRef]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009. [Google Scholar] [CrossRef]
- Franz, S.; Ertel, A.; Engel, K.M.; Simon, J.C.; Saalbach, A. Overexpression of S100A9 in Obesity Impairs Macrophage Differentiation via TLR4-NFkB-Signaling Worsening Inflammation and Wound Healing. Theranostics 2022, 12, 1659–1682. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Engel, K.M.; Sampels, S.; Dzyuba, B.; Podhorec, P.; Policar, T.; Dannenberger, D.; Schiller, J. Swimming at Different Temperatures: The Lipid Composition of Sperm from Three Freshwater Fish Species Determined by Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy. Chem. Phys. Lipids. 2019, 221, 65–72. [Google Scholar] [CrossRef]
- Paroutoglou, K.; Papadavid, E.; Christodoulatos, G.S.; Dalamaga, M. Deciphering the Association between Psoriasis and Obesity: Current Evidence and Treatment Considerations. Curr. Obes. Rep. 2020, 9, 165–178. [Google Scholar] [CrossRef]
- Ford, A.R.; Siegel, M.; Bagel, J.; Cordoro, K.M.; Garg, A.; Gottlieb, A.; Green, L.J.; Gudjonsson, J.E.; Koo, J.; Lebwohl, M.; et al. Dietary Recommendations for Adults With Psoriasis or Psoriatic Arthritis From the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2018, 154, 934. [Google Scholar] [CrossRef]
- Henderson, G.C. Plasma Free Fatty Acid Concentration as a Modifiable Risk Factor for Metabolic Disease. Nutrients 2021, 13, 2590. [Google Scholar] [CrossRef]
- Consuegra-Fernández, M.; Julià, M.; Martínez-Florensa, M.; Aranda, F.; Català, C.; Armiger-Borràs, N.; Arias, M.-T.; Santiago, F.; Guilabert, A.; Esteve, A.; et al. Genetic and Experimental Evidence for the Involvement of the CD6 Lymphocyte Receptor in Psoriasis. Cell Mol. Immunol. 2018, 15, 898–906. [Google Scholar] [CrossRef]
- De la Calle-Martin, O.; Hernandez, M.; Ordi, J.; Casamitjana, N.; Arostegui, J.I.; Caragol, I.; Ferrando, M.; Labrador, M.; Rodriguez-Sanchez, J.L.; Espanol, T. Familial CD8 Deficiency Due to a Mutation in the CD8α Gene. J. Clin. Investig. 2001, 108, 117–123. [Google Scholar] [CrossRef]
- Howie, D.; Simarro, M.; Sayos, J.; Guirado, M.; Sancho, J.; Terhorst, C. Molecular Dissection of the Signaling and Costimulatory Functions of CD150 (SLAM): CD150/SAP Binding and CD150-Mediated Costimulation. Blood 2002, 99, 957–965. [Google Scholar] [CrossRef]
- Dogra, S.; Shabeer, D.; Rajagopalan, M. Anti-CD6 MAbs for the Treatment of Psoriasis. Expert Opin. Biol. Ther. 2020, 20, 1215–1222. [Google Scholar] [CrossRef]
- Furue, K.; Ito, T.; Tsuji, G.; Nakahara, T.; Furue, M. The CCL20 and CCR6 Axis in Psoriasis. Scand. J. Immunol. 2020, 91, 12846. [Google Scholar] [CrossRef]
- Getschman, A.E.; Imai, Y.; Larsen, O.; Peterson, F.C.; Wu, X.; Rosenkilde, M.M.; Hwang, S.T.; Volkman, B.F. Protein Engineering of the Chemokine CCL20 Prevents Psoriasiform Dermatitis in an IL-23–Dependent Murine Model. Proc. Natl. Acad. Sci. USA 2017, 114, 12460–12465. [Google Scholar] [CrossRef]
- Lanna, C.; Mancini, M.; Gaziano, R.; Cannizzaro, M.V.; Galluzzo, M.; Talamonti, M.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Wang, Y.; et al. Skin Immunity and Its Dysregulation in Psoriasis. Cell Cycle 2019, 18, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Vranova, M.; Friess, M.C.; Haghayegh Jahromi, N.; Collado-Diaz, V.; Vallone, A.; Hagedorn, O.; Jadhav, M.; Willrodt, A.-H.; Polomska, A.; Leroux, J.-C.; et al. Opposing Roles of Endothelial and Leukocyte-Expressed IL-7Rα in the Regulation of Psoriasis-like Skin Inflammation. Sci. Rep. 2019, 9, 11714. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.L.; Huang, Y.; Gao, Y.L.; Sun, Y.Z.; Han, Y.; Chen, H.D.; Gao, X.H.; Qi, R.Q. Interleukin-18 Exacerbates Skin Inflammation and Affects Microabscesses and Scale Formation in a Mouse Model of Imiquimod-Induced Psoriasis. Chin. Med. J. 2019, 132, 690–698. [Google Scholar] [CrossRef]
- Wilsmann-Theis, D.; Wagenpfeil, J.; Holzinger, D.; Roth, J.; Koch, S.; Schnautz, S.; Bieber, T.; Wenzel, J. Among the S100 Proteins, S100A12 Is the Most Significant Marker for Psoriasis Disease Activity. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1165–1170. [Google Scholar] [CrossRef]
- Delehedde, M.; Vidyasagar, R.; Fernig, D.G.; Lyon, M.; McDonnell, T.J. Hepatocyte Growth Factor/Scatter Factor Binds to Small Heparin-Derived Oligosaccharides and Stimulates the Proliferation of Human HaCaT Keratinocytes. J. Biol. Chem. 2002, 277, 12456–12462. [Google Scholar] [CrossRef]
- Hisadome, M.; Ohnishi, T.; Kakimoto, K.; Kusuyama, J.; Bandow, K.; Kanekura, T.; Matsuguchi, T. Hepatocyte Growth Factor Reduces CXCL10 Expression in Keratinocytes. FEBS Lett. 2016, 590, 3595–3605. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Miccono, A.; Naso, M.; Nichetti, M.; Riva, A.; Guerriero, F.; De Gregori, M.; Peroni, G.; Perna, S. Food Pyramid for Subjects with Chronic Pain: Foods and Dietary Constituents as Anti-Inflammatory and Antioxidant Agents. Nutr. Res. Rev. 2018, 31, 131–151. [Google Scholar] [CrossRef]

| PASI w4 | PASI w8 | TAG w4 | TAG w8 | BMI w4 | BMI w8 | ||
|---|---|---|---|---|---|---|---|
| PASI | r | 0.197 | 0.342 | −0.059 | 0.169 | ||
| p | 0.316 | 0.069 | 0.767 | 0.382 | |||
| WHR | r | −0.021 | −0.291 | −0.128 | 0.087 | 0.089 | 0.144 |
| p | 0.914 | 0.125 | 0.518 | 0.655 | 0.651 | 0.456 | |
| BMI | r | −0.059 | 0.169 | 0.375 * | 0.460 * | ||
| p | 0.767 | 0.382 | 0.049 | 0.012 | |||
| TAG | r | 0.197 | 0.342 | 0.375 * | 0.460 * | ||
| p | 0.316 | 0.069 | 0.049 | 0.012 | |||
| IL8 | r | 0.187 | 0.215 | 0.226 | 0.192 | −0.339 | −0.280 |
| p | 0.341 | 0.262 | 0.249 | 0.319 | 0.078 | 0.141 | |
| VEGFA | r | 0.207 | −0.100 | 0.262 | 0.149 | −0.091 | 0.069 |
| p | 0.290 | 0.604 | 0.178 | 0.440 | 0.646 | 0.723 | |
| CD8A | r | 0.138 | −0.011 | 0.317 | 0.167 | −0.378 * | −0.103 |
| p | 0.483 | 0.956 | 0.100 | 0.387 | 0.047 | 0.596 | |
| MCP-3 | r | 0.170 | −0.091 | 0.170 | −0.073 | −0.405 * | −0.246 |
| p | 0.386 | 0.640 | 0.386 | 0.705 | 0.032 | 0.198 | |
| GDNF | r | 0.067 | −0.125 | −0.006 | 0.004 | −0.116 | 0.168 |
| p | 0.737 | 0.519 | 0.976 | 0.982 | 0.555 | 0.384 | |
| CDCP1 | r | 0.132 | 0.346 | 0.250 | 0.467 * | −0.234 | 0.180 |
| p | 0.503 | 0.066 | 0.200 | 0.011 | 0.231 | 0.351 | |
| CD244 | r | 0.123 | 0.051 | 0.180 | 0.119 | −0.318 | −0.242 |
| p | 0.534 | 0.792 | 0.360 | 0.538 | 0.100 | 0.205 | |
| IL-7 | r | −0.008 | 0.429 * | 0.300 | 0.219 | −0.144 | −0.107 |
| p | 0.967 | 0.020 | 0.121 | 0.254 | 0.464 | 0.579 | |
| OPG | r | 0.097 | 0.317 | 0.263 | 0.340 | −0.012 | −0.083 |
| p | 0.622 | 0.094 | 0.177 | 0.071 | 0.953 | 0.668 | |
| LAPTGF-beta-1 | r | 0.201 | 0.400 * | 0.097 | 0.341 | 0.055 | 0.133 |
| p | 0.305 | 0.032 | 0.622 | 0.070 | 0.781 | 0.493 | |
| uPA | r | 0.089 | 0.244 | 0.025 | 0.075 | −0.348 | −0.289 |
| p | 0.652 | 0.202 | 0.901 | 0.698 | 0.070 | 0.128 | |
| IL6 | r | 0.118 | 0.561 ** | 0.054 | 0.243 | −0.005 | −0.172 |
| p | 0.549 | 0.002 | 0.785 | 0.204 | 0.981 | 0.372 | |
| IL-17C | r | 0.143 | 0.024 | 0.105 | 0.117 | 0.099 | 0.272 |
| p | 0.468 | 0.901 | 0.597 | 0.546 | 0.616 | 0.154 | |
| MCP-1 | r | 0.234 | 0.046 | 0.223 | 0.154 | −0.099 | 0.064 |
| p | 0.232 | 0.813 | 0.253 | 0.426 | 0.616 | 0.741 | |
| IL-17A | r | 0.120 | 0.152 | 0.232 | 0.264 | 0.087 | 0.281 |
| p | 0.544 | 0.431 | 0.235 | 0.166 | 0.661 | 0.140 | |
| CXCL11 | r | 0.235 | 0.296 | 0.035 | 0.066 | −0.309 | −0.337 |
| p | 0.229 | 0.119 | 0.860 | 0.734 | 0.109 | 0.074 | |
| AXIN1 | r | 0.368 | 0.018 | 0.039 | −0.092 | −0.223 | −0.053 |
| p | 0.054 | 0.925 | 0.845 | 0.636 | 0.253 | 0.786 | |
| TRAIL | r | −0.056 | −0.022 | 0.178 | −0.014 | −0.103 | −0.205 |
| p | 0.779 | 0.909 | 0.364 | 0.941 | 0.602 | 0.287 | |
| IL-20RA | r | 0.045 | −0.279 | 0.122 | 0.164 | −0.227 | −0.067 |
| p | 0.819 | 0.142 | 0.536 | 0.397 | 0.246 | 0.731 | |
| CXCL9 | r | 0.149 | 0.158 | 0.102 | 0.088 | −0.411 * | −0.058 |
| p | 0.449 | 0.414 | 0.607 | 0.649 | 0.030 | 0.765 | |
| CST5 | r | 0.380 * | −0.019 | 0.485 ** | −0.006 | 0.130 | 0.035 |
| p | 0.046 | 0.923 | 0.009 | 0.976 | 0.509 | 0.856 | |
| OSM | r | 0.208 | 0.154 | 0.257 | 0.027 | 0.247 | 0.132 |
| p | 0.288 | 0.426 | 0.186 | 0.889 | 0.204 | 0.496 | |
| CXCL1 | r | −0.119 | 0.008 | 0.010 | −0.265 | −0.134 | −0.420 * |
| p | 0.546 | 0.968 | 0.960 | 0.166 | 0.496 | 0.023 | |
| CCL4 | r | 0.160 | 0.075 | 0.362 | 0.185 | −0.116 | 0.113 |
| p | 0.415 | 0.698 | 0.058 | 0.337 | 0.558 | 0.560 | |
| CD6 | r | 0.439 * | 0.175 | 0.447 * | 0.055 | −0.087 | 0.062 |
| p | 0.019 | 0.363 | 0.017 | 0.778 | 0.661 | 0.751 | |
| SCF | r | −0.037 | −0.091 | 0.125 | −0.237 | 0.036 | −0.151 |
| p | 0.853 | 0.640 | 0.526 | 0.216 | 0.856 | 0.435 | |
| IL-18 | r | 0.402 * | 0.433 * | 0.266 | 0.287 | −0.297 | 0.033 |
| p | 0.034 | 0.019 | 0.172 | 0.131 | 0.125 | 0.865 | |
| SLAMF1 | r | 0.171 | 0.200 | 0.314 | 0.118 | 0.020 | 0.398 * |
| p | 0.384 | 0.299 | 0.103 | 0.541 | 0.921 | 0.032 | |
| TGF-alpha | r | 0.088 | 0.063 | 0.101 | 0.017 | 0.121 | 0.079 |
| p | 0.657 | 0.745 | 0.610 | 0.929 | 0.539 | 0.683 | |
| MCP-4 | r | 0.127 | 0.068 | 0.373 | 0.099 | −0.211 | −0.019 |
| p | 0.520 | 0.726 | 0.050 | 0.609 | 0.280 | 0.920 | |
| CCL11 | r | 0.199 | −0.040 | 0.301 | −0.050 | −0.130 | 0.011 |
| p | 0.309 | 0.837 | 0.120 | 0.796 | 0.510 | 0.957 | |
| TNFSF14 | r | 0.172 | 0.015 | 0.078 | 0.008 | 0.036 | 0.075 |
| p | 0.382 | 0.939 | 0.694 | 0.968 | 0.855 | 0.700 | |
| MMP-1 | r | −0.007 | −0.116 | −0.095 | −0.057 | −0.363 | −0.258 |
| p | 0.971 | 0.548 | 0.630 | 0.770 | 0.058 | 0.176 | |
| LIF-R | r | −0.015 | 0.321 | 0.365 | 0.289 | −0.128 | −0.005 |
| p | 0.938 | 0.090 | 0.056 | 0.128 | 0.515 | 0.978 | |
| FGF-21 | r | −0.244 | −0.135 | 0.027 | −0.219 | 0.060 | 0.084 |
| p | 0.211 | 0.485 | 0.890 | 0.253 | 0.764 | 0.665 | |
| CCL19 | r | 0.188 | 0.436 * | 0.388 * | 0.301 | 0.001 | 0.131 |
| p | 0.339 | 0.018 | 0.041 | 0.112 | 0.997 | 0.499 | |
| IL-10RB | r | 0.211 | 0.458 * | 0.606 ** | 0.500 ** | 0.103 | 0.188 |
| p | 0.282 | 0.012 | 0.001 | 0.006 | 0.601 | 0.328 | |
| IL-18R1 | r | 0.377 * | 0.385 * | 0.509 ** | 0.398 * | 0.191 | 0.252 |
| p | 0.048 | 0.039 | 0.006 | 0.032 | 0.331 | 0.186 | |
| PD-L1 | r | 0.240 | 0.159 | 0.276 | 0.322 | −0.282 | 0.000 |
| p | 0.219 | 0.411 | 0.154 | 0.089 | 0.146 | 0.999 | |
| CXCL5 | r | −0.090 | 0.111 | 0.022 | −0.079 | −0.075 | −0.334 |
| p | 0.651 | 0.565 | 0.912 | 0.684 | 0.706 | 0.076 | |
| TRANCE | r | −0.128 | −0.203 | 0.227 | −0.062 | −0.068 | 0.020 |
| p | 0.517 | 0.290 | 0.246 | 0.751 | 0.732 | 0.917 | |
| HGF | r | 0.146 | 0.186 | 0.291 | 0.033 | 0.119 | 0.142 |
| p | 0.457 | 0.333 | 0.134 | 0.867 | 0.547 | 0.461 | |
| IL-12B | r | 0.137 | 0.029 | 0.514 ** | 0.268 | −0.066 | 0.019 |
| p | 0.488 | 0.883 | 0.005 | 0.159 | 0.740 | 0.920 | |
| MMP-10 | r | −0.077 | 0.323 | 0.276 | 0.204 | −0.114 | 0.177 |
| p | 0.697 | 0.088 | 0.155 | 0.289 | 0.565 | 0.357 | |
| IL-10 | r | 0.105 | 0.309 | 0.445 * | 0.485 ** | 0.070 | 0.244 |
| p | 0.594 | 0.103 | 0.018 | 0.008 | 0.725 | 0.201 | |
| TNF | r | 0.255 | 0.193 | 0.390 * | 0.207 | −0.230 | −0.078 |
| p | 0.191 | 0.316 | 0.040 | 0.282 | 0.239 | 0.689 | |
| CCL23 | r | −0.135 | −0.137 | 0.017 | −0.145 | −0.447 * | −0.281 |
| p | 0.493 | 0.479 | 0.932 | 0.452 | 0.017 | 0.139 | |
| CD5 | r | 0.083 | 0.210 | 0.348 | −0.011 | −0.183 | −0.027 |
| p | 0.673 | 0.273 | 0.070 | 0.953 | 0.351 | 0.889 | |
| CCL3 | r | 0.162 | 0.184 | 0.530 ** | 0.180 | −0.064 | 0.032 |
| p | 0.409 | 0.340 | 0.004 | 0.349 | 0.747 | 0.868 | |
| Flt3L | r | 0.199 | 0.322 | 0.562 ** | 0.319 | 0.307 | 0.255 |
| p | 0.311 | 0.088 | 0.002 | 0.092 | 0.112 | 0.182 | |
| CXCL6 | r | 0.015 | 0.019 | 0.111 | 0.011 | −0.135 | −0.246 |
| p | 0.938 | 0.923 | 0.575 | 0.956 | 0.494 | 0.199 | |
| CXCL10 | r | 0.125 | 0.312 | 0.195 | 0.156 | −0.273 | −0.032 |
| p | 0.525 | 0.100 | 0.320 | 0.420 | 0.161 | 0.869 | |
| 4E-BP1 | r | 0.232 | −0.192 | 0.033 | −0.249 | −0.158 | −0.319 |
| p | 0.235 | 0.319 | 0.866 | 0.192 | 0.423 | 0.091 | |
| SIRT2 | r | 0.336 | 0.014 | −0.015 | −0.140 | −0.154 | −0.278 |
| p | 0.081 | 0.941 | 0.938 | 0.469 | 0.434 | 0.145 | |
| DNER | r | 0.284 | 0.104 | 0.527 ** | 0.182 | 0.069 | 0.014 |
| p | 0.143 | 0.590 | 0.004 | 0.345 | 0.728 | 0.941 | |
| EN-RAGE(S100A12) | r | 0.429 * | 0.198 | 0.074 | −0.276 | −0.286 | −0.367 |
| p | 0.023 | 0.304 | 0.709 | 0.147 | 0.140 | 0.050 | |
| CD40 | r | 0.229 | −0.079 | 0.292 | 0.044 | −0.157 | 0.078 |
| p | 0.241 | 0.683 | 0.131 | 0.819 | 0.424 | 0.686 | |
| IFN-gamma | r | 0.228 | 0.437 * | 0.242 | 0.231 | −0.333 | −0.157 |
| p | 0.243 | 0.018 | 0.214 | 0.228 | 0.083 | 0.416 | |
| FGF-19 | r | 0.316 | −0.110 | 0.340 | 0.130 | 0.114 | 0.131 |
| p | 0.102 | 0.571 | 0.076 | 0.503 | 0.565 | 0.497 | |
| LIF | r | 0.366 | −0.036 | −0.250 | −0.143 | −0.079 | −0.205 |
| p | 0.055 | 0.853 | 0.200 | 0.460 | 0.691 | 0.287 | |
| MCP-2 | r | 0.208 | 0.096 | 0.394 * | 0.095 | −0.156 | −0.102 |
| p | 0.289 | 0.620 | 0.038 | 0.626 | 0.427 | 0.599 | |
| CASP8 | r | 0.451 * | −0.028 | 0.108 | −0.194 | −0.193 | −0.287 |
| p | 0.016 | 0.887 | 0.586 | 0.313 | 0.324 | 0.131 | |
| CCL25 | r | 0.231 | 0.077 | 0.632 ** | 0.153 | 0.147 | 0.325 |
| p | 0.237 | 0.690 | 0.000 | 0.429 | 0.456 | 0.085 | |
| CX3CL1 | r | 0.022 | −0.003 | 0.279 | −0.008 | −0.260 | −0.179 |
| p | 0.910 | 0.986 | 0.150 | 0.966 | 0.181 | 0.353 | |
| TNFRSF9 | r | 0.210 | −0.035 | 0.312 | −0.135 | −0.178 | −0.300 |
| p | 0.283 | 0.855 | 0.106 | 0.485 | 0.364 | 0.114 | |
| TWEAK | r | 0.070 | −0.021 | 0.141 | −0.069 | −0.322 | −0.307 |
| p | 0.725 | 0.913 | 0.475 | 0.722 | 0.095 | 0.105 | |
| CCL20 | r | 0.165 | 0.249 | 0.234 | −0.208 | 0.075 | 0.057 |
| p | 0.403 | 0.193 | 0.230 | 0.278 | 0.706 | 0.767 | |
| ST1A1 | r | −0.064 | −0.137 | −0.045 | −0.145 | −0.110 | 0.196 |
| p | 0.746 | 0.479 | 0.821 | 0.454 | 0.578 | 0.309 | |
| STAMBP | r | 0.348 | −0.058 | 0.004 | −0.219 | −0.117 | −0.312 |
| p | 0.069 | 0.766 | 0.985 | 0.253 | 0.553 | 0.100 | |
| ADA | r | 0.285 | 0.118 | 0.201 | 0.209 | −0.113 | −0.034 |
| p | 0.142 | 0.543 | 0.304 | 0.277 | 0.568 | 0.862 | |
| TNFB | r | 0.146 | −0.023 | 0.083 | −0.143 | −0.462 * | −0.397 * |
| p | 0.459 | 0.905 | 0.674 | 0.460 | 0.013 | 0.033 | |
| CSF-1 | r | 0.205 | 0.103 | 0.104 | −0.096 | −0.328 | −0.159 |
| p | 0.296 | 0.595 | 0.599 | 0.620 | 0.088 | 0.410 |
| w4 | b-Value | p-Value |
| lgIL-18 | 0.285 | 0.107 |
| lgCST5 | 0.319 | 0.078 |
| lgIL6 | 0.397 | 0.03 |
| lg IL-18R | 0.299 | 0.104 |
| lg SIRT2 | 0.444 | 0.019 |
| lgENRAGE | 0.457 | 0.013 |
| lgCASP8 | 0.452 | 0.017 |
| w8 | b-value | p-value |
| lgIL-18 | 0.415 | 0.028 |
| lgIL-18R | 0.329 | 0.099 |
| lgIL-7 | 0.432 | 0.03 |
| lgIL-6 | 0.621 | 0.001 |
| lgLAPTGFb | 0.255 | 0.186 |
| lgCCL19 | 0.236 | 0.237 |
| lgIL-10RB | 0.355 | 0.057 |
| lgINFγ | 0.522 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saalbach, A.; Seitz, A.-T.; Kohlmann, J.; Kalweit, L.; Vogt, L.; Selig, L.; Engel, K.M.; Simon, J.C. Modulation of Dietary Fatty Acids in an Open-Label Study Improves Psoriasis and Dampens the Inflammatory Activation Status. Nutrients 2023, 15, 1698. https://doi.org/10.3390/nu15071698
Saalbach A, Seitz A-T, Kohlmann J, Kalweit L, Vogt L, Selig L, Engel KM, Simon JC. Modulation of Dietary Fatty Acids in an Open-Label Study Improves Psoriasis and Dampens the Inflammatory Activation Status. Nutrients. 2023; 15(7):1698. https://doi.org/10.3390/nu15071698
Chicago/Turabian StyleSaalbach, Anja, Anna-Theresa Seitz, Johannes Kohlmann, Lena Kalweit, Lisa Vogt, Lars Selig, Kathrin M. Engel, and Jan C. Simon. 2023. "Modulation of Dietary Fatty Acids in an Open-Label Study Improves Psoriasis and Dampens the Inflammatory Activation Status" Nutrients 15, no. 7: 1698. https://doi.org/10.3390/nu15071698
APA StyleSaalbach, A., Seitz, A.-T., Kohlmann, J., Kalweit, L., Vogt, L., Selig, L., Engel, K. M., & Simon, J. C. (2023). Modulation of Dietary Fatty Acids in an Open-Label Study Improves Psoriasis and Dampens the Inflammatory Activation Status. Nutrients, 15(7), 1698. https://doi.org/10.3390/nu15071698






