Role of Zerumbone, a Phytochemical Sesquiterpenoid from Zingiber zerumbet Smith, in Maintaining Macrophage Polarization and Redox Homeostasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Western Blotting Analysis
2.4. NO Assay
2.5. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.6. Phagocytosis Assay
2.7. Statistical Analysis
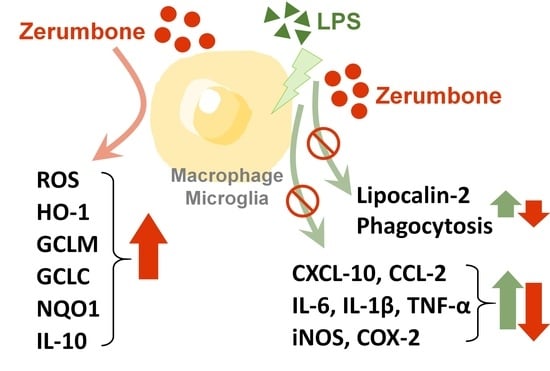
3. Results
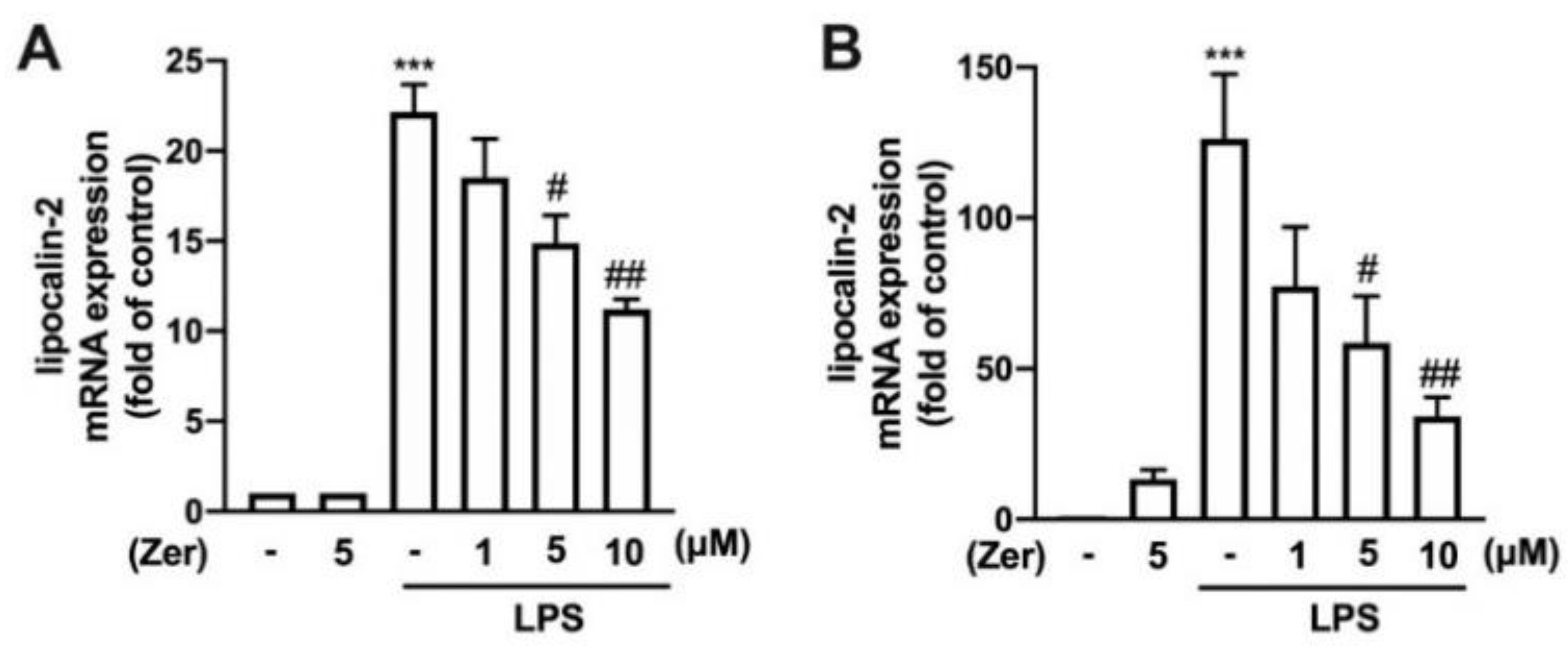
3.1. Zerumbone Lowers the Expression of Lipocalin-2 in Macrophages and Microglial Cells
3.2. Supplement with Zerumbone Decreases H2O2, ROO•, and HO• Production in Microglial Cells
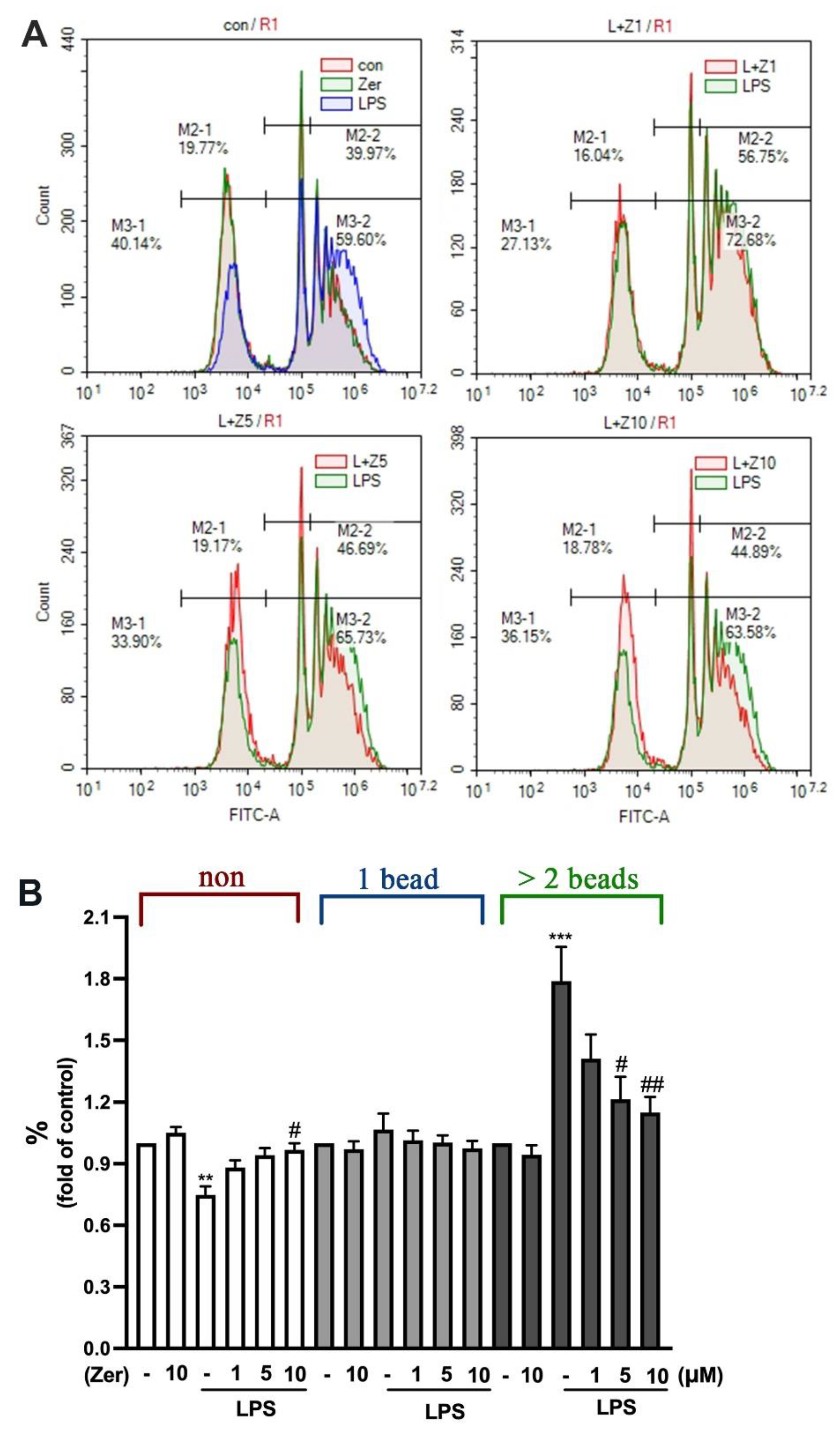
3.3. Inhibitory Effect of Zerumbone against Phagocytic Activity in Microglial Cells
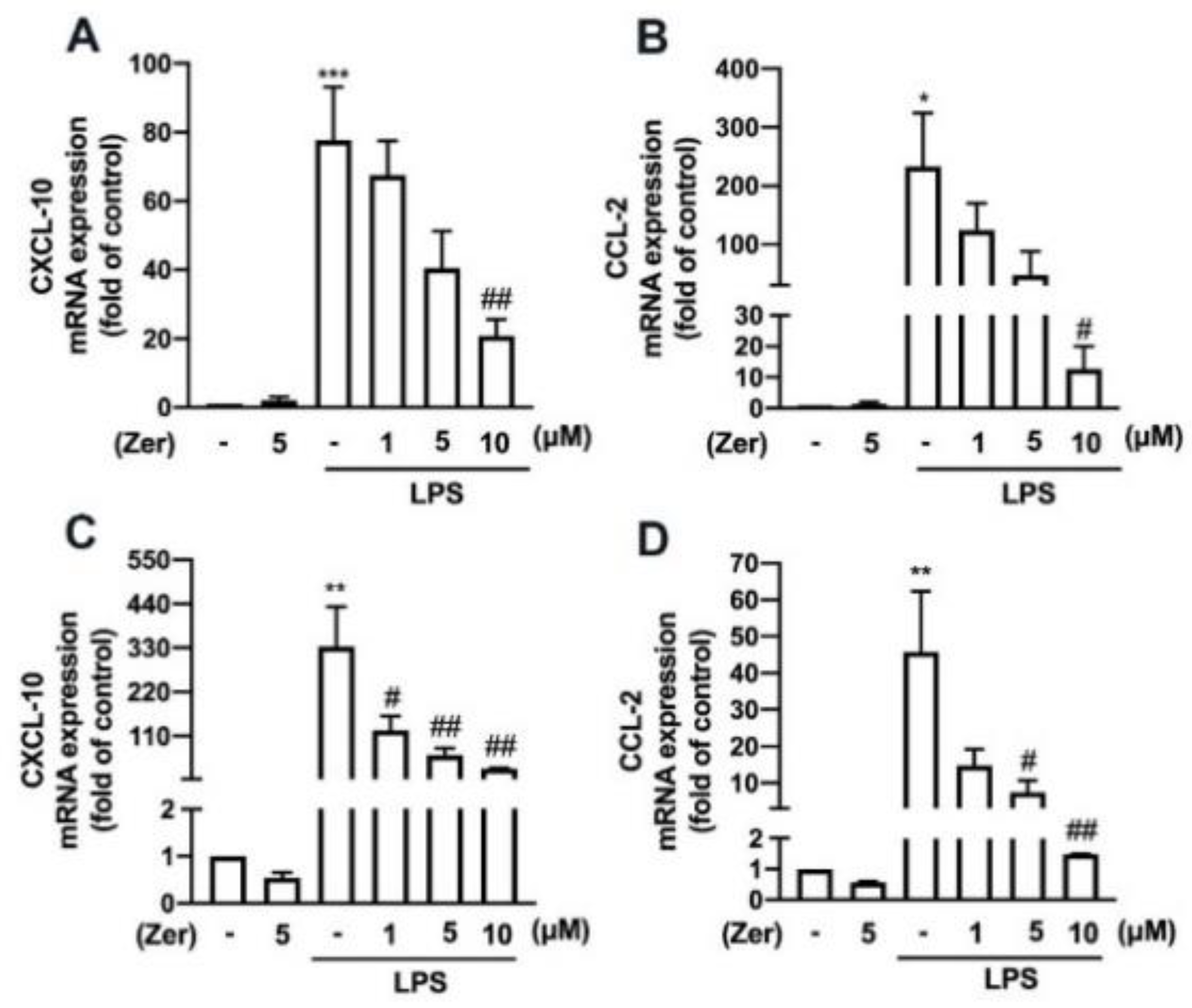
3.4. Zerumbone Reduces the Expression of Proinflammatory Mediators and M1-Macrophage Polarization Markers in Macrophages and Microglial Cells
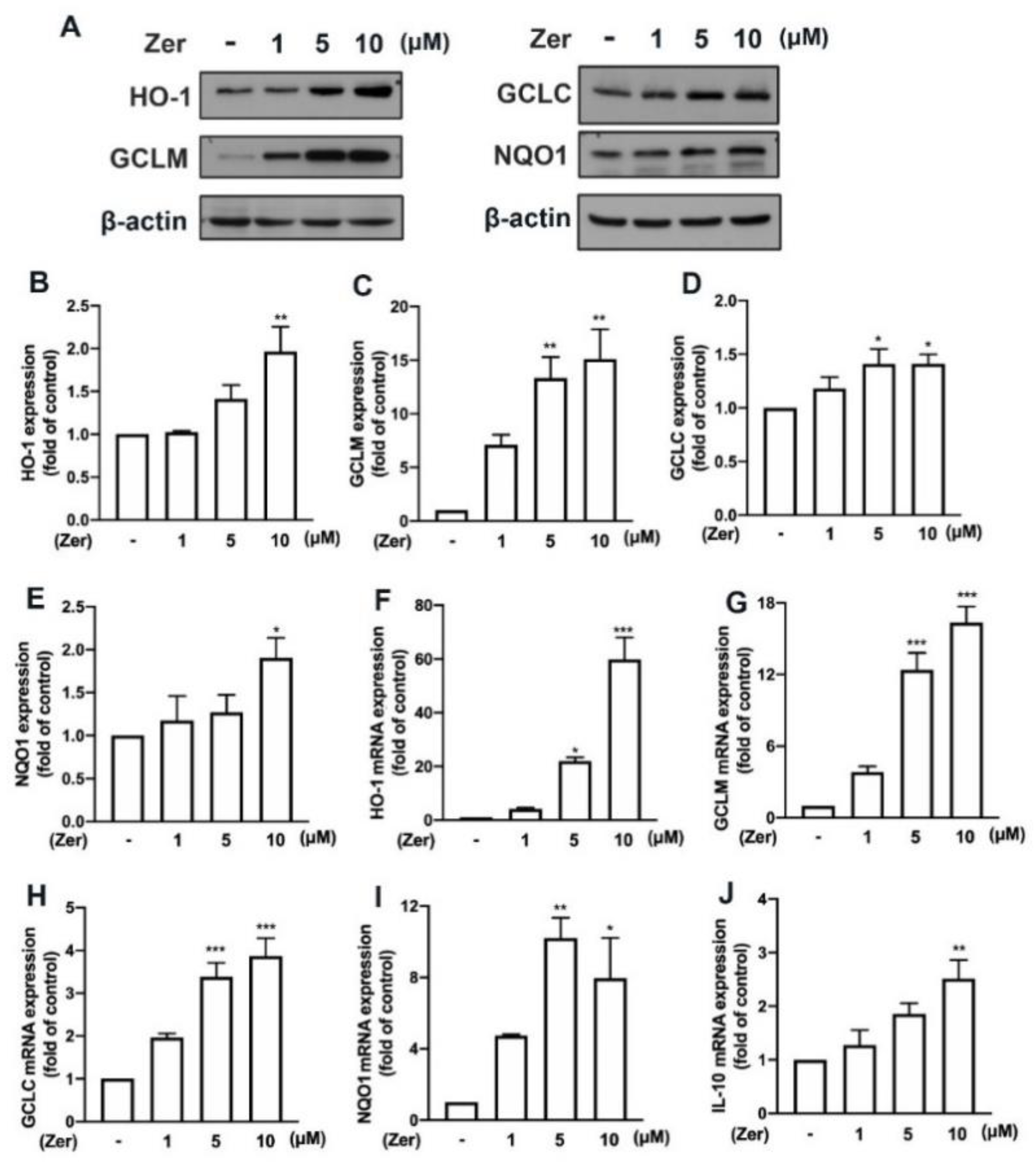
3.5. Zerumbone Promotes Endogenous Antioxidant Production and IL-10 Expression in Microglial Cells
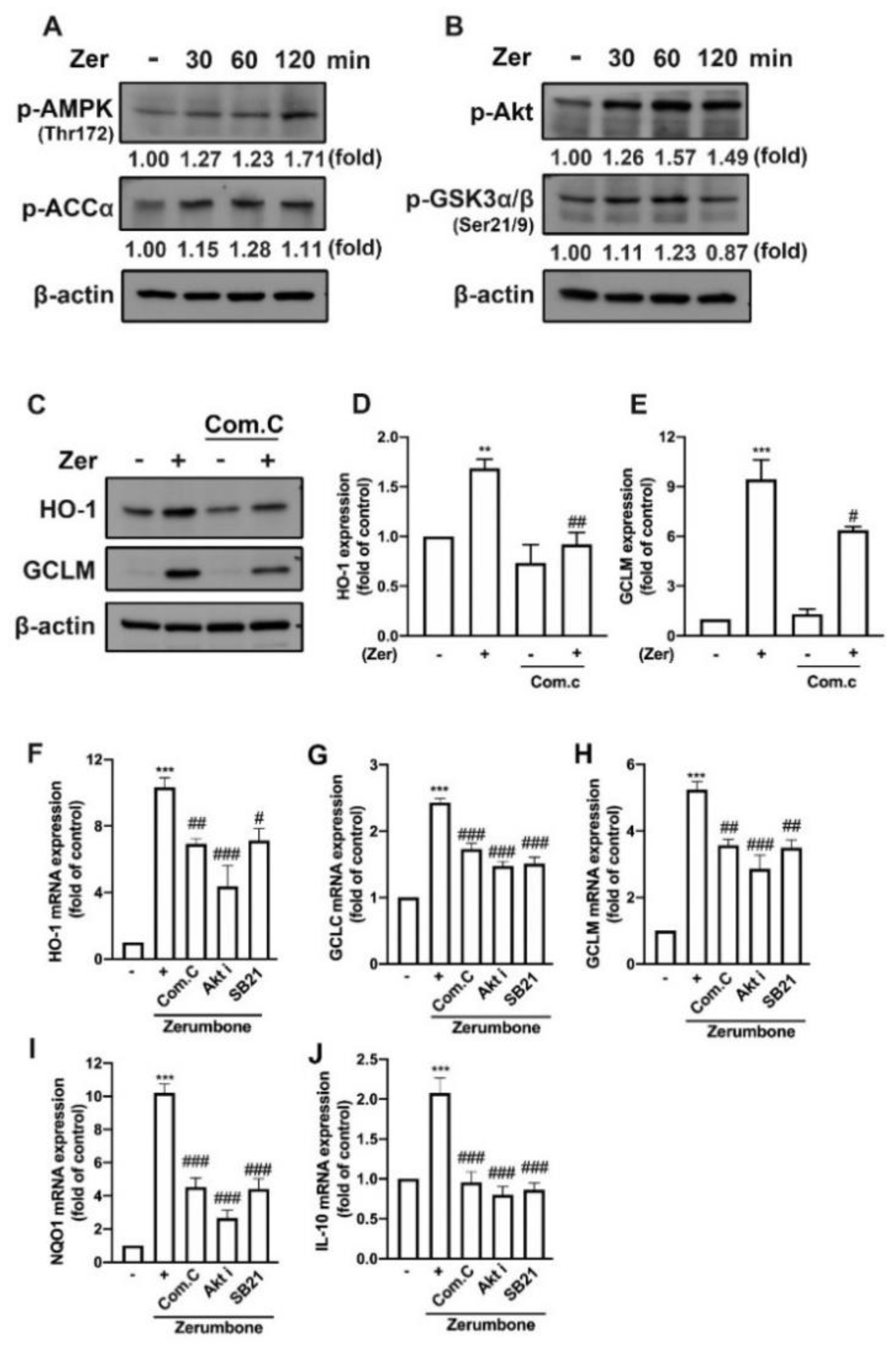
3.6. AMPK and Akt/GSK3 Signaling Pathways Mediate Zerumbone-Stimulated Production of Endogenous Antioxidants in Microglial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, M.K.; Lee, W.H.; Suk, K. Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochem. Pharmacol. 2016, 103, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Colton, C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009, 4, 399–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Allen, J.; Hankey-Giblin, P.A. Ontogeny and polarization of macrophages in inflammation: Blood monocytes versus tissue macrophages. Front. Immunol. 2015, 5, 683. [Google Scholar] [CrossRef] [Green Version]
- Bashir, S.; Sharma, Y.; Elahi, A.; Khan, F. Macrophage polarization: The link between inflammation and related diseases. Inflamm. Res. 2016, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Labonte, A.C.; Tosello-Trampont, A.C.; Hahn, Y.S. The role of macrophage polarization in infectious and inflammatory diseases. Mol. Cells 2014, 37, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Chiba, K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol. Ther. 2015, 154, 21–35. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Nieto, C.; Domínguez-Soto, A.; Barroso, R.; Sánchez-Mateos, P.; Puig-Kroger, A.; López-Bravo, M.; Joven, J.; Ardavín, C.; Rodríguez-Fernández, J.L.; et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef]
- Mills, C.D.; Ley, K. M1 and M2 macrophages: The chicken and the egg of immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef] [PubMed]
- West, M. Dead adipocytes and metabolic dysfunction: Recent progress. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Suganami, T.; Yamauchi, A.; Degawa-Yamauchi, M.; Tanaka, M.; Kouyama, R.; Kobayashi, Y.; Nitta, N.; Yasuda, K.; Hirata, Y.; et al. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J. Biol. Chem. 2008, 283, 35715–35723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.; Ji, H.H.; Li, Y.J.; Guo, S.D. Macrophage plasticity and atherosclerosis therapy. Front. Mol. Biosci. 2021, 8, 679797. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, Y.; Li, C. SIRT1 inhibits hepatocellular carcinoma metastasis by promoting M1 macrophage polarization via NF-κb pathway. OncoTargets Ther. 2019, 12, 2519–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.H.; Chung, E.; Chi, G.F.; Ahn, W.; Lim, J.E.; Hong, H.S.; Kim, D.W.; Choi, H.; Kim, J.; Son, Y. Substance P induces M2-type macrophages after spinal cord injury. Neuroreport 2012, 23, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Huang, B.R.; Lin, C.J.; Shen, C.K.; Lai, S.W.; Chen, C.W.; Lin, H.J.; Lin, C.H.; Hsieh, Y.C.; Lu, D.Y. Paliperidone inhibits glioblastoma growth in mouse brain tumor model and reduces PD-L1 expression. Cancers 2021, 13, 4357. [Google Scholar] [CrossRef]
- Tsai, C.F.; Chen, G.W.; Chen, Y.C.; Shen, C.K.; Lu, D.Y.; Yang, L.Y.; Chen, J.H.; Yeh, W.L. Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients 2021, 14, 67. [Google Scholar] [CrossRef]
- Lee, S.; Jha, M.K.; Suk, K. Lipocalin-2 in the inflammatory activation of brain astrocytes. Crit. Rev. Immunol. 2015, 35, 77–84. [Google Scholar] [CrossRef]
- Borkham-Kamphorst, E.; Drews, F.; Weiskirchen, R. Induction of lipocalin-2 expression in acute and chronic experimental liver injury moderated by pro-inflammatory cytokines interleukin-1β through nuclear factor-κb activation. Liver Int. 2011, 31, 656–665. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Weiskirchen, S.; Weiskirchen, R. Lipocalin 2 (LCN2) expression in hepatic malfunction and therapy. Front. Physiol. 2016, 7, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, E.; Lee, S.; Kim, J.H.; Kim, J.H.; Seo, J.W.; Lee, W.H.; Mori, K.; Nakao, K.; Suk, K. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 2013, 27, 1176–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Weng, Y.C.; Chiang, I.C.; Huang, Y.T.; Liao, Y.C.; Chen, Y.C.; Kao, C.Y.; Liu, Y.L.; Lee, T.H.; Chou, W.H. Neutralization of lipocalin-2 diminishes stroke-reperfusion injury. Int. J. Mol. Sci. 2020, 21, 6253. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.P.; Girisa, S.; Banik, K.; Ghosh, S.; Swathi, P.; Deka, M.; Padmavathi, G.; Kotoky, J.; Sethi, G.; Fan, L. Potential application of zerumbone in the prevention and therapy of chronic human diseases. J. Funct. Foods 2019, 53, 248–258. [Google Scholar] [CrossRef]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of zerumbone as an anti-cancer agent. Molecules 2019, 24, 734. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.A.; Jantan, I.; Harikrishnan, H. Zerumbone suppresses the activation of inflammatory mediators in LPS-stimulated U937 macrophages through Myd88-dependent NF-κb/MAPK/PI3K-Akt signaling pathways. Int. Immunopharmacol. 2018, 55, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Moreira da Silva, T.; Pinheiro, C.D.; Puccinelli Orlandi, P.; Pinheiro, C.C.; Soares Pontes, G. Zerumbone from Zingiber zerumbet (L.) Smith: A potential prophylactic and therapeutic agent against the cariogenic bacterium Streptococcus mutans. BMC Complement. Altern. Med. 2018, 18, 301. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, S.I.; Abdul, A.B.; Devi, N.; Taha, M.M.; Al-zubairi, A.S.; Mohan, S.; Mariod, A.A. Regression of cervical intraepithelial neoplasia by zerumbone in female Balb/c mice prenatally exposed to diethylstilboestrol: Involvement of mitochondria-regulated apoptosis. Exp. Toxicol. Pathol. 2010, 62, 461–469. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Perimal, E.K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Tasrip, N.A.; Mokhtar, F.; Zakaria, Z.A.; Lajis, N.H.; Israf, D.A. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia 2010, 81, 855–858. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Yeap, S.K.; Abdul Samad, N.; Andas, R.J.; Muhammad Nadzri, N.; Anasamy, T.; et al. Acute toxicity study of zerumbone-loaded nanostructured lipid carrier on BALB/c mice model. BioMed Res. Int. 2014, 2014, 563930. [Google Scholar] [CrossRef]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Maggi, F.; Nicoletti, M.; Baldan, V.; Dall Acqua, S. New drugs from old natural compounds: Scarcely investigated sesquiterpenes as new possible therapeutic agents. Curr. Med. Chem. 2018, 25, 1241–1258. [Google Scholar] [CrossRef] [PubMed]
- Jafarian, S.; Ling, K.H.; Hassan, Z.; Perimal-Lewis, L.; Sulaiman, M.R.; Perimal, E.K. Effect of zerumbone on scopolamine-induced memory impairment and anxiety-like behaviours in rats. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, X.H.; Zhao, X.J.; Xu, L.; Pan, C.L.; Zhang, Z.Y. Zerumbone ameliorates behavioral impairments and neuropathology in transgenic APP/PS1 mice by suppressing MAPK signaling. J. Neuroinflammation 2020, 17, 61. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.H.; Huang, B.R.; Lai, S.W.; Lin, C.; Lin, H.Y.; Yang, L.Y.; Lu, D.Y. SIRT1 activation by minocycline on regulation of microglial polarization homeostasis. Aging 2020, 12, 17990–18007. [Google Scholar] [CrossRef]
- Chuang, J.-Y.; Chang, P.-C.; Shen, Y.-C.; Lin, C.; Tsai, C.-F.; Chen, J.-H.; Yeh, W.-L.; Wu, L.-H.; Lin, H.-Y.; Liu, Y.-S.; et al. Regulatory effects of fisetin on microglial activation. Molecules 2014, 19, 8820–8839. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-F.; Kuo, Y.-H.; Yeh, W.-L.; Wu, C.Y.-J.; Lin, H.-Y.; Lai, S.-W.; Liu, Y.-S.; Wu, L.-H.; Lu, J.-K.; Lu, D.-Y. Regulatory effects of caffeic acid phenethyl ester on neuroinflammation in microglial cells. Int. J. Mol. Sci. 2015, 16, 5572–5589. [Google Scholar] [CrossRef] [Green Version]
- Shrikanth, C.B.; Chilkunda, N.D. Zerumbone ameliorates high glucose-induced reduction in AMP-activated protein kinase phosphorylation in tubular kidney cells. J. Agric. Food Chem. 2017, 65, 9208–9216. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Jung, C.H.; Choi, W.H.; Ha, T.Y. Zerumbone ameliorates high-fat diet-induced adiposity by restoring AMPK-regulated lipogenesis and microRNA-146b/SIRT1-mediated adipogenesis. Oncotarget 2017, 8, 36984–36995. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.C.; Lee, S.S.; Yang, M.L.; Huang-Liu, R.; Lee, C.Y.; Li, Y.C.; Kuan, Y.H. Zerumbone reduced the inflammatory response of acute lung injury in endotoxin-treated mice via Akt-NFκB pathway. Chem. Biol. Interact. 2017, 271, 9–14. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Sun, Y.; Wang, Z.; Qian, Y.; Duraisamy, V.; Antary, T.M.A. Zerumbone-induced reactive oxygen species-mediated oxidative stress re-sensitizes breast cancer cells to paclitaxel. Biotechnol. Appl. Biochem. 2022; ahead of print. [Google Scholar]
- Hu, Z.; Zeng, Q.; Zhang, B.; Liu, H.; Wang, W. Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells. Biochimie 2014, 107 Pt B, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, A.; Ahuja, N.; Mercado, A.L.; Diagaradjane, P.; Raju, U.; Patel, N.; Mohindra, P.; Diep, N.; Guha, S.; Krishnan, S. Zerumbone increases oxidative stress in a thiol-dependent ROS-independent manner to increase DNA damage and sensitize colorectal cancer cells to radiation. Cancer Med. 2015, 4, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Singh, C.B.; Dey, S.; Mandal, S.; Ghosh, J.; Mallick, S.; Hussain, A.; Swapana, N.; Ross, S.A.; Pal, C. Induction of apoptosis by zerumbone isolated from Zingiber zerumbet (L.) smith in protozoan parasite Leishmania donovani due to oxidative stress. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2016, 20, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbuZahra, H.M.; Rajendran, P.; Ismail, M.B. Zerumbone exhibit protective effect against zearalenone induced toxicity via ameliorating inflammation and oxidative stress induced apoptosis. Antioxidants 2021, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Niu, J.; Ou, L.; Deng, B.; Wang, Y.; Li, S. Zerumbone protects against Carbon Tetrachloride (CCL(4))-induced acute liver injury in mice via inhibiting oxidative stress and the inflammatory response: Involving the TLR4/NF-κB/COX-2 pathway. Molecules 2019, 24, 1964. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, E.T.; Selmanovic, D.; Maltry, M.; Morano, R.; Franco-Villanueva, A.; Estrada, C.M.; Solomon, M.B. Endocrine stress responsivity and social memory in 3xTg-AD female and male mice: A tale of two experiments. Horm. Behav. 2020, 126, 104852. [Google Scholar] [CrossRef]
- Uppin, V.; Acharya, P.; Bettadaiah Bheemanakere, K.; Talahalli, R.R. Hyperlipidemia downregulate brain antioxidant defense enzymes and neurotrophins in rats: Assessment of the modulatory potential of EPA+DHA and zerumbone. Mol. Nutr. Food Res. 2020, 64, 2000381. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Jeong, E.A.; Lee, J.Y.; An, H.S.; Jang, H.M.; Ahn, Y.J.; Lee, J.; Kim, K.E.; Roh, G.S. Lipocalin-2 deficiency reduces oxidative stress and neuroinflammation and results in attenuation of kainic acid-induced hippocampal cell death. Antioxidants 2021, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.-H.; Kim, J.-H.; Seo, J.-W.; Han, H.-S.; Lee, W.-H.; Mori, K.; Nakao, K.; Barasch, J.; Suk, K. Lipocalin-2 is a chemokine inducer in the central nervous system: Role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J. Biol. Chem. 2011, 286, 43855–43870. [Google Scholar]
- Jha, M.K.; Jeon, S.; Jin, M.; Ock, J.; Kim, J.H.; Lee, W.H.; Suk, K. The pivotal role played by lipocalin-2 in chronic inflammatory pain. Exp. Neurol. 2014, 254, 41–53. [Google Scholar] [CrossRef]
- Naudé, P.J.; Nyakas, C.; Eiden, L.E.; Ait-Ali, D.; van der Heide, R.; Engelborghs, S.; Luiten, P.G.; De Deyn, P.P.; den Boer, J.A.; Eisel, U.L. Lipocalin 2: Novel component of proinflammatory signaling in Alzheimer’s disease. FASEB J. 2012, 26, 2811–2823. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Jeong, K.H.; Kim, J.H.; Jin, M.; Kim, J.H.; Lee, M.G.; Choi, D.K.; Won, S.Y.; McLean, C.; Jeon, M.T.; et al. Pathogenic upregulation of glial lipocalin-2 in the parkinsonian dopaminergic system. J. Neurosci. 2016, 36, 5608–5622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.; Jeong, J.H.; Song, J. Lipocalin 2 regulates iron homeostasis, neuroinflammation, and insulin resistance in the brains of patients with dementia: Evidence from the current literature. CNS Neurosci. Ther. 2021, 27, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Xie, S.-Y.; Chen, C.-W.; Lu, D.-Y. Electroacupuncture improves repeated social defeat stress-elicited social avoidance and anxiety-like behaviors by reducing lipocalin-2 in the hippocampus. Mol. Brain 2021, 14, 150. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Z.; Huang, A.; Zhu, D.; Sun, P.; Duan, Y. Lipocalin 2 is a regulator during macrophage polarization induced by soluble worm antigens. Front. Cell. Infect. Microbiol. 2021, 11, 747135. [Google Scholar] [CrossRef]
- Yan, A.; Zhang, Y.; Lin, J.; Song, L.; Wang, X.; Liu, Z. Partial depletion of peripheral M1 macrophages reverses motor deficits in MPTP-treated mouse by suppressing neuroinflammation and dopaminergic neurodegeneration. Front. Aging Neurosci. 2018, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Starossom, S.C.; Mascanfroni, I.D.; Imitola, J.; Cao, L.; Raddassi, K.; Hernandez, S.F.; Bassil, R.; Croci, D.O.; Cerliani, J.P.; Delacour, D.; et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 2012, 37, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxidative Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef] [Green Version]
- Ming, X.F.; Rajapakse, A.G.; Yepuri, G.; Xiong, Y.; Carvas, J.M.; Ruffieux, J.; Scerri, I.; Wu, Z.; Popp, K.; Li, J.; et al. Arginase II promotes macrophage inflammatory responses through mitochondrial reactive oxygen species, contributing to insulin resistance and atherogenesis. J. Am. Heart Assoc. 2012, 1, e000992. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Liu, N.; Zhu, X.; Wang, L.; Zhao, Y.; Liu, Q.; Gao, C.; Li, J. Subanesthetic isoflurane abates ROS-activated MAPK/NF-κB signaling to repress ischemia-induced microglia inflammation and brain injury. Aging 2020, 12, 26121–26139. [Google Scholar] [CrossRef]
- Makhezer, N.; Ben Khemis, M.; Liu, D.; Khichane, Y.; Marzaioli, V.; Tlili, A.; Mojallali, M.; Pintard, C.; Letteron, P.; Hurtado-Nedelec, M.; et al. NOX1-derived ROS drive the expression of lipocalin-2 in colonic epithelial cells in inflammatory conditions. Mucosal Immunol. 2019, 12, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zou, S.; Cui, Z.; Guo, P.; Meng, Q.; Shi, X.; Gao, Y.; Yang, G.; Han, Z. Zerumbone protects INS-1 rat pancreatic beta cells from high glucose-induced apoptosis through generation of reactive oxygen species. Biochem. Biophys. Res. Commun. 2015, 460, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.M.; Chen, C.L.; Yang, C.M.; Wu, P.H.; Tsou, C.J.; Chiang, K.W.; Wu, W.B. The carotenoid lutein enhances matrix metalloproteinase-9 production and phagocytosis through intracellular ROS generation and ERK1/2, p38 MAPK, and RARβ activation in murine macrophages. J. Leukoc. Biol. 2013, 93, 723–735. [Google Scholar] [CrossRef]
- Su, C.C.; Wang, S.C.; Chen, I.C.; Chiu, F.Y.; Liu, P.L.; Huang, C.H.; Huang, K.H.; Fang, S.H.; Cheng, W.C.; Huang, S.P.; et al. Zerumbone suppresses the LPS-induced inflammatory response and represses activation of the NLRP3 inflammasome in macrophages. Front. Pharmacol. 2021, 12, 652860. [Google Scholar] [CrossRef]
- Neumann, H.; Kotter, M.R.; Franklin, R.J. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain 2009, 132, 288–295. [Google Scholar] [CrossRef]
- Anwar, S.; Rivest, S. Alzheimer’s disease: Microglia targets and their modulation to promote amyloid phagocytosis and mitigate neuroinflammation. Expert Opin. Ther. Targets 2020, 24, 331–344. [Google Scholar] [CrossRef]
- Goldmann, T.; Prinz, M. Role of microglia in CNS autoimmunity. Clin. Dev. Immunol. 2013, 2013, 208093. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Liang, L.; Li, J.; Xiong, Q.; Yu, X.; Yu, H. Lipocalin-2 alleviates LPS-induced inflammation through alteration of macrophage properties. J. Inflamm. Res. 2021, 14, 4189–4203. [Google Scholar] [CrossRef]
- Wan, T.; Zhu, W.; Zhao, Y.; Zhang, X.; Ye, R.; Zuo, M.; Xu, P.; Huang, Z.; Zhang, C.; Xie, Y.; et al. Astrocytic phagocytosis contributes to demyelination after focal cortical ischemia in mice. Nat. Commun. 2022, 13, 1134. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de Puig, I.; Miró, F.; Salas-Perdomo, A.; Bonfill-Teixidor, E.; Ferrer-Ferrer, M.; Márquez-Kisinousky, L.; Planas, A.M. IL-10 deficiency exacerbates the brain inflammatory response to permanent ischemia without preventing resolution of the lesion. J. Cereb. Blood Flow Metab. 2013, 33, 1955–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Jiang, Z.; Fitzgerald, D.C.; Ma, C.; Yu, S.; Li, H.; Zhao, Z.; Li, Y.; Ciric, B.; Curtis, M.; et al. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J. Clin. Investig. 2009, 119, 3678–3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyota, T.; Ingraham, K.L.; Swan, R.J.; Jacobsen, M.T.; Andrews, S.J.; Ikezu, T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012, 19, 724–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricchetti, G.A.; Williams, L.M.; Foxwell, B.M. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J. Leukoc. Biol. 2004, 76, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, H.-Y.; Chen, J.-H.; Tseng, W.-P.; Ko, P.-Y.; Liu, Y.-S.; Yeh, W.-L.; Lu, D.-Y. Effects of paeonol on anti-neuroinflammatory responses in microglial cells. Int. J. Mol. Sci. 2015, 16, 8844–8860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.-L.; Lee, C.-L.; Korivi, M.; Liao, J.-W.; Rajendran, P.; Wu, J.-J.; Hseu, Y.-C. Zerumbone protects human skin keratinocytes against UVA-irradiated damages through Nrf2 induction. Biochem. Pharmacol. 2018, 148, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.R.; Chang, P.C.; Yeh, W.L.; Lee, C.H.; Tsai, C.F.; Lin, C.; Lin, H.Y.; Liu, Y.S.; Wu, C.S.; Hsu, H.C.; et al. Anti-neuroinflammatory effects of the calcium channel blocker nicardipine on microglial cells: Implications for neuroprotection. PLoS ONE 2014, 9, e91167. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.Y.; Huang, B.R.; Yeh, W.L.; Lee, C.H.; Huang, S.S.; Lai, C.H.; Lin, H.; Lu, D.Y. Antineuroinflammatory effects of lycopene via activation of adenosine monophosphate-activated protein kinase-α1/heme oxygenase-1 pathways. Neurobiol. Aging 2014, 35, 191–202. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, W.-L.; Huang, B.-R.; Chen, G.-W.; Charoensaensuk, V.; Tsai, C.-F.; Yang, L.-Y.; Lu, D.-Y.; Chen, M.-K.; Lin, C. Role of Zerumbone, a Phytochemical Sesquiterpenoid from Zingiber zerumbet Smith, in Maintaining Macrophage Polarization and Redox Homeostasis. Nutrients 2022, 14, 5402. https://doi.org/10.3390/nu14245402
Yeh W-L, Huang B-R, Chen G-W, Charoensaensuk V, Tsai C-F, Yang L-Y, Lu D-Y, Chen M-K, Lin C. Role of Zerumbone, a Phytochemical Sesquiterpenoid from Zingiber zerumbet Smith, in Maintaining Macrophage Polarization and Redox Homeostasis. Nutrients. 2022; 14(24):5402. https://doi.org/10.3390/nu14245402
Chicago/Turabian StyleYeh, Wei-Lan, Bor-Ren Huang, Guan-Wei Chen, Vichuda Charoensaensuk, Cheng-Fang Tsai, Liang-Yo Yang, Dah-Yuu Lu, Mao-Kai Chen, and Chingju Lin. 2022. "Role of Zerumbone, a Phytochemical Sesquiterpenoid from Zingiber zerumbet Smith, in Maintaining Macrophage Polarization and Redox Homeostasis" Nutrients 14, no. 24: 5402. https://doi.org/10.3390/nu14245402