The Impact of a Polyphenol-Rich Extract from the Berries of Aronia melanocarpa L. on Collagen Metabolism in the Liver: A Study in an In Vivo Model of Human Environmental Exposure to Cadmium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
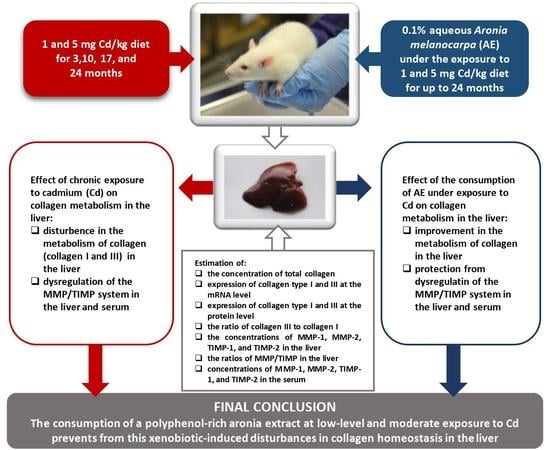
2.2. Diets Containing Cd
2.3. Extract from the Berries of A. melanocarpa
2.4. Study Design
- Control: Received water without the AE addition (<0.05 μg Cd/L) and the standard feed.
- AE: Received the 0.1% aqueous solution of AE as the only drinking fluid.
- Cd1: Intoxicated with Cd at the concentration of 1 mg/kg diet.
- Cd1 + AE: Received the 0.1% AE and the feed containing 1 mg Cd/kg.
- Cd5: Treated with Cd at the concentration of 5 mg/kg diet.
- Cd5 + AE: Received the 0.1% AE and the feed containing 5 mg Cd/kg.
2.5. Assay of the Concentration of Total Collagen in the Liver
2.6. Determination of the Expression of Collagen I and III at the mRNA Level in the Liver
2.6.1. Isolation of Total Ribonucleic Acid (RNA) from the Liver Tissue
2.6.2. Synthesis of cDNA and Quantitative Real-Time Polymerase Chain Reaction (Real-Time PCR)
2.7. Determination of Collagen I and III Expression at the Protein Level in the Liver
2.8. Assessment of the Concentrations of MMPs and TIMPs in the Liver and Serum
2.8.1. Preparation of the Aliquots of the Hepatic Tissue
2.8.2. Assay of MMPs and TIMPs in the Aliquots of the Liver Tissue and in the Serum
2.9. Statistical Analysis
3. Results
3.1. The Concentration of Total Collagen in the Liver
3.2. The Expression of Collagen I and III at the mRNA Level in the Liver
3.3. The Expression of Collagen I and III at the Protein Level in the Liver
3.4. The Ratio of the Expression of Collagen III to Collagen I in the Liver
3.5. The Concentrations of MMPs and TIMPs in the Liver
3.5.1. The Concentrations of MMP-1 and MMP-2 in the Liver
3.5.2. The Concentrations of TIPM-1 and TIMP-2 in the Liver
3.5.3. The Ratios of the Concentrations of MMP-1/TIMP-1, MMP-2/TIPM-2, and MMP-2/TIMP-1 in the Liver
3.6. The Concentrations of MMPs and TIMPs in the Serum
3.6.1. The Concentrations of MMP-1 and MMP-2 in the Serum
3.6.2. The Concentrations of TIMP-1 and TIMP-2 in the Serum
3.7. Mutual Dependencies between Collagen, MMPs, and TIMPs in the Liver and Serum
3.8. Mutual Dependencies between Indices of Collagen Metabolism in the Liver and Cd Concentration in This Organ
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | extract from Aronia melanocarpa L. berries |
| bp | base pair |
| b.w. | body weight |
| ANOVA | one-way analysis of variance |
| ANOVA/MANOVA | two-way analysis of variance |
| Cd | cadmium |
| Cd2+ | cadmium ion |
| cDNA | complementary deoxyribonucleic acid |
| CCl4 | carbon tetrachloride |
| CV | coefficient of variation |
| ECM | extracellular matrix |
| FR | free radicals |
| HSC | hepatic stellate cells |
| MMP-1 | matrix metalloproteinase-1 |
| MMP-2 | matrix metalloproteinase-2 |
| MMPs | matrix metalloproteinases |
| mRNA | messenger ribonucleic acid |
| NaCl | sodium chloride |
| p | level of statistical significance |
| r | correlation coefficient |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| Real-Time PCR | real-time polymerase chain reaction |
| RT-PCR | reverse transcriptase polymerase chain reaction |
| SE | standard error |
| TGF-β1 | trans-forming growth factor-β1 |
| TIMPs | tissue inhibitors of metalloproteinases |
| TIMP-1 | tissue inhibitor of metalloproteinase-1 |
| TIMP-2 | tissue inhibitor of metalloproteinase-2 |
| TNF-α | tumor necrosis factor-α |
| w/v | weight/volume |
| vs. | versus |
| v/v | volume/volume |
| α-SMA | α-smooth muscle actin |
References
- Mężyńska, M.; Brzóska, M.M. Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J. Appl. Toxicol. 2019, 39, 117–145. [Google Scholar] [CrossRef] [Green Version]
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef] [Green Version]
- Borowska, S.; Brzóska, M.M.; Tomczyk, M. Complexation of bioelements and toxic metals by polyphenolic compounds—Implications for health. Curr. Drug Targets 2018, 19, 1612–1638. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Tomczyk, M.; Strawa, J.W.; Brzóska, M.M. Estimation of the chelating ability of an extract from Aronia melanocarpa L. berries and its main polyphenolic ingredients towards ions of zinc and copper. Molecules 2020, 25, 1507. [Google Scholar] [CrossRef] [Green Version]
- Nemes, A.; Homoki, J.R.; Kiss, R.; Hegedűs, C.; Kovács, D.; Peitl, B.; Gál, F.; Stündl, L.; Szilvássy, Z.; Remenyik, J. Effect of anthocyanin-rich tart cherry extract on inflammatory mediators and adipokines involved in type 2 diabetes in a high fat diet induced obesity mouse model. Nutrients 2019, 11, 1966. [Google Scholar] [CrossRef] [Green Version]
- Castro-Barquero, S.; Tresserra-Rimbau, A.; Vitelli-Storelli, F.; Doménech, M.; Salas-Salvadó, J.; Martín-Sánchez, V.; Rubín-García, M.; Buil-Cosiales, P.; Corella, D.; Fitó, M.; et al. Dietary polyphenol intake is associated with HDL-cholesterol and a better profile of other components of the metabolic syndrome: A PREDIMED-plus sub-study. Nutrients 2020, 12, 689. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary cadmium intake and sources in the US. Nutrients 2019, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Satarug, S. Dietary cadmium intake and its effects on kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Mężyńska, M.; Brzóska, M.M. Environmental exposure to cadmium—A risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. 2018, 25, 3211–3232. [Google Scholar] [CrossRef]
- Hyder, O.; Chung, M.; Cosgrove, D.; Herman, J.M.; Li, Z.; Firoozmand, A.; Gurakar, A.; Koteish, A.; Pawlik, T.M. Cadmium exposure and liver disease among US adults. J. Gastrointest. Surg. 2013, 17, 1265–1273. [Google Scholar] [CrossRef]
- Kang, M.Y.; Cho, S.H.; Lim, Y.H.; Seo, J.C.; Hong, Y.C. Effects of environmental cadmium exposure on liver function in adults. Occup. Environ. Med. 2013, 70, 268–273. [Google Scholar] [CrossRef]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Piłat-Marcinkiewicz, B. Extract from Aronia melanocarpa L. berries prevents cadmium-induced oxidative stress in the liver: A study in a rat model of low-level and moderate lifetime human exposure to this toxic metal. Nutrients 2019, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Galicka, A. Extract from Aronia melanocarpa L. berries protects against cadmium-induced lipid peroxidation and oxidative damage to proteins and DNA in the liver: A study in a rat model of environmental human exposure to this xenobiotic. Nutrients 2019, 11, 758. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowski, A.; Onopiuk, B.M.; Car, H.; Onopiuk, P.; Dąbrowska, Z.N.; Rogalska, J.; Brzóska, M.M.; Dąbrowska, E. Beneficial impact of an extract from the berries of Aronia melanocarpa L. on the oxidative-reductive status of the submandibular gland of rats exposed to cadmium. Antioxidants 2020, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Brzóska, M.M.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Jurczuk, M.; Roszczenko, A.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols against cadmium-induced disorders in bone metabolism: A study in a rat model of lifetime human exposure to this heavy metal. Chem. Biol. Interact. 2015, 229, 132–146. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Roszczenko, A.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Mężyńska, M. Protective effect of chokeberry (Aronia melanocarpa L.) extract against cadmium impact on the biomechanical properties of the femur: A study in a rat model of low and moderate lifetime women exposure to this heavy metal. Nutrients 2017, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Jurczuk, M.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols on cadmium accumulation in the body: A study in a rat model of human exposure to this metal. Curr. Drug Targets 2015, 16, 1470–1487. [Google Scholar] [CrossRef]
- Kirshnakumar, N.; Prabu, S.M.; Sulfikkarali, N.K. Quercetin protects against cadmium induced biochemical and structural changes in rat liver revealed by FT-IR spectroscopy. Biomed. Prev. Nutr. 2012, 2, 179–185. [Google Scholar] [CrossRef]
- Li, Y.; Shen, R.; Wu, H.; Yu, L.; Wang, Z.; Wang, D. Liver changes induced by cadmium poisoning distinguished by confocal Raman imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 15, 117483. [Google Scholar] [CrossRef]
- Del Carmen, E.M.; Souza, V.; Bucio, L.; Hernández, E.; Damián-Matsumura, P.; Zaga, V.; Gutiérrez-Ruiz, M.C. Cadmium induces alpha(1)collagen (I) and metallothionein II gene and alters the antioxidant system in rat hepatic stellate cells. Toxicology 2002, 170, 63–73. [Google Scholar] [CrossRef]
- De Lima, E.C.; de Moura, C.; Silva, M.; Vilegas, W.; Santamarina, A.B.; Pisani, L.P.; de Oliveira, F.; Ribeiro, D.A. Therapeutical properties of Mimosa caesalpiniifolia in rat liver intoxicated with cadmium. Env. Sci. Pollut. Res. Int. 2020, 27, 10981–10989. [Google Scholar] [CrossRef]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef] [Green Version]
- Karsdal, M.A.; Manon-Jensen, T.; Genovese, F.; Kristensen, J.H.; Nielsen, M.J.; Sand, J.M.; Hansen, N.U.; Bay-Jensen, A.C.; Bager, C.L.; Krag, A.; et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G807–G830. [Google Scholar] [CrossRef] [Green Version]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Byrne, M.; Krane, S.; Jaenisch, R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 1852–1856. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, K.; Kucich, U.; Whitbeck, C.; Levin, R.M.; Howard, P.S. Functional changes in bladder tissue from type III collagen deficient mice. Mol. Cell. Biochem. 2006, 283, 107–114. [Google Scholar] [CrossRef]
- Asgari, M.; Latifi, N.; Heris, H.K.; Vali, H.; Mongeau, L. In vitro fibrillogenesis of tropocollagen type III in collagen type I affects its relative fibrillar topology and mechanics. Sci. Rep. 2017, 7, 1392. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Qi, K.; Zhang, L.; Bai, Z.; Ren, C.; Xu, X.; Zhang, Z.; Li, X. Glutathione might attenuate cadmium-induced liver oxidative stress and hepatic stellate cell activation. Biol. Trace. Elem. Res. 2019, 191, 443–452. [Google Scholar] [CrossRef]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef]
- Yamano, T.; DeCicco, L.A.; Rikans, L.E. Attenuation of cadmium-induced liver injury in senescent male Fischer 344 rats: Role of Kupffer cells and inflammatory cytokines. Toxicol. Appl. Pharm. 2000, 162, 68–75. [Google Scholar] [CrossRef]
- Kim, K.S.; Lim, H.J.; Lim, J.S.; Son, J.Y.; Lee, J.; Lee, B.M.; Chang, S.C.; Kim, H.S. Curcumin ameliorates cadmium-induced nephrotoxicity in Sprague-Dawley rats. Food Chem. Toxicol. 2018, 114, 34–40. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, Q.; Chen, J.; Liu, D. Potential ameliorative effects of grape seed-derived polyphenols against cadmium induced prostatic deficits. Biomed. Pharm. 2017, 91, 707–713. [Google Scholar] [CrossRef]
- Heinemeier, K.M.; Olesen, J.L.; Haddad, F.; Langberg, H.; Kjaer, M.; Baldwin, K.M.; Schjerling, P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J. Physiol. 2007, 582, 1303–1316. [Google Scholar] [CrossRef]
- Saneyasu, T.; Akhtar, R.; Sakai, T. Molecular cues guiding matrix stiffness in liver fibrosis. Biomed. Res. Int. 2016, 2016, 2646212. [Google Scholar] [CrossRef] [Green Version]
- Diehl, A.M.; Chute, J. Underlying potential: Cellular and molecular determinants of adult liver repair. J. Clin. Investig. 2013, 123, 1858–1860. [Google Scholar] [CrossRef]
- De Moura, C.F.; Ribeiro, F.A.; Handan, B.A.; Aguiar, O.; Oshima, C.T.; Ribeiro, D.A. Grape juice concentrate protects rat liver against cadmium intoxication: Histopathology, cytochrome c and metalloproteinases expression. Drug. Res. 2016, 66, 339–344. [Google Scholar] [CrossRef]
- Ala-Kokko, L.; Pihlajaniemi, T.; Myers, J.C.; Kivirikko, K.I.; Savolainen, E.R. Gene expression of type I, III, IV collagens in hepatic fibrosis induced by dimethylnitrosamine in the rat. Biochem. J. 1987, 244, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Aycock, R.S.; Seyer, J.M. Collagens of normal and cirrhotic human liver. Connect. Tissue Res. 1989, 23, 19–31. [Google Scholar] [CrossRef]
- Masuda, H.; Fukumoto, M.; Hirayoshi, K.; Nagata, K. Coexpression of the collagen-binding stress protein HSP47 gene and the alpha 1(I) and alpha 1(III) collagen genes in carbon tetrachloride-induced rat liver fibrosis. J. Clin. Investig. 1994, 94, 2481–2488. [Google Scholar] [CrossRef]
- Rojkind, M.; Perez-Tamayo, R. Liver fibrosis. Int. Rev. Connect. Tissue Res. 1983, 10, 333–393. [Google Scholar] [CrossRef]
- Hahn, E.G.; Schuppan, D. Ethanol and Fibrogenesis in the Liver. In Alcohol Related Diseases in Gastroenterology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1985; pp. 124–153. [Google Scholar]
- Kivirikko, K.I.; Kuivaniemi, H. Posttranslational modifications of collagen and their alterations in heritable diseases. In Connective Tissue Disease: Molecular Pathology of the Extracellular Matrix; Uitto, J., Perejda, A.J., Eds.; Marcel Dekker: New York, NY, USA, 1978; pp. 263–292. [Google Scholar]
- Che, J.; Chan, E.S.; Cronstein, B.N. Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Mol. Pharmacol. 2007, 72, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Praktiknjo, M.; Lehmann, J.; Nielsen, M.J.; Schierwagen, R.; Uschner, F.E.; Meyer, C.; Thomas, D.; Strassburg, C.P.; Bendtsen, F.; Møller, S.; et al. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol. Commun. 2018, 2, 211–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenger, M.P.E.; Bozec, L.; Horton, M.A.; Mesquida, P. Mechanical Properties of Collagen Fibrils. Biophys. J. 2007, 93, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Nieto, N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology 2006, 44, 1487–1501. [Google Scholar] [CrossRef]
- Hemmann, S.; Graf, J.; Roderfeld, M.; Roeb, E. Expression of MMPs and TIMPs in liver fibrosis—A systematic review with special emphasis on anti-fibrotic strategies. J. Hepatol. 2007, 46, 955–975. [Google Scholar] [CrossRef]
- Fomenko, O.; Shiyntum, H.; Shaulska, O.; Shevtsova, A.; Ushakova, G. Effects of cadmium on the activity of matrix metalloproteinases and metallothionein level in the rat brain. Neurophysiology 2017, 49, 154–157. [Google Scholar] [CrossRef]
- Olszowski, T.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Pro-inflammatory properties of cadmium. Acta Biochim. Pol. 2012, 59, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Lacorte, L.M.; Rinaldi, J.C.; Justulin, L.A., Jr.; Delella, F.K.; Moroz, A.; Felisbino, S.L. Cadmium exposure inhibits MMP2 and MMP9 activities in the prostate and testis. Biochem. Biophys. Res. Commun. 2015, 457, 538–541. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gao, J.; Yu, W.; Hao, R.; Fan, J.; Wei, J. The effects and mechanism of Aronia melanocarpa Elliot anthocyanins on hepatic fibrosis. J. Funct. Foods 2020, 68, 103897. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Y.; Long, C.; He, P.; Gu, J.; Yang, L.; Liang, Y.; Wang, Y. Anthocyanins isolated from blueberry ameliorates CCl4 induced liver fibrosis by modulation of oxidative stress, inflammation and stellate cell activation in mice. Food Chem. Toxicol. 2018, 120, 491–499. [Google Scholar] [CrossRef]
- Choi, J.H.; Hwang, Y.P.; Choi, C.Y.; Chung, Y.C.; Jeong, H.G. Anti-fibrotic effects of the anthocyanins isolated from the purple-fleshed sweet potato on hepatic fibrosis induced by dimethylnitrosamine administration in rats. Food Chem. Toxicol. 2010, 48, 3137–3143. [Google Scholar] [CrossRef]
- Jiang, X.W.; Shen, T.R.; Tang, X.L.; Yang, W.Q.; Guo, H.H.; Ling, W.H. Cyanidin-3-O-β-glucoside combined with its metabolite protocatechuic acid attenuated the activation of mice hepatic stellate cells. Food Funct. 2017, 8, 2945–2957. [Google Scholar] [CrossRef]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef]
- Yang, F.; Luo, L.; Zhu, Z.D.; Zhou, X.; Wang, Y.; Xue, J.; Zhang, J.; Cai, X.; Chen, Z.L.; Ma, Q.; et al. Chlorogenic acid inhibits liver fibrosis by blocking the miR-21-regulated TGF-β1/Smad7 signaling pathway in vitro and in vivo. Front. Pharmacol. 2017, 8, 929. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.U.H.; Uttra, A.M.; Qasim, S.; Ikram, J.; Saleem, M.; Niazi, Z.R. Phytochemicals targeting matrix metalloproteinases regulating tissue degradation in inflammation and rheumatoid arthritis. Phytomedicine 2020, 66, 153134. [Google Scholar] [CrossRef]
- Yang, N.; Dang, S.; Shi, J.; Wu, F.; Li, M.; Zhang, X.; Li, Y.; Jia, X.; Zhai, S. Caffeic acid phenethyl ester attenuates liver fibrosis via inhibition of TGF-β1/Smad3 pathway and induction of autophagy pathway. Biochem. Biophys. Res. Commun. 2017, 486, 22–28. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, N.; Hou, N.; Dong, L.; Li, J. Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017, 46, 68–73. [Google Scholar] [CrossRef]
- Singla, E.; Dharwal, V.; Naura, S.A. Gallic acid protects against the COPD-linked lung inflammation and emphysema in mice. Inflamm. Res. 2020, 69, 423–434. [Google Scholar] [CrossRef]
- Khatami, P.G.; Soleimani, A.; Sharifi, N.; Aghadavod, E.; Asemi, Z. The effects of high-dose vitamin E supplementation on biomarkers of kidney injury, inflammation, and oxidative stress in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. J. Clin. Lipidol. 2016, 10, 922–929. [Google Scholar] [CrossRef]
- Bonaventura, P.; Lamboux, A.; Albarède, F.; Miossec, P. Regulatory effects of zinc on cadmium-induced cytotoxicity in chronic inflammation. PLoS ONE 2017, 12, e0180879. [Google Scholar] [CrossRef] [Green Version]
- Marcellin, P.; Kutala, B.K. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018, 38 (Suppl. 1), 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Duration (Months) | 1 mg Cd/kg Diet + AE | 5 mg Cd/kg Diet + AE | ||||||
|---|---|---|---|---|---|---|---|---|
| Main Effect of Cd | Main Effect of AE | Interactive Effect of Cd + AE | Cd + AE Effect vs. Cd Effect + AE Effect Possible Character of Cd–AE Interaction | Main Effect of Cd | Main Effect of AE | Interactive Effect of Cd + AE | Cd + AE Effect vs. Cd Effect + AE Effect Possible Character of Cd–AE Interaction | |
| Collagen I mRNA | ||||||||
| 3 | 9.219 ** | NS | 22.12 *** | 0 vs. −59 3 + (−37) 0 vs. −96 Antagonistic action | 21.07 *** | NS | 21.44 *** | −37 vs. −64 + (−37) −37 vs. −101 Antagonistic action |
| 10 | 7.277 * | NS | 14.76 ** | 0 vs. −51 + 0 0 vs. −51 Antagonistic action | 20.82 *** | NS | 8.056 * | −34 vs. −67 + 0 −34 vs. −67 Antagonistic action |
| 17 | 26.75 *** | 8.803 ** | 42.48 *** | 0 vs. 2-fold + (+1.33-fold) 0 vs. +3.33-fold Antagonistic action | 14.57 ** | NS | 9.321 ** | +43 vs. +85 + (+33) +43 vs. +118 Antagonistic action |
| 24 | 23.86 *** | 12.69 ** | 19.75 *** | 0 vs. +4-fold + 0 0 vs. +4-fold Antagonistic action | 23.76 *** | 5.898 * | 11.92 ** | 0 vs. +3.3-fold + 0 0 vs. +3.3-fold Antagonistic action |
| Collagen III mRNA | ||||||||
| 3 | 25.52 *** | 18.81 *** | NS | No interaction | 13.81 ** | NS | NS | No interaction |
| 10 | 24.68 *** | 4.851 * | 11.26 ** | +1.43-fold vs. +2.3-fold + 0 +1.43-fold vs. +2.3-fold Antagonistic action | 122.5 *** | NS | NS | No interaction |
| 17 | 8.179 ** | NS | 17.69 *** | 0 vs. +80 + 0 0 vs. +80 Antagonistic action | 44.79 *** | NS | 15.19 *** | +1.64-fold vs. +2.3-fold + 0 +1.64-fold vs. +2.3-fold Antagonistic action |
| 24 | 8.021 * | 10.07 ** | NS | No interaction | 39.39 *** | 6.196 * | NS | No interaction |
| Duration (Months) | 1 mg Cd/kg Diet + AE | 5 mg Cd/kg Diet + AE | ||||||
|---|---|---|---|---|---|---|---|---|
| Main Effect of Cd | Main Effect of AE | Interactive Effect of Cd + AE | Cd + AE Effect vs. Cd Effect + AE Effect Possible Character of Cd–AE Interaction | Main Effect of Cd | Main Effect of AE | Interactive Effect of Cd + AE | Cd + AE Effect vs. Cd Effect + AE Effect Possible Character of Cd–AE Interaction | |
| Collagen I | ||||||||
| 3 | NS | NS | 13.88 ** | 0 vs. −19 3 + 0 0 vs. −19 Antagonistic action | 14.47 ** | 9.928 ** | 25.37 *** | 0 vs. −31 + 0 0 vs. −31 Antagonistic action |
| 10 | 232.4 *** | 72.18 *** | 50.73 *** | −10 vs. −34 + 0 −10 vs. −34 Antagonistic action | 138.8 *** | 34.42 *** | 25.38 *** | −15 vs. −43 + 0 −15 vs. −43 Antagonistic action |
| 17 | 19.91 *** | 4.297 * | 31.09 *** | +12 vs. +50 + (+17) +12 vs. +67 Antagonistic action | 12.57 ** | NS | 17.61 *** | +14 vs. +33 + (+17) +14 vs. +50 Antagonistic action |
| 24 | 9.685 ** | 7.746 * | 9.121 ** | 0 vs. −27 + 0 0 vs. −27 Antagonistic action | 14.23 ** | 16.55 *** | 19.22 *** | 0 vs. −32 + 0 0 vs. −32 Antagonistic action |
| Collagen III | ||||||||
| 3 | 18.05 *** | 5.407 * | 27.16 *** | +33 vs. +51 + (+37) +33 vs. +88 Antagonistic action | 10.82 ** | 39.33 *** | NS | No interaction |
| 10 | 14.09 ** | NS | 38.58 *** | 0 vs. +64 + (+33) 0 vs. +97 Antagonistic action | 98.93 *** | NS | 6.636 * | 2-fold vs. +2.2-fold + (+1.33-fold) 2-fold vs. 3.53-fold Antagonistic action |
| 17 | 148.9 *** | 43.15 *** | 217.8 *** | +17 vs. +80 + (+24) +17 vs. +104 Antagonistic action | 150.3 *** | 20.02 *** | 121.2 *** | +29 vs. +86 + (+24) +29 vs. +110 Antagonistic action |
| 24 | 14.62 ** | 8.997 ** | NS | No interaction | 54.56 *** | 9.717 ** | NS | No interaction |
| Duration (Months) | 1 mg Cd/kg Diet + AE | 5 mg Cd/kg Diet + AE | ||||||
|---|---|---|---|---|---|---|---|---|
| Main Effect of Cd | Main Effect of AE | Interactive Effect of Cd + AE | Cd + AE Effect vs. Cd Effect + AE Effect Possible Character of Cd–AE Interaction | Main Effect of Cd | Main Effect of AE | Interactive Effect of Cd + AE | Cd + AE Effect vs. Cd Effect + AE Effect Possible Character of Cd–AE Interaction | |
| Collagen III/Collagen I Expression at the mRNA Level | ||||||||
| 3 | 14.45 ** | 7.268 * | 13.65 ** | 0 vs. 3.8-fold + 0 0 vs. 3.8-fold Antagonistic action | 30.75 *** | 6.776 * | 14.81 ** | +1.89-fold vs. +3.8-fold + 0 +1.89-fold vs. +3.8-fold Antagonistic action |
| 10 | 26.67 *** | 13.19 ** | 24.01 *** | +1.55-fold vs. +4.6-fold + 0 +1.55-fold vs. +4.6-fold Antagonistic action | 40.22 *** | 4.861 * | 8.448 ** | +3.7-fold vs. +7-fold + 0 +3.7-fold vs. +7-fold Antagonistic action |
| 17 | - | - | - | - | - | - | - | - |
| 24 | 13.15 ** | NS | 6.586 * | 0 vs. −88 3 + 0 0 vs. −88 Antagonistic action | 21.11 *** | NS | NS | No interaction |
| Collagen III/Collagen I Expression at the Protein Level | ||||||||
| 3 | 18.96 *** | NS | 41.80 *** | +31 vs. +81 + (+47) +31 vs. +128 Antagonistic action | 22.27 *** | NS | 12.72 ** | +58 vs. +77 + (+47) +58 vs. +124 Antagonistic action |
| 10 | 94.62 *** | 32.18 *** | 92.83 *** | +1.32-fold vs. +2.5-fold + (+1.3-fold) +1.32-fold vs. +3.8-fold Antagonistic action | 167.54 *** | 13.65 ** | 32.73 *** | 2.4-fold vs. +3.8-fold + (+1.3-fold) +2.4-fold vs. +5.1-fold Antagonistic action |
| 17 | 5.054* | NS | 6.845 * | 0 vs. +21 + 0 0 vs. +21 Antagonistic action | 20.59 *** | 4.459 * | 10.90 ** | 0 vs. +41 + 0 0 vs. +41 Antagonistic action |
| 24 | - | - | - | - | - | - | - | - |
| Group | Experiment Duration | |||
|---|---|---|---|---|
| 3 Months | 10 Months | 17 Months | 24 Months | |
| MMP-1 (ng/mL) | ||||
| Control | 12.94 ± 0.223 | 12.20 ± 0.196 | 14.58 ± 0.410 | 15.66 ± 0.563 |
| AE | 13.18 ± 0.481 | 12.14 ± 0.171 | 14.12 ± 0.349 | 16.39 ± 1.128 |
| Cd1 | 12.21 ± 0.455 | 12.50 ± 0.141 | 16.04 ± 0.564 * | 14.91 ± 0.626 ** |
| Cd1 + AE | 12.11 ± 0.737 | 11.57 ± 0.533 | 14.53 ± 0.404 † | 15.29 ± 0.841 |
| Cd5 | 12.95 ± 0.229 | 10.85 ± 0.501 | 16.35 ± 0.351 **,‡ | 18.54 ± 0.370 **,## |
| Cd5 + AE | 12.58 ± 0.197 | 12.09 ± 0.282 | 14.27 ± 0.504 †† | 15.38 ± 0.355 †† |
| MMP-2 (ng/mL) | ||||
| Control | 10.36 ± 0.452 | 8.368 ± 0.372 | 11.89 ± 0.486 | 11.25 ± 0.363 |
| AE | 12.81 ± 0.654 | 9.088 ± 0.579 | 15.36 ± 1.098 | 12.59 ± 0.764 |
| Cd1 | 10.96 ± 0.663 | 10.07 ± 0.572 | 15.38 ± 0.590 | 15.77 ± 0.725 ** |
| Cd1 + AE | 13.03 ± 0.513 | 9.698 ± 1.1391 | 14.17 ± 0.558 | 13.16 ± 0.679 |
| Cd5 | 11.43 ± 0.646 | 10.48 ± 0.409 | 23.43 ± 1.841 ***,## | 16.31 ± 0.561 *** |
| Cd5 + AE | 11.15 ± 1.027 | 8.704 ± 0.645 | 12.56 ± 1.071 ††† | 10.81 ± 0.929 ††,# |
| Group | Experiment Duration | |||
|---|---|---|---|---|
| 3 Months | 10 Months | 17 Months | 24 Months | |
| TIMP-1 (ng/mL) | ||||
| Control | 2.298 ± 0.059 | 2.216 ± 0.086 | 4.819 ± 0.170 | 2.624 ± 0.232 |
| AE | 2.158 ± 0.106 | 2.049 ± 0.169 | 3.968 ± 0.155 | 2.014 ± 0.209 |
| Cd1 | 2.324 ± 0.156 | 1.971 ± 0.112 | 2.533 ± 0.135 ***,‡ | 1.469 ± 0.181 * |
| Cd1 + AE | 2.061 ± 0.176 | 2.075 ± 0.075 | 3.780 ± 0.178 † | 2.152 ± 0.216 † |
| Cd5 | 2.235 ± 0.113 | 2.136 ± 0.106 | 2.643 ± 0.104 ***,‡ | 1.342 ± 0.143 ** |
| Cd5 + AE | 2.460 ± 0.102 | 2.115 ± 0.094 | 3.229 ± 0.211 *,† | 2.413 ± 0.226 † |
| TIMP-2 (ng/mL) | ||||
| Control | 1.722 ± 0.064 | 1.833 ± 0.027 | 1.622 ± 0.012 | 1.676 ± 0.048 |
| AE | 1.670 ± 0.010 | 1.712 ± 0.041 | 1.616 ± 0.027 | 1.730 ± 0.046 |
| Cd1 | 1.548 ± 0.052 | 1.563 ± 0.085 * | 1.589 ± 0.023 | 1.597 ± 0.068 |
| Cd1 + AE | 1.643 ± 0.013 | 1.855 ± 0.035 † | 1.720 ± 0.032 † | 1.728 ± 0.034 |
| Cd5 | 1.317 ± 0.240 | 1.553 ± 0.019 ** | 1.613 ± 0.027 | 1.278 ± 0.064 *,‡,## |
| Cd5 + AE | 1.652 ± 0.056 | 1.825 ± 0.065 † | 1.774 ± 0.041 † | 1.846 ± 0.048 †††,# |
| PARAMETER | MMP-1 Concentration | MMP-2 Concentration | TIMP-1 Concentration | TIMP-2 Concentration | |
|---|---|---|---|---|---|
| Total Collagen Concentration | 0.233 *** | 0.332 *** | NS | 0.184 * | |
| Collagen I mRNA Expression | 0.163 * | 0.313 *** | −0.280 *** | NS | |
| Collagen III mRNA Expression | NS | 0.498 *** | −0.460 *** | NS | |
| Collagen I Protein Expression | NS | −0.285 *** | NS | NS | |
| Collagen III Protein Expression | NS | −0.186 * | 0.513 *** | 0.325 *** | |
| MMP-1 Concentration | - | 0.448 *** | 0.268 *** | 0.603 *** | |
| MMP-2 Concentration | - | - | NS | 0.446 *** | |
| TIMP-1 Concentration | - | - | - | 0.498 *** | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, M.; Brzóska, M.M.; Rogalska, J.; Galicka, A. The Impact of a Polyphenol-Rich Extract from the Berries of Aronia melanocarpa L. on Collagen Metabolism in the Liver: A Study in an In Vivo Model of Human Environmental Exposure to Cadmium. Nutrients 2020, 12, 2766. https://doi.org/10.3390/nu12092766
Kozłowska M, Brzóska MM, Rogalska J, Galicka A. The Impact of a Polyphenol-Rich Extract from the Berries of Aronia melanocarpa L. on Collagen Metabolism in the Liver: A Study in an In Vivo Model of Human Environmental Exposure to Cadmium. Nutrients. 2020; 12(9):2766. https://doi.org/10.3390/nu12092766
Chicago/Turabian StyleKozłowska, Magdalena, Małgorzata M. Brzóska, Joanna Rogalska, and Anna Galicka. 2020. "The Impact of a Polyphenol-Rich Extract from the Berries of Aronia melanocarpa L. on Collagen Metabolism in the Liver: A Study in an In Vivo Model of Human Environmental Exposure to Cadmium" Nutrients 12, no. 9: 2766. https://doi.org/10.3390/nu12092766