Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Determination of Biochemical Markers
2.3. Hematoxylin and Eosin Staining
2.4. Serum Lipidomics Analyses
2.4.1. Serum Lipid Extraction
2.4.2. UPLC-QTOF/MS
2.4.3. Lipid Identification
2.5. Statistical Analysis
3. Results
3.1. Effect of RB on Body Weight and Masses of Liver, Omental Fat, and Epididymal Fat in Rats with HED-Induced Obesity
3.2. Effect of RB on Serum Biochemical Parameters of Rats with HED-Induced Obesity
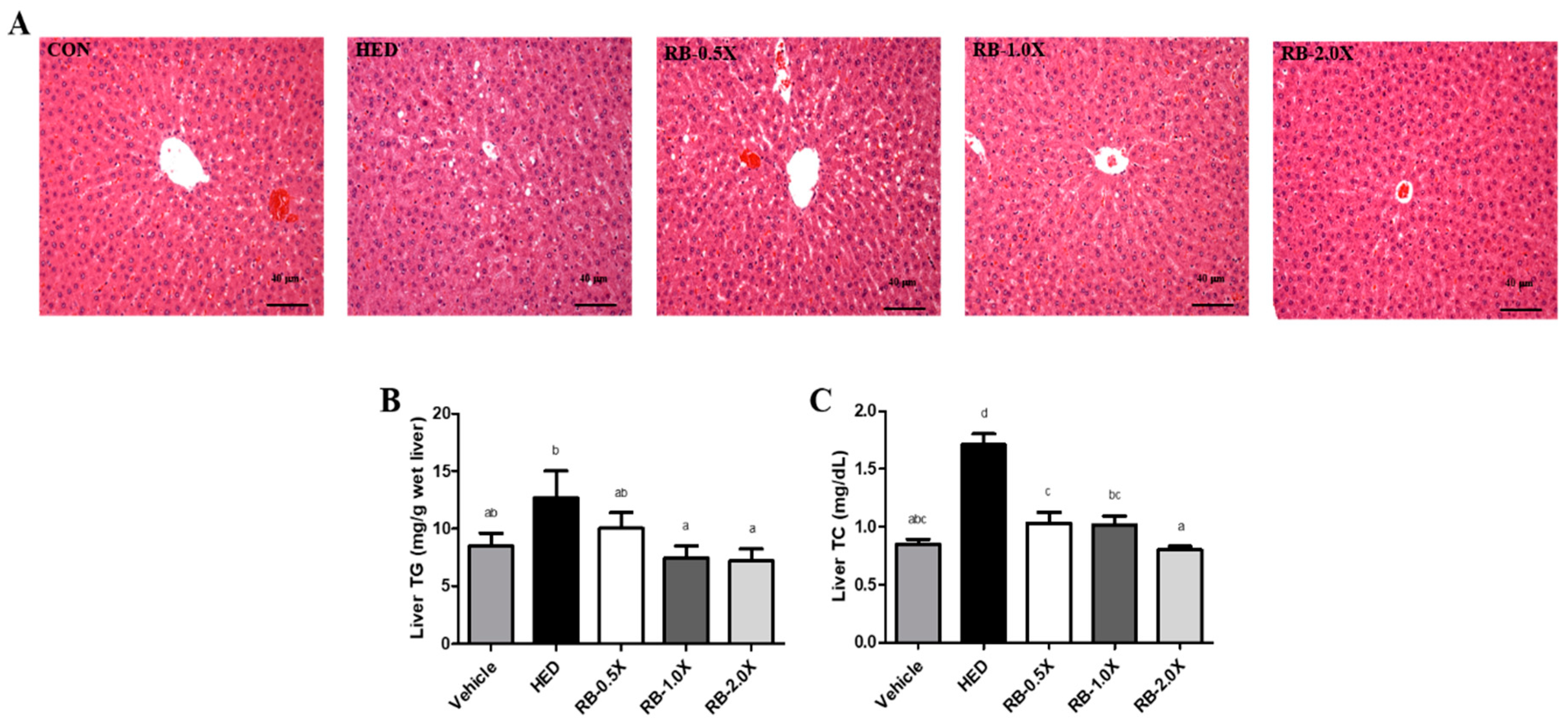
3.3. Effect of RB on Hepatic Lipid Accumulation in Rats with HED-Induced Obesity
3.4. Effect of RB on Adipocyte Size Distribution in Rats with HED-Induced Obesity
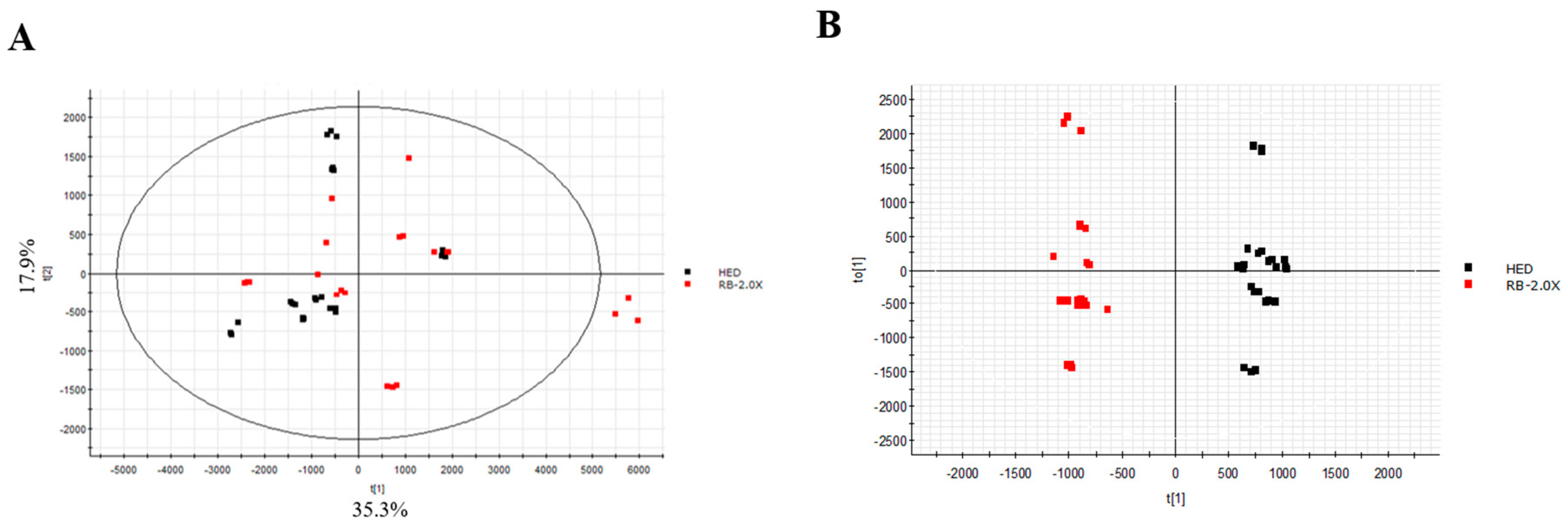
3.5. Effect of RB on Lipidomics in Rats with HED-Induced Obesity Analyses of Lipidomic Profiles
3.6. Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. (Lond.) 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- National Task Force on the Prevention and Treatment of Obesity. Overweight, Obesity, And Health Risk. Arch. Intern. Med. 2000, 160, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, C.Y.; Oh, S.W.; Yoo, H.J. Prevalence of obesity and metabolic syndrome in Korean adults. Obes. Rev. 2008, 9, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Cheung, B.C.; Cheung, T.T.; Samaranayake, N.R. Safety of antiobesity drugs. Eur. PMC. 2013, 4, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: A systematic review. BMC Med. 2016, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food. Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.; Ju, Y.H. A review on rice bran protein: Its properties and extraction methods. Crit. Rev. Food. Sci. Nutr. 2011, 51, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.Y.; Han, S.Y.; Kim, S.R.; Kim, I.H.; Lee, H.Y.; Kim, H.K. Bioactive components in rice bran oil improve lipid profiles in rats fed a high-cholesterol diet. Nutr. Res. 2005, 25, 597–606. [Google Scholar] [CrossRef]
- Islam, M.S.; Nagasaka, R.; Ohara, K.; Hosoya, T.; Ozaki, H.; Ushio, H.; Hori, M. Biological abilities of rice bran-derived antioxidant phytochemicals for medical therapy. Curr. Top. Med. Chem. 2011, 11, 1847–1853. [Google Scholar] [CrossRef]
- Jariwalla, R.J. Rice-bran products: Phytonutrients with potential applications in preventive and clinical medicine. Drugs Exp. Clin. Res. 2001, 27, 17–26. [Google Scholar] [PubMed]
- Hagl, S.; Berressem, D.; Grewal, R.; Sus, N.; Frank, J.; Eckert, G.P. Rice bran extract improves mitochondrial dysfunction in brains of aged NMRI mice. Nutr. Neurosci. 2016, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McPeak, P.; Cheruvanky, R.; Cherukuri, R.S.V. Supportive therapy for diabetes, hyperglycemia and hypoglycemia. U.S. Patent No. 6,303,586, 16 October 2001. [Google Scholar]
- Lin, S.; Yang, Z.; Liu, H.; Tang, L.; Cai, Z. Beyond glucose: Metabolic shifts in responses to the effects of the oral glucose tolerance test and the high-fructose diet in rats. Mol. Biosyst. 2011, 7, 1537–1548. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Huang, C.C.; Tung, Y.T.; Huang, W.C.; Chen, Y.M.; Hsu, Y.J.; Hsu, M.C. Beneficial effects of cocoa, coffee, green tea, and garcinia complex supplement on diet induced obesity in rats. BMC Complement. Altern. Med. 2016, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.A.D.S.C.; Bassinello, P.Z.; Penteado, M.d.V.C.J. Nutritional composition of rice bran submitted to different stabilization procedures. Braz. J. Pharm. Sci. 2012, 48, 651–657. [Google Scholar] [CrossRef]
- Yamagishi, T.; Tsuboi, T.; Kikuchi, K. Potent natural immunomodulator, rice water-soluble polysaccharide fractions with anticomplementary activity. Cereal Chem. 2003, 80, 5–8. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Sami, S.A.; Khan, F.A. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus Types I and II. J. Nutr. Biochem. 2002, 13, 175–187. [Google Scholar] [CrossRef]
- Sugano, M.; Tsuji, E. Rice bran oil and cholesterol metabolism. J. Nutr. 1997, 127, 521s–524s. [Google Scholar] [CrossRef]
- Xu, Z.; Hua, N.; Godber, J.S. Antioxidant activity of tocopherols, tocotrienols, and gamma-oryzanol components from rice bran against cholesterol oxidation accelerated by 2, 2’-azobis(2-methylpropionamidine) dihydrochloride. J. Agric. Food Chem. 2001, 49, 2077–2081. [Google Scholar] [CrossRef]
- Saghatelian, A.; Trauger, S.A.; Want, E.J.; Hawkins, E.G.; Siuzdak, G.; Cravatt, B.F. Assignment of endogenous substrates to enzymes by global metabolite profiling. Biochemistry 2004, 43, 14332–14339. [Google Scholar] [CrossRef]
- Szczygielska, A.; Widomska, S.; Jaraszkiewicz, M.; Knera, P.; Muc, K. Blood lipids profile in obese or overweight patients. Ann. Univ. Mariae. Curie. Sklodowska. Med. 2003, 58, 343–349. [Google Scholar]
- Nam, M.; Choi, M.S.; Jung, S.H.; Jung, Y.G.; Choi, J.Y.; Ryu, D.H.; Hwang, G.S. Lipidomic profiling of liver tissue from obesity-prone and obesity-resistant mice fed a high fat diet. Sci. Rep. 2015, 5, 16984. [Google Scholar] [CrossRef]
- Eisinger, K.; Krautbauer, S.; Hebel, T.; Schmitz, G.; Aslanidis, C.; Liebisch, G.; Buechler, C. Lipidomic analysis of the liver from high-fat diet induced obese mice identifies changes in multiple lipid classes. Exp. Mol. Pathol. 2014, 97, 37–43. [Google Scholar] [CrossRef]
- Holland, W.L.; Brozinick, J.T.; Wang, L.P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef]
- Khamzina, L.; Veilleux, A.; Bergeron, S.; Marette, A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: Possible involvement in obesity-linked insulin resistance. J. Endocrinol. 2005, 146, 1473–1481. [Google Scholar] [CrossRef]
- Murphy, N.J.; Schraer, C.D.; Thiele, M.C.; Boyko, E.J.; Bulkow, L.R.; Doty, B.J.; Lanier, A.P. Dietary change and obesity associated with glucose intolerance in Alaska Natives. J. Am. Diet. Assoc. 1995, 95, 676–682. [Google Scholar] [CrossRef]
- Qatanani, M.; Lazar, M.A. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes. Dev. 2007, 21, 1443–1455. [Google Scholar] [CrossRef]
- Steppan, C.M.; Lazar, M.A. Resistin and obesity-associated insulin resistance. Trends. Endocrinol. Metab. 2002, 13, 18–23. [Google Scholar] [CrossRef]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef]
- Magkos, F.; Su, X.; Bradley, D.; Fabbrini, E.; Conte, C.; Eagon, J.C.; Varela, J.E.; Brunt, E.M.; Patterson, B.W.; Klein, S. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology 2012, 142, 1444–1446. [Google Scholar] [CrossRef]


| Vehicle | HED | RB-0.5X | RB-1.0X | RB-2.0X | |
|---|---|---|---|---|---|
| Water (mL) | 45.3 ± 0.8 b | 26.3 ± 0.4 a | 25.8 ± 0.2 a | 26.7± 0.3 a | 25.0 ± 0.3 a |
| Food intake (g/day) | 23.3 ± 0.4 a | 23.5 ± 0.4 a | 23.6 ± 0.4 a | 23.0 ± 0.3 a | 23.2 ± 0.4 a |
| Energy intake (kcal/day) | 77.9 ± 1.3 a | 99.2 ± 1.6 b | 99.9 ± 1.9 b | 98.2 ± 1.5 b | 99.4 ± 1.7 b |
| Feed efficiency 1 | 0.5 ± 0.2 a | 0.9 ± 0.2 a | 0.8 ± 0.2 a | 0.8 ± 0.2 a | 0.8 ± 0.1 a |
| Final body weight (g) | 416 ± 8 a | 487 ± 5 c | 472 ± 4b c | 466 ± 8 b | 463 ± 5 b |
| Body weight gain (g) | 97.1 ± 4.4a | 168.1 ± 5.4 b | 149.1 ± 2.5 b | 148.4 ± 6.1 b | 146.8 ± 14.3 b |
| Liver | 11.55 ± 0.41 a | 15.34 ± 0.28 b | 14.09 ± 0.23 b | 14.09 ± 0.23 b | 14.24 ± 0.42 b |
| Omental fat | 3.74 ± 0.33 a | 7.03 ± 0.57 b | 6.40 ± 0.40 b | 7.10 ± 1.14 b | 6.48 ± 0.64 b |
| Epididymal fat | 3.33 ± 0.36 a | 7.56 ± 0.53 b | 6.62 ± 0.26 b | 6.75 ± 0.75 b | 6.96 ± 0.65 b |
| Total fat | 9.51 ± 0.88 a | 24.40 ± 1.50 b | 22.65 ± 0.76 b | 23.76 ± 1.81 b | 23.36 ± 1.80 b |
| Vehicle | HED | RB-0.5X | RB-1.0X | RB-2.0X | |
|---|---|---|---|---|---|
| Uric acid (mg/dL) | 3.0 ± 0.4 a | 6.4 ± 0.6 c | 4.5 ± 0.2 b | 3.8 ± 0.6 ab | 2.6 ± 0.3 a |
| GOT (U/L) | 64 ± 2 a | 66 ± 2 a | 63 ± 2 a | 62 ± 1 a | 64 ± 1 a |
| FFA (mmol/L) | 0.62 ± 0.03 a | 1.04 ± 0.06 b | 0.95 ± 0.07 b | 0.88 ± 0.09 b | 1.03 ± 0.07 b |
| TC (mg/dL) | 59 ± 4 a | 75 ± 4 b | 72 ± 5 b | 68 ± 4 ab | 69 ± 6 ab |
| TG (mg/dL) | 40 ± 3 a | 155 ± 13 b | 135 ± 13 b | 137 ± 16 b | 133 ± 10 b |
| HDL (mg/dL) | 35.3 ± 2.3 a | 35.4 ± 0.8 a | 37.8 ± 2.5 a | 34.8 ± 1.8 a | 36.1 ± 2.6 a |
| LDL (mg/dL) | 14.0 ± 1.1 a | 11.5 ± 0.8 a | 11.8 ± 1.1 a | 11.4 ± 1.1 a | 11.4 ± 1.1 a |
| Glucose (mg/dL) | 153 ± 8 a | 341 ± 25 c | 250 ± 14 b | 283 ± 24 b | 283 ± 8 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-C.; Huang, W.-C.; Ng, X.E.; Lee, M.-C.; Hsu, Y.-J.; Huang, C.-C.; Wu, H.-H.; Yeh, C.-L.; Shirakawa, H.; Budijanto, S.; et al. Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity. Nutrients 2019, 11, 2033. https://doi.org/10.3390/nu11092033
Yang S-C, Huang W-C, Ng XE, Lee M-C, Hsu Y-J, Huang C-C, Wu H-H, Yeh C-L, Shirakawa H, Budijanto S, et al. Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity. Nutrients. 2019; 11(9):2033. https://doi.org/10.3390/nu11092033
Chicago/Turabian StyleYang, Suh-Ching, Wen-Ching Huang, Xin Er Ng, Mon-Chien Lee, Yi-Ju Hsu, Chi-Chang Huang, Hai-Hsin Wu, Chiu-Li Yeh, Hitoshi Shirakawa, Slamet Budijanto, and et al. 2019. "Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity" Nutrients 11, no. 9: 2033. https://doi.org/10.3390/nu11092033
APA StyleYang, S.-C., Huang, W.-C., Ng, X. E., Lee, M.-C., Hsu, Y.-J., Huang, C.-C., Wu, H.-H., Yeh, C.-L., Shirakawa, H., Budijanto, S., Tung, T.-H., & Tung, Y.-T. (2019). Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity. Nutrients, 11(9), 2033. https://doi.org/10.3390/nu11092033








