Conjugated Linoleic Acid Isomers Affect Profile of Lipid Compounds and Intensity of Their Oxidation in Heart of Rats with Chemically-Induced Mammary Tumors—Preliminary Study
Abstract
:1. Introduction
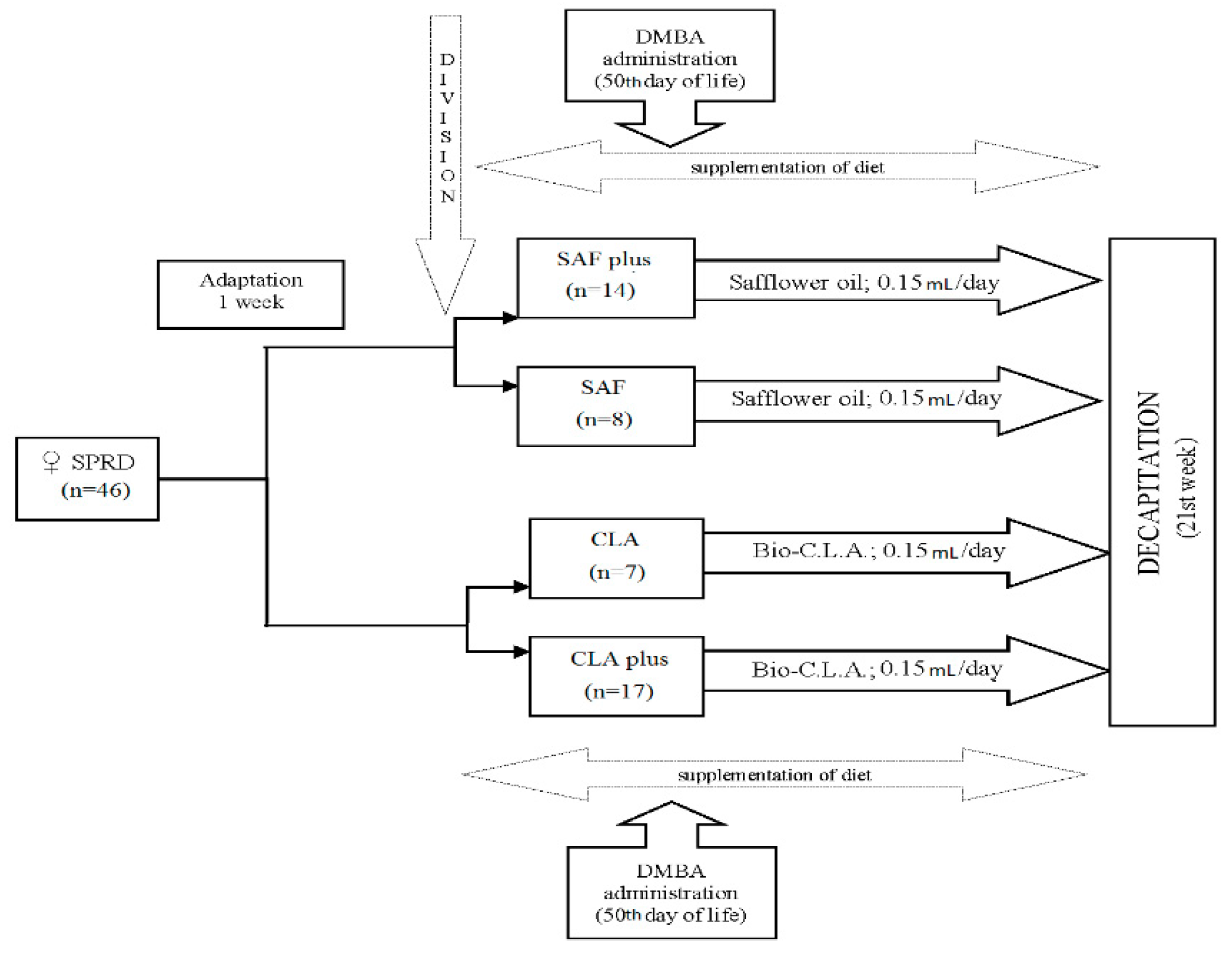
2. Materials and Methods
2.1. Ethics Approval Statement
2.2. Dietary Ingredients
2.3. Animal Experiment
2.4. Fatty Acids (Fas) and Conjugated Fatty Acids (Cfas) Profile in Hearts
- (1)
- Peroxidability index (PI)
- (2)
- Index of atherogenicity (AI)
- (3)
- Index of thrombogenicity (TI)
- (4)
- Hypo/hypercholesterolemic index (HH)
2.5. Total Cholesterol and Oxysterols Content in Hearts
2.6. Malondialdehyde (MDA) Concentration in Hearts
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization—WHO. Available online: http://www.who.int/cardiovascular_diseases/en/ (accessed on 20 November 2018).
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaes, A.; Prizment, A.; Koene, R.J.; Konety, S. Cardio-oncology Related to Heart Failure: Common Risk Factors Between Cancer and Cardiovascular Disease. Heart Fail. Clin. 2017, 13, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.Y.; Agha, A.M.; Lopez-Mattei, J.; Palaskas, N.; Kim, P.; Thompson, K.; Mouhayar, E.; Marmagkiolis, K.; Hassan, S.A.; Karimzad, K.; et al. Interventional Cardio-Oncology: Adding a New Dimension to the Cardio-Oncology Field. Front. Cardiovasc. Med. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Venneri, L.; Caliccio, F.; Manivarmane, R. Subclinical myocardial dysfunction in cancer patients: Is there a direct effect of tumour growth? Eur. Heart J. Cardiovasc. Imaging 2015, 16 (Suppl. 2), ii127. [Google Scholar]
- Asteggiano, R.; Suter, T.; Bax, J.J. Cardio-oncology: Principles and organisational issues. E-Journal Cardiol. Pract. 2019, 16, 37. [Google Scholar]
- López-Fernandez, T.; Van der Meer, P. Cardio-oncology: It is not only heart failure! E-J. Cardiol. Pract. 2019, 16. [Google Scholar] [CrossRef]
- Marmot, M.; Atinmo, T.; Byers, T.; Chen, J.; Hirohata, T.; Jackson, A.; James, W.; Kolonel, L.; Kumanyika, S.; Leitzmann, C.; et al. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; AICR: Washington, DC, USA, 2007. [Google Scholar]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar]
- Patel, A.; Pathak, Y.; Patel, J.; Sutariya, V. Role of nutritional factors in pathogenesis of cancer. Food Qual. Saf. 2018, 2, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Ferrini, K.; Ghelfi, F.; Mannucci, R.; Titta, L. Lifestyle, nutrition and breast cancer: Facts and presumptions for consideration. Ecancermedicalscience 2015, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pariza, M.W.; Ashoor, S.H.; Chu, F.S.; Lund, D.B. Effects of temperature and time on mutagen formation in pan-fried hamburger. Cancer Lett. 1979, 7, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pariza, M.W.; Loretz, L.J.; Storkson, J.M.; Holland, N.C. Mutagens and modulator of mutagenesis in fried ground beef. Cancer Res. 1983, 43 (Suppl. 5), 2444–2446. [Google Scholar]
- Pariza, M.W.; Hargraves, W.A. A beef-derived mutagenesis modulator inhibits initiation of mouse epidermal tumors by 7,12-dimethylbenz[a]anthracene. Carcinogenesis 1985, 6, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Tokarz, A. Źródła Pokarmowe Oraz Efekty Prozdrowotne Sprzężonych Dienów Kwasu Linolowego (CLA). Biul. Wydz. Farm. Warsz. Uniw. Med. 2009, 1, 1–12. [Google Scholar]
- Białek, A.; Zagrodzki, P.; Tokarz, A. Chemometric analysis of the interactions among different parameters describing health conditions, breast cancer risk and fatty acids profile in serum of rats supplemented with conjugated linoleic acids. Prostaglandins Leukot. Essent. Fat. Acids 2016, 106, 1–10. [Google Scholar] [CrossRef]
- Zock, P.L.; Katan, M.B. Linoleic acid intake and cancer risk. A review. Am. J. Clin Nutr. 1998, 68, 142–153. [Google Scholar] [PubMed]
- Białek, A.; Tokarz, A.; Zagrodzki, P. Conjugated linoleic acids in diet of female rats inhibit the breast cancer formation in their offspring. J. Food Nutr. Res. 2014, 53, 39–50. [Google Scholar]
- Białek, A.; Tokarz, A.; Zagrodzki, P. Conjugated Linoleic Acids (CLA) Decrease the Breast Cancer Risk in DMBA-Treated Rats. Acta Pol. Pharm. 2015, 73, 315–327. [Google Scholar]
- Kelley, D.S.; Bartolini, G.L.; Newman, J.W.; Vemuri, M.; Mackey, B.E. Fatty acid composition of liver, adipose tissue, spleen, and heart of mice fed diets containing t10, c12-, and c9, t11-conjugated linoleic acid. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 331–338. [Google Scholar] [CrossRef]
- Yuan, G.-F.; Sinclair, A.J.; Sun, H.-Y.; Li, D. Fatty Acid Composition in Tissues of Mice Fed Diets Containing Conjugated Linolenic Acid and Conjugated Linoleic Acid. J. Food Lipids 2009, 16, 148–163. [Google Scholar] [CrossRef]
- Alasnier, C.; Berdeaux, O.; Chardigny, J.M.; Sébédio, J.L. Fatty acid composition and conjugated linoleic acid content of different tissues in rats fed individual conjugated linoleic acid isomers given as triacylglycerols. J. Nutr. Biochem. 2002, 13, 337–345. [Google Scholar]
- Diniz, Y.S.; Santos, P.P.; Assalin, H.B.; Souza, G.A.; Rocha, K.K.H.R.; Ebaid, G.M.X.; Seiva, F.R.F.; Amauchi, J.F.; Novelli Filho, J.L.V.B.; Novelli, E.L.B. Conjugated linoleic acid and cardiac health: Oxidative stress and energetic metabolism in standard and sucrose-rich diets. Eur. J. Pharmacol. 2008, 579, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Arab, L. Biomarkers of fat and fatty acid intake. J. Nutr. 2003, 133, 925S–932S. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Korniluk, K.; Wasowska, I. Improved saponification then mild base and acid-catalyzed methylation is a useful method for quantifying fatty acids, with special emphasis on conjugated dienes. Acta Chromatogr. 2007, 18, 59–71. [Google Scholar]
- Białek, M.; Czauderna, M.; Białek, A. Partial replacement of rapeseed oil with fish oil, and dietary antioxidants supplementation affects concentrations of biohydrogenation products and conjugated fatty acids in rumen and selected lamb tissues. Anim. Feed Sci. Technol. 2018, 241, 63–74. [Google Scholar] [CrossRef]
- Lopes, L.D.; Böger, B.R.; Cavalli, K.F.; dos, S.; Silveira-Júnior, J.F.; Osório, D.V.C.L.; de Oliveira, D.F.; Luchetta, L.; Tonial, I.B. Fatty acid profile, quality lipid index and bioactive compounds of flour from grape residues. Cienc. e Investig. Agrar. 2014, 41, 225–234. [Google Scholar] [CrossRef]
- Ghaeni, M.; Ghahfarokhi, K.N. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices in Leiognathusbindus and Upeneussulphureus. J. Mar. Sci. Res. Dev. 2013, 3, 3–5. [Google Scholar] [CrossRef]
- Bialek, A.; Bialek, M.; Jelinska, M.; Tokarz, A. Fatty acid composition and oxidative characteristics of novel edible oils in Poland. CyTA-Food 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Czauderna, M.; Marounek, M.; Duskova, D.; Kowalczyk, J. The sensitive and simple measurement of underivatized cholesterol and its oxygen derivatives in biological materials by capillary gas chromatography coupled to a mass-selective detector. Acta Chromatogr. 2013, 25, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Czauderna, M.; Kowalczyk, J.; Marounek, M. The simple and sensitive measurement of malondialdehyde in selected specimens of biological origin and some feed by reversed phase high performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2251–2258. [Google Scholar] [CrossRef]
- Statistica Data Analysis Software System, version 13, StaSoft Inc.: Tulsa, OK, USA, 2016.
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Adami, H.-O.; Day, N.E.; Trichopoulos, D.; Willett, W.C. Primary and secondary prevention in the reduction of cancer morbidity and mortality. Eur. J. Cancer 2001, 37, S118–S127. [Google Scholar] [CrossRef]
- S.717—21st Century Cancer ALERT (Access to Life-Saving Early detection, Research and Treatment) Act. Available online: https://www.congress.gov/bill/111th-congress/senate-bill/717/text (accessed on 21 July 2019).
- Bougnoux, P.; Hajjaji, N.; Maheo, K.; Couet, C.; Chevalier, S. Fatty acids and breast cancer: Sensitization to treatments and prevention of metastatic re-growth. Prog. Lipid Res. 2010, 49, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Tappia, P.S.; Mangat, R.; Gabriel, C.; Dent, M.R.; Aroutiounova, N.; Weiler, H. Gender differences in the cardiac response to dietary conjugated linoleic acid isomers. Can. J. Physiol. Pharmacol. 2006, 84, 257–264. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Ann, D.K. When fats commit crimes: Fatty acid metabolism, cancer stemness and therapeutic resistance. Cancer Commun. 2018, 38, 1–12. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Ayalew-Pervanchon, A.; Rousseau, D.; Moreau, D.; Assayag, P.; Weill, P.; Grynberg, A. Long-term effect of dietary α-linolenic acid or decosahexaenoic acid on incorporation of decosahexaenoic acid in membranes and its influence on rat heart in vivo. Am. J. Physiol. Circ. Physiol. 2007, 293, H2296–H2304. [Google Scholar] [CrossRef] [PubMed]
- Brochot, A.; Guinot, M.; Auchere, D.; MacAire, J.P.; Weill, P.; Grynberg, A. Effects of alpha-linolenic acid vs. docosahexaenoic acid supply on the distribution of fatty acids among the rat cardiac subcellular membranes after a short- or long-term dietary exposure. Nutr. Metab. 2009, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Van Bilsen, M.; Planavila, A. Fatty acids and cardiac disease: Fuel carrying a message. Acta Physiol. 2014, 211, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Stawarska, A.; Tokarz, A.; Czuba, K.; Konarska, A.; Mazurkiewicz, M.; Stanimirova-Daszykowska, I. Enrichment of maternal diet with conjugated linoleic acids influences desaturases activity and fatty acids profile in livers and hepatic microsomes of the offspring with 7,12-dimethylbenz[A]anthracene-induced mammary tumors. Acta Pol. Pharm-Drug Res. 2014, 71, 747–761. [Google Scholar]
- Tsuzuki, T.; Ikeda, I. Slow Absorption of Conjugated Linoleic Acid in Rat Intestines, and Similar Absorption Rates of 9 c,11 t -Conjugated Linoleic Acid and 10 t,12 c -Conjugated Linoleic Acid. Biosci. Biotechnol. Biochem. 2007, 71, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Parra, P.; Serra, F.; Palou, A. Conjugated Linoleic Acid Supplementation under a High-Fat Diet Modulates Stomach Protein Expression and Intestinal Microbiota in Adult Mice. PLoS ONE 2015, 10, e0125091. [Google Scholar] [CrossRef] [PubMed]
- Kamlage, B.; Hartmann, L.; Gruhl, B.; Blaut, M. Intestinal microorganisms do not supply associated gnotobiotic rats with conjugated linoleic acid. J. Nutr. 1999, 129, 2212–2217. [Google Scholar] [CrossRef]
- Jelińska, M.; Białek, A.; Gielecińska, I.; Mojska, H.; Tokarz, A. Impact of conjugated linoleic acid administered to rats prior and after carcinogenic agent on arachidonic and linoleic acid metabolites in serum and tumors. Prostaglandins Leukot. Essent. Fat. Acids 2017, 126, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gonenc, A.; Ozkan, Y.; Torun, M.; Simsek, B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J. Clin. Pharm. Ther. 2003, 26, 141–144. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Wąsowska, I.; Niedźwiedzka, K.; Pastuszewska, B. The effects of selenium and conjugated linoleic acid (CLA) isomers on fatty acid composition, CLA isomer content in tissues, and growth of rats. J. Anim. Feed Sci. 2003, 12, 865–881. [Google Scholar] [CrossRef]
- Khosla, P.; Fungwe, T.V. Conjugated linoleic acid: Effects on plasma lipids and cardiovascular function. Curr. Opin. Lipidol. 2001, 12, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Leonarduzzi, G.; Testa, G.; Gargiulo, S.; Poli, G.; Biasi, F. Lipid Oxidation Derived Aldehydes and Oxysterols Between Health and Disease. Eur. J. Lipid Sci. Technol. 2019, 121, 1–16. [Google Scholar] [CrossRef]
- Kulig, W.; Cwiklik, L.; Jurkiewicz, P.; Rog, T.; Vattulainen, I. Cholesterol oxidation products and their biological importance. Chem. Phys. Lipids 2016, 199, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Brzeska, M.; Szymczyk, K.; Szterk, A. Current Knowledge about Oxysterols: A Review. J. Food Sci. 2016, 81, R2299–R2308. [Google Scholar] [CrossRef] [PubMed]
- Grandgirard, A.; Demaison-Meloche, J.C.C.; Demaison, L. Incorporation of oxyphytosterols in tissues of hamster. Reprod. Nutr. Dev. 2004, 4544, 599–608. [Google Scholar] [CrossRef]
- Adachi, J.; Kudo, R.; Ueno, Y.; Hunter, R.; Rajendram, R.; Want, E.; Preedy, V.R. Heart 7-Hydroperoxycholesterol and Oxysterols Are Elevated in Chronically Ethanol-Fed Rats. J. Nutr. 2001, 131, 2916–2920. [Google Scholar] [CrossRef] [Green Version]
- Combe, N.; Clouet, P.; Chardigny, J.M.; Lagarde, M.; Léger, C.L. Trans fatty acids, conjugated linoleic acids, and cardiovascular diseases. Eur. J. Lipid Sci. Technol. 2007, 109, 945–953. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Jefferis, B.J.; Lennon, L.; Papacosta, O.; Whincup, P.H.; Hingorani, A.D. Serum conjugated linoleic acid and risk of incident heart failure in older men: The British regional heart study. J. Am. Heart Assoc. 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | Labofeed H | Safflower Oil | Bio-C.L.A. |
|---|---|---|---|
| C6:0 (μg/g) | 10.4 | nd | nd |
| C8:0 (mg/g) | nd | nd | 1.23 |
| C10:0 (μg/g) | nd | nd | 917 |
| C12:0 (μg/g) | 4.8 | nd | 29.2 |
| C14:0 (μg/g) | 18.6 | 209 | 209 |
| C15:0 (μg/g) | 10.0 | 53.8 | nd |
| C16:0 (mg/g) | 1.05 | 13.9 | 16.2 |
| c7 C16:1 (μg/g) | 11.9 | 167 | 31.8 |
| c9 C16:1 (μg/g) | 16.4 | 266 | 235 |
| C17:0 (μg/g) | 10.0 | 61.4 | 77.5 |
| c6C17:1 (μg/g) | 6.2 | nd | nd |
| c9C17:1 (μg/g) | nd | 69.0 | 54.2 |
| C18:0 (mg/g) | 0.44 | 0.01 | 6.48 |
| t11C18:1 (μg/g) | nd | nd | 53.4 |
| c9 C18:1 (mg/g) | 1.12 | 130 | 37.0 |
| c11 C18:1 (mg/g) | 0.04 | 2.12 | 2.66 |
| t9c12 C18:2 (μg/g) | nd | nd | 202 |
| c9c12 C18:2 (LA) (mg/g) | 4.12 | 75.3 | 42.7 |
| c9c12c15 C18:3 (ALA) (mg/g) | 2.21 | 0.95 | 0.00 |
| C20:0 (μg/g) | 10.5 | 957 | 820 |
| c9t11C18:2 (mg/g) | nd | nd | 99.6 |
| t7c9C18:2 (μg/g) | nd | nd | 944 |
| t10c12C18:2 (mg/g) | nd | nd | 97.6 |
| c11c13C18:2 (mg/g) | nd | nd | 4.13 |
| c9c11C18:2 (μg/g) | nd | nd | 699 |
| c11C20:1 (mg/g) | 10.4 | 619 | nd |
| c8c11c14c17C20:4n-3 (μg/g) | nd | 672 | nd |
| C22:0 (μg/g) | 5.4 | nd | 359 |
| C24:0 (μg/g) | nd | 202 | 64.9 |
| c15 C24:1 (μg/g) | nd | 240 | 187 |
| Conjugated fatty acids (mg/g) | |||
| ƩCFA | nd | 0.49 | 192 |
| ƩCD | nd | 0.23 | 189 |
| tt CD | nd | 0.17 | 5.18 |
| ct/tc CD | nd | 0.05 | 178 |
| cc CD | nd | nd | 6.23 |
| ƩCT: | 0.00 | 0.26 | 3.00 |
| ttt CT | nd | 0.22 | 2.78 |
| ttc CT | nd | 0.02 | 0.22 |
| cct CT | 0.00 | 0.02 | 0.00 |
| Cholesterol (μg/g) | 155 | nd | nd |
| 7AOH (μg/g) | nd | nd | nd |
| 7BOH (μg/g) | nd | nd | nd |
| 5,6AE (μg/g) | nd | nd | nd |
| 7K (μg/g) | nd | nd | nd |
| MDA (ng/g) | nd | nd | nd |
| Diet | SAF Oil | Bio-C.L.A. | p-Values for Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Group | SAF | SAFplus | CLA | CLAplus | Diet (D) | Mammary Tumors (MT) | Interaction | |
| Variables | (D × MT) | |||||||
| Total number of animals | 8 | 14 | 7 | 17 | - | - | - | |
| Number of animals with tumors | 0 | 13 | 0 | 15 | - | - | - | |
| Cancer incidence (%) | 0 | 93 | 0 | 88 | - | - | - | |
| Final body weight (g) | 223 ± 10.9 | 212 ± 10.5 | 226 ± 15.8 | 223 ± 18.1 | 0.15 | 0.13 | 0.39 | |
| Mass of heart (g) | 0.78 ± 0.05 | 0.94 ± 0.10 | 0.84 ± 0.08 | 0.95 ± 0.10 | 0.19 | 0 | 0.38 | |
| Heart mass – body mass ratio (%) | 0.35 ± 0.02 | 0.44 ± 0.05 | 0.37 ± 0.02 | 0.43 ± 0.04 | 0.71 | 0 | 0.12 | |
| First tumor appearance (week of life) | 0 | 18 ± 1.79 | 0 | 20 ± 1.64 | 0.26 | 0 | 0.26 | |
| Total number of tumors | 0 | 63c | 0a | 28b | 0.0326 | 0 | 0.0326 | |
| Number of tumors per animal | 0 | 4.5 | 0 | 1.6 | - | - | - | |
| Average tumor mass (g) | 0 | 5.4 ± 1.7 | 0 | 2.5 ± 0.4 | 0.0195 | 0.0017 | 0.19 | |
| Tumor mass – body mass ratio (%) | 0 | 2.6 | 0 | 1.1 | 0.17 | 0.0008 | 0.17 | |
| Diet | SAF Oil | Bio-C.L.A. | p-Values for Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Group | SAF (n = 8) | SAFplus | CLA (n = 7) | CLAplus | Diet (D) | Mammary Tumors (MT) | Interaction | |
| Variables | (n = 14) | (n = 17) | (D × MT) | |||||
| ƩFAs (mg/g) | 6.79 ± 19 | 6.42 ± 0.84 | 6.31 ± 0.74 | 6.73 ± 0.59 | 0.7 | 0.9 | 0.09 | |
| C8:0 (μg/g) | 48.2 ± 17.0b | 0.00 ± 0.00a | 30.7 ± 12.5ab | 23.7 ± 9.32ab | 0.55 | 0 | 0 | |
| C12:0 (μg/g) | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 8.80 ± 3.95b | 0 | 0 | 0 | |
| C14:0 (μg/g) | 22.0 ± 4.95 | 16.9 ± 5.31 | 20.9 ± 6.32 | 19.6 ± 5.96 | 0.69 | 0.11 | 0.34 | |
| C15:0 (μg/g) | 12.1 ± 2.80ab | 11.7 ± 5.12ab | 8.27 ± 3.19a | 16.1 ± 7.42b | 0.87 | 0.07 | 0.048 | |
| C16:0 (μg/g) | 970 ± 44.4 | 927 ± 127 | 958 ± 61.0 | 963 ± 105 | 0.73 | 0.57 | 0.48 | |
| C17:0 (μg/g) | 43.2 ± 6.10 | 39.3 ± 14.9 | 40.9 ± 10.3 | 40.9 ± 10.7 | 0.92 | 0.63 | 0.62 | |
| C18:0 (mg/g) | 2.06 ± 0.18 | 2.00 ± 0.31 | 1.97 ± 0.16 | 1.95 ± 0.17 | 0.37 | 0.59 | 0.8 | |
| C20:0 (μg/g) | 9.74 ± 5.18 | 7.13 ± 3.88 | 7.36 ± 3.13 | 10.5 ± 5.65 | 0.75 | 0.86 | 0.08 | |
| C21:0 (μg/g) | 6.35 ± 3.29 | 10.7 ± 5.48 | 3.92 ± 2.53 | 7.66 ± 4.53 | 0.13 | 0.0302 | 0.86 | |
| Ʃ SFA (mg/g) | 3.11 ± 0.16 | 3.00 ± 0.45 | 3.03 ± 0.23 | 3.03 ± 0.27 | 0.81 | 0.61 | 0.61 | |
| A-SFA (μg/g) | 992 ± 46.4 | 943 ± 128 | 976 ± 64.5 | 985 ± 108 | 0.72 | 0.57 | 0.41 | |
| T-SFA (mg/g) | 3.00 ± 0.16 | 2.94 ± 0.43 | 2.95 ± 0.23 | 2.93 ± 0.26 | 0.78 | 0.73 | 0.85 | |
| c7C16:1 (μg/g) | 12.2 ± 4.24 | 12.3 ± 4.58 | 11.3 ± 6.51 | 14.6 ± 8.26 | 0.75 | 0.46 | 0.49 | |
| c9C16:1 (μg/g) | 12.0 ± 4.63 | 12.4 ± 8.07 | 10.4 ± 2.61 | 13.3 ± 6.62 | 0.88 | 0.45 | 0.57 | |
| t9C18:1 (μg/g) | 16.1 ± 4.25b | 0.00 ± 0.00a | 8.23 ± 4.99ab | 14.3 ± 4.24b | 0.0171 | 0.0005 | 0 | |
| c9C18:1 (μg/g) | 269 ± 27.3b | 199 ± 60.5ab | 181 ± 37.9a | 196 ± 43.6ab | 0.0072 | 0.1 | 0.0109 | |
| c11C18:1 (μg/g) | 141 ± 6.89ab | 131 ± 16.4ab | 117 ± 19.5a | 144 ± 14.3b | 0.3 | 0.11 | 0.0012 | |
| Ʃ MUFA (μg/g) | 449 ± 24.5b | 356 ± 79.8ab | 327 ± 62.1a | 371 ± 62.0ab | 0.0198 | 0.27 | 0.0036 | |
| LA (mg/g) | 1.79 ± 0.89 | 1.67 ± 0.29 | 1.59 ± 0.28 | 1.80 ± 0.26 | 0.66 | 0.56 | 0.07 | |
| ALA (μg/g) | 69.9 ± 11.5 | 27.9 ± 7.39 | 21.2 ± 8.16 | 32.9 ± 9.66 | 0.17 | 0.34 | 0.09 | |
| c9t11C18:2 (μg/g) | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.15 ± 7.99 | 13.4 ± 6.32 | 0 | 0.21 | 0.21 | |
| t10c12C18:2 (μg/g) | 0.00 ± 0.00 | 0.00 ± 0.00 | 9.38 ± 6.19 | 13.6 ± 7.31 | 0 | 0.21 | 0.21 | |
| c11c14C20:2 (μg/g) | 6.49 ± 2.04 | 14.8 ± 7.92 | 7.20 ± 4.73 | 13.7 ± 4.74 | 0.93 | 0.0005 | 0.65 | |
| DGLA (μg/g) | 17.8 ± 6.66 | 16.9 ± 5.51 | 12.3 ± 4.66 | 18.6 ± 6.38 | 0.33 | 0.18 | 0.07 | |
| AA (μg/g) | 907 ± 58.0 | 851 ± 126 | 823 ± 107 | 892 ± 109 | 0.54 | 0.85 | 0.09 | |
| EPA (μg/g) | 12.7 ± 10.6 | 12.1 ± 2.06 | 15.5 ± 8.03 | 14.3 ± 6.06 | 0.41 | 0.76 | 0.91 | |
| DPA (μg/g) | 106 ± 4.77abc | 92.6 ± 12.7a | 94.3 ± 10.8ab | 124 ± 20.2c | 0.06 | 0.12 | 0.0001 | |
| DHA (μg/g) | 248 ± 44.9 | 381 ± 61.4 | 264 ± 78.7 | 412 ± 49.3 | 0.22 | 0.0433 | 0.69 | |
| Ʃ PUFA (mg/g) | 3.23 ± 0.16 | 3.06 ± 0.46 | 2.95 ± 0.47 | 3.33 ± 0.33 | 0.96 | 0.41 | 0.0435 | |
| n-3 PUFA (μg/g) | 498 ± 47.0 | 505 ± 71.2 | 504 ± 109 | 583 ± 57.5 | 0.09 | 0.09 | 0.15 | |
| n-6 PUFA (mg/g) | 2.73 ± 0.13 | 2.56 ± 0.41 | 2.43 ± 0.38 | 2.72 ± 0.31 | 0.56 | 0.6 | 0.0504 | |
| Indices | ||||||||
| PI | 6892 ± 402 | 6629 ± 935 | 6447 ± 1038 | 7232 ± 653 | 0.77 | 0.34 | 0.06 | |
| AI | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.07 | 0.5 | 0.17 | 0.41 | |
| TI | 0.96 ± 0.08ab | 1.00 ± 0.14ab | 1.04 ± 0.12b | 0.89 ± 0.07a | 0.64 | 0.13 | 0.013 | |
| HH | 3.37 ± 0.44 | 3.44 ± 0.46 | 3.16 ± 0.34 | 3.55 ± 0.27 | 0.68 | 0.07 | 0.21 | |
| Diet | SAF Oil | Bio-C.L.A. | p-Values for Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Group | SAF (n = 8) | SAFplus (n = 14) | CLA (n = 7) | CLAplus | Diet (D) | Mammary Tumors (MT) | Interaction (D × MT) | |
| Variables | (n = 17) | |||||||
| CFA (μg/g) | 19.0 ± 4.19ab | 13.8 ± 1.71a | 74.1 ± 63.7c | 32.0 ± 13.3bc | 0.0002 | 0.0108 | 0.0429 | |
| CD | 12.1 ± 2.79a | 11.5 ± 2.35a | 58.3 ± 51.1b | 28.1 ± 11.1b | 0.0001 | 0.0325 | 0.0393 | |
| tt | 7.72 ± 1.82a | 8.06 ± 1.18a | 30.2 ± 26.9b | 13.5 ± 5.54b | 0.0006 | 0.0343 | 0.0278 | |
| ct/tc | 2.86 ± 0.81 | 2.11 ± 0.55 | 25.6 ± 22.9 | 13.3 ± 5.52 | 0 | 0.0521 | 0.08 | |
| cc | 1.03 ± 0.22ab | 0.90 ± 0.27a | 2.52 ± 1.64b | 1.31 ± 0.47ab | 0.0004 | 0.0096 | 0.0326 | |
| CT | 6.90 ± 1.51b | 2.81 ± 0.84a | 15.7 ± 12.7b | 3.26 ± 1.15a | 0.0113 | 0 | 0.0211 | |
| ttt | 4.55 ± 1.05b | 1.02 ± 0.53a | 11.7 ± 10.9b | 1.12 ± 0.35a | 0.0165 | 0 | 0.0194 | |
| ttc | 0.20 ± 0.05 | 0.72 ± 0.61 | 0.22 ± 0.14 | 0.62 ± 0.44 | 0.78 | 0.0039 | 0.69 | |
| cct | 2.10 ± 0.19bc | 1.26 ± 0.22a | 3.63 ± 1.51c | 1.60 ± 0.62ab | 0.0006 | 0 | 0.0218 | |
| Diet | SAF Oil | Bio-C.L.A. | p-Values for Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Group | SAF (n = 8) | SAFplus (n = 14) | CLA (n = 7) | CLAplus (n = 17) | Diet (D) | Mammary Tumors (MT) | Interaction (D × MT) | |
| Variables | ||||||||
| MDA (ng/g) | 7182 ± 1018 | 8205 ± 1798 | 6171 ± 1548 | 6125 ± 1052 | 0.0012 | 0.28 | 0.24 | |
| Cholesterol (mg/g) | 2.97 ± 0.10 | 3.05 ± 0.17 | 2.85 ± 0.15 | 2.95 ± 0.19 | 0.06 | 0.14 | 0.88 | |
| Oxysterols | ||||||||
| 7AOH (μg/g) | 0.94 ± 0.27 | 1.16 ± 0.51 | 0.79 ± 0.10 | 0.91 ± 0.27 | 0.12 | 0.19 | 0.68 | |
| 7BOH (μg/g) | 0.16 ± 0.08 | 0.14 ± 0.03 | 0.10 ± 0.01 | 0.14 ± 0.07 | 0.18 | 0.87 | 0.19 | |
| 5,6AE (μg/g) | 0.65 ± 0.17 | 0.80 ± 0.39 | 0.63 ± 0.08 | 0.66 ± 0.14 | 0.35 | 0.31 | 0.51 | |
| 7K (μg/g) | 1.65 ± 0.46 | 2.84 ± 1.41 | 1.38 ± 0.22 | 1.86 ± 0.64 | 0.06 | 0.0139 | 0.27 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białek, M.; Białek, A.; Czauderna, M. Conjugated Linoleic Acid Isomers Affect Profile of Lipid Compounds and Intensity of Their Oxidation in Heart of Rats with Chemically-Induced Mammary Tumors—Preliminary Study. Nutrients 2019, 11, 2032. https://doi.org/10.3390/nu11092032
Białek M, Białek A, Czauderna M. Conjugated Linoleic Acid Isomers Affect Profile of Lipid Compounds and Intensity of Their Oxidation in Heart of Rats with Chemically-Induced Mammary Tumors—Preliminary Study. Nutrients. 2019; 11(9):2032. https://doi.org/10.3390/nu11092032
Chicago/Turabian StyleBiałek, Małgorzata, Agnieszka Białek, and Marian Czauderna. 2019. "Conjugated Linoleic Acid Isomers Affect Profile of Lipid Compounds and Intensity of Their Oxidation in Heart of Rats with Chemically-Induced Mammary Tumors—Preliminary Study" Nutrients 11, no. 9: 2032. https://doi.org/10.3390/nu11092032





