Short-Term Betanin Intake Reduces Oxidative Stress in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Feed Stuff Processing
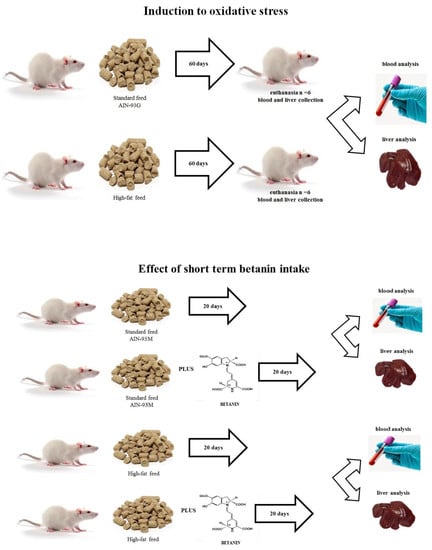
2.3. Animals
2.4. Plasma Analyses
2.4.1. Biochemical Analyses
2.4.2. Antioxidant Enzyme Activities
2.5. Tissue Analyses
Thiobarbituric Acid Reactive Substances (TBARS)
2.6. Histopathological Analyses
2.7. Statistical Analysis
3. Results
3.1. Biochemical Parameter Analyses
3.2. Antioxidant Enzyme Activities
3.3. Thiobarbituric Acid Reactive Substances (TBARS) in Liver Tissue
3.4. Histopathological Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fearon, I.M.; Faux, S.P. Oxidative stress and cardiovascular disease: Novel tools give (free) radical insight. J. Mol. Cell. Cardiol. 2009, 47, 372–381. [Google Scholar] [CrossRef]
- World Health Organization, Media Centre, Cardiovascular Diseases (CVDs). Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 20 March 2019).
- Celermajer, D.S.; Chow, C.K.; Marijon, E.; Anstey, N.M.; Woo, K.S. Cardiovascular disease in the developing world: Prevalences, patterns, and the potential of early disease detection. J. Am. Coll. Cardiol. 2012, 60, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Lassegue, B.; Griendling, K.K. Reactive Oxygen Species in Hypertension. Am. J. Hypertens. 2004, 17, 852–860. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Yang, R.L.; Shi, Y.H.; Hao, G.; Wu, L.; Guo-Wei, L. Increasing oxidative stress with progressive hyperlipidemia in human: Relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Sugamura, K.; Keaney, J.F., Jr. Reactive oxygen species in cardiovascular disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Long. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Maytin, M.; Leopold, J.; Loscalzo, J. Oxidant stress in the vasculature. Curr. Ather. Rep. 1999, 1, 156–164. [Google Scholar] [CrossRef]
- Robinett, N.G.; Peterson, R.L.; Culotta, C.V. Eukaryotic copper-only superoxide dismutases (SODs): A new class of SOD enzymes and SOD-like protein domains. J. Biol. Chem. 2018, 293, 4636–4643. [Google Scholar] [CrossRef]
- Flohé, L. The impact of thiol peroxidases on redox regulation. Free Radic. Res. 2015, 50, 1–7. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 1–11. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P. Nutrients and oxidative stress: Friend or foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef] [PubMed]
- Ballal, K.; Wilson, C.R.; Harmancey, R.; Taegtmeyer, H. Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Mol. Cell. Biochem. 2010, 344, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.T.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Bruce, K.D.; Cagampang, F.R.; Argenton, M.; Zhang, J.; Ethirajan, P.L.; Burdge, G.C.; Bateman, A.C.; Clough, G.F.; Poston, L.; Hanson, M.A.; et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 2009, 50, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Venezuela, R.; Echeverria, F.; Ortiz, M.; Rincón-Cervera, M.A.; Espinosa, A.; Hernandez-Rodas, M.A.; Illesca, P.; Valenzuela, A.; Videla, L.A. Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids Health Dis. 2017, 16, 1–16. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, 1–14. [Google Scholar] [CrossRef]
- Pollock, R.L. The effect of green leafy and cruciferous vegetable intake on the incidence of cardiovascular disease: A meta-analysis. J. R. Soc. Med. Cardiovasc. Dis. 2016, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Ushida, Y.; Shiozawa, H.; Umeda, R.; Tsuruya, K.; Aoki, Y.; Suganuma, H.; Nishizaki, Y. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J. Gastroenterol. 2015, 21, 12457–12467. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef]
- Baião, D.S.; Silva, D.V.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. In Food Additives, 1st ed.; InTechOpen: Rijeka, Croatia, 2017; Volume v.x, pp. 1–24. [Google Scholar] [CrossRef]
- Silva, D.V.T.; Baião, D.S.; Silva, O.S.; Alves, G.; Perrone, D.; Del Aguila, E.M.; Paschoalin, V.M.F. Betanin, a natural food additive: Stability, bioavailability, antioxidant and preservative ability assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef]
- Gliszczyńska-Swigło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; D’Alessio, P. Antioxidant Betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression. Ann. N.Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.; Szaefer, H.; Baer-Dubowska, W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Tan, C.; Wang, Y.; Yang, S.; Tan, D. Betanin reduces the accumulation and cross-links of collagen in high-fructose-fed rat heart through inhibiting non-enzymatic glycation. Chem. Biol. Interact. 2015, 227, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Dhananjayan, I.; Kathiroli, S.; Subramani, S.; Veerasamy, V. Ameliorating effect of betanin, a natural chromoalkaloid by modulating hepatic carbohydrate metabolic enzyme activities and glycogen content in streptozotocin—Nicotinamide induced experimental rats. Biomed. Pharmacother. 2017, 88, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.M. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Wheeler, C.R.; Salzman, J.A.; Elsayed, N.M.; Omaye, S.T.; Korte, D.W., Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990, 184, 193–199. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Jung, S.; Nam, C.K.; Jo, C. Detection of malondialdehyde in processed meat products without interference from the ingredients. Food Chem. 2016, 209, 90–94. [Google Scholar] [CrossRef]
- Yu, T.; Jhun, B.S.; Yoon, Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid. Redox. Signal. 2011, 14, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Klafke, J.Z.; Porto, F.G.; Batista, R.; Bochi, G.V.; Moresco, R.N.; da Luz, P.L.; Viecili, P.R. Association between hypertriglyceridemia and protein oxidation and proinflammatory markers in normocholesterolemic and hypercholesterolemic individuals. Clin. Chim. Acta 2015, 448, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sarna, L.K.; Sid, V.; Wang, P.; Siow, Y.L.; House, J.D.; Karmin, O. Tyrosol attenuates high fat diet-induced hepatic oxidative stress: Potential involvement of cystathionine β-synthase and cystathionine γ-lyase. Lipids 2016, 51, 583–590. [Google Scholar] [CrossRef] [PubMed]
- González-Mañán, D.; D’Espessailles, A.; Dossi, C.G.; San Martín, M.; Mancilla, R.A.; Tapia, G.S. Rosa mosqueta oil prevents oxidative stress and inflammation through the upregulation of PPAR-α and NRF2 in C57BL/6J mice fed a high-fat diet. J. Nutr. 2017, 147, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Juśkiewicz, J.; Wiczkowski, W. Using the SPE and Micro-HPLC-MS/MS Method for the Analysis of Betalains in Rat Plasma after Red Beet Administration. Molecules 2017, 22, 2137. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska, M.; Juskiewicz, J.; Wiczkowski, W. Physiological properties of beetroot crisps applied in standard and dyslipidaemic diets of rats. Lipids Health Dis. 2011, 10, 178. [Google Scholar] [CrossRef]
- Hirano, T.; Mamo, J.C.; Furukawa, S.; Nagano, S.; Takahashi, T. Effect of acute hyperglycemia on plasma triglyceride concentration and triglyceride secretion rate in non-fasted rats. Diabetes Res. Clin. Pract. 1990, 9, 231–238. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Everhart, J.E. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009, 136, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Westerbacka, J.; Cornér, A.; Tiikkainen, M.; Vehkavaara, S.; Häkkinen, A.M.; Fredriksson, J.; Yki-Järvinen, H. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: Implications for sex differences in markers of cardiovascular risk. Diabetologia 2004, 47, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Gao, C.; Yang, S.; Wang, J.; Tan, D. Betanin attenuates carbon tetrachloride (CCl4)-induced liver injury in common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2013, 40, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Minhajuddin, M.; Beg, Z.H.; Iqbal, J. Hypolipidemic and antioxidant properties of tocotrienol rich fraction isolated from rice bran oil in experimentally induced hyperlipidemic rats. Food Chem. Toxicol. 2005, 43, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Le, G.; Li, A.; Zheng, J.; Shi, Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition 2006, 22, 1185–1191. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Bai, B.; Yang, X.; Han, J. Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat. Food Chem. Toxicol. 2015, 78, 141–146. [Google Scholar] [CrossRef]
- Sutariya, B.; Saraf, M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J. Ethnopharmacol. 2017, 198, 432–443. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Yang, S.; Wang, J.; Yang, X.; Tan, D. Betanin attenuates paraquat-induced liver toxicity through a mitochondrial pathway. Food Chem. Toxicol. 2014, 70, 100–106. [Google Scholar] [CrossRef]
- Sutariya, B.; Taneja, N.; Badgujar, L.; Saraf, M. Modulatory effect of betanin on high glucose induced epithelial to mesenchymal transition in renal proximal tubular cells. Biomed. Pharmacother. 2017, 89, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Baião, D.S.; d’El-Rei, J.; Alves, G.; Neves, M.F.; Perrone, D.; Del Aguila, E.M.; Paschoalin, V.M.F. Chronic effects of nitrate supplementation with a newly designed beetroot formulation on biochemical and hemodynamic parameters of individuals presenting risk factors for cardiovascular diseases: A pilot study. J. Func. Foods 2019, 58, 85–94. [Google Scholar] [CrossRef]




| Ingredients | Standard AIN-93G | High-Fat AIN-93G |
|---|---|---|
| Casein (g) | 20 | 20 |
| Starch (g) | 52.9 | 27.95 |
| Soy oil (g) | 7 | 7 |
| Lard (g) | 0 | 25 |
| Sugar (sucrose, g) | 10 | 10 |
| Minerals (mg) | 1.52 | 1.52 |
| Calcium | 500 | 500 |
| Phosphorus | 300 | 300 |
| Magnesium | 50 | 50 |
| Sodium | 104 | 104 |
| Potassium | 360 | 360 |
| Chloride | 163 | 163 |
| Sulfur | 30 | 30 |
| Iron | 4 | 4 |
| Zinc | 4 | 4 |
| Manganese | 1 | 1 |
| Vitamins | 5.5 | 5.5 |
| Nicotinic acid (mg) | 1.5 | 1.5 |
| Pantothenic acid (mg) | 1.5 | 1.5 |
| Pyridoxine (mg) | 0.6 | 0.6 |
| Thiamine (mg) | 0.5 | 0.5 |
| Riboflavin (mg) | 0.6 | 0.6 |
| Folic acid (mg) | 0.2 | 0.2 |
| Biotin (mg) | 0.002 | 0.002 |
| Vitamin B12 (µg) | 2.5 | 2.5 |
| Vitamin K (µg) | 90 | 90 |
| Vitamin E (µg) | 400 | 400 |
| Vitamin A (µg) | 120 | 120 |
| Vitamin D (µg) | 3 | 3 |
| l-Cysteine (g) | 0.3 | 0.3 |
| Colin (g) | 0.25 | 0.25 |
| Cellulose (g) | 5 | 5 |
| Total | 100 | 100 |
| PTN (g) | 17.3 | 17.3 |
| CHO (g) | 54.2 | 29.2 |
| LIP (g) | 7 | 32 |
| SFA (g) | 1.1 | 10.9 |
| MUFA (g) | 1.7 | 12.9 |
| PUFA (g) | 3.6 | 6.4 |
| Cholesterol (g) | 0 | 0.02 |
| Total fiber (g) | 5 | 5 |
| kcal | 349 | 474 |
| Phase 1 Study | ||
|---|---|---|
| Parameters | CONT 60 | HF 60 |
| Glucose (mg·dL−1) | 83.3 ± 3.1 | 120.2 ± 8.0* |
| Insulin (µUi·mL−1) | 32.9 ± 2.6 | 38.5 ± 3.6 |
| HOMA-IR (mmol·L−1) | 6.7 ± 0.5 | 11.3 ± 1.2* |
| TC (mg·dL−1) | 47.6 ± 2.0 | 58.1 ± 2.1* |
| TG (mg·dL−1) | 27.1 ± 1.9 | 31.7 ± 4.4* |
| AST (U·L−1) | 133.1 ± 8.4 | 216.2 ± 10.5* |
| ALT (U·L−1) | 40.0 ± 3.2 | 48.4 ± 6.2 |
| Phase 2 Study | ||||
|---|---|---|---|---|
| Biochemical Parameters | CONT 80 | CONT 80+BET | HF 80 | HF 80+BET |
| Glucose (mg·dL−1) | 124.9 ± 12.3a | 112.6 ± 16.5a | 137.2 ± 23.4b | 106.6 ± 8.3a |
| Insulin (µUi·mL−1) | 42.7 ± 5.3a | 36.7 ± 6.9a | 59.8 ± 4.9b | 39.4 ± 7.1a |
| HOMA-IR (mmol·L−1) | 13.2 ± 2.3a | 10.2 ± 2.1a | 20.4 ± 4.9b | 10.9 ± 1.7a |
| TC (mg·dL−1) | 43.7 ± 7.7a | 45.7 ± 3.9a | 69.7 ± 10.8b | 71.7 ± 5.5b |
| TG (mg·dL−1) | 20.3 ± 5.1a | 19.6 ± 3.1a | 38.8 ± 8.6b | 28.6 ± 5.9a |
| AST (U·L−1) | 193.0 ± 74.5a | 172.0 ± 49.5a | 265.5 ± 60.3b | 137.6 ± 27.3a |
| ALT (U·L−1) | 68.4 ± 2.8a | 56.1 ± 10.4b | 78.2 ± 7.3c | 37.6 ± 7.2b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, D.V.T.; Pereira, A.D.; Boaventura, G.T.; Ribeiro, R.S.d.A.; Verícimo, M.A.; Carvalho-Pinto, C.E.d.; Baião, D.d.S.; Del Aguila, E.M.; Paschoalin, V.M.F. Short-Term Betanin Intake Reduces Oxidative Stress in Wistar Rats. Nutrients 2019, 11, 1978. https://doi.org/10.3390/nu11091978
da Silva DVT, Pereira AD, Boaventura GT, Ribeiro RSdA, Verícimo MA, Carvalho-Pinto CEd, Baião DdS, Del Aguila EM, Paschoalin VMF. Short-Term Betanin Intake Reduces Oxidative Stress in Wistar Rats. Nutrients. 2019; 11(9):1978. https://doi.org/10.3390/nu11091978
Chicago/Turabian Styleda Silva, Davi Vieira Teixeira, Aline D’Avila Pereira, Gilson Teles Boaventura, Roberto Stefan de Almeida Ribeiro, Maurício Afonso Verícimo, Carla Eponina de Carvalho-Pinto, Diego dos Santos Baião, Eduardo Mere Del Aguila, and Vania M. Flosi Paschoalin. 2019. "Short-Term Betanin Intake Reduces Oxidative Stress in Wistar Rats" Nutrients 11, no. 9: 1978. https://doi.org/10.3390/nu11091978
APA Styleda Silva, D. V. T., Pereira, A. D., Boaventura, G. T., Ribeiro, R. S. d. A., Verícimo, M. A., Carvalho-Pinto, C. E. d., Baião, D. d. S., Del Aguila, E. M., & Paschoalin, V. M. F. (2019). Short-Term Betanin Intake Reduces Oxidative Stress in Wistar Rats. Nutrients, 11(9), 1978. https://doi.org/10.3390/nu11091978