Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells
Abstract
:1. Introduction
2. Materials and Methods
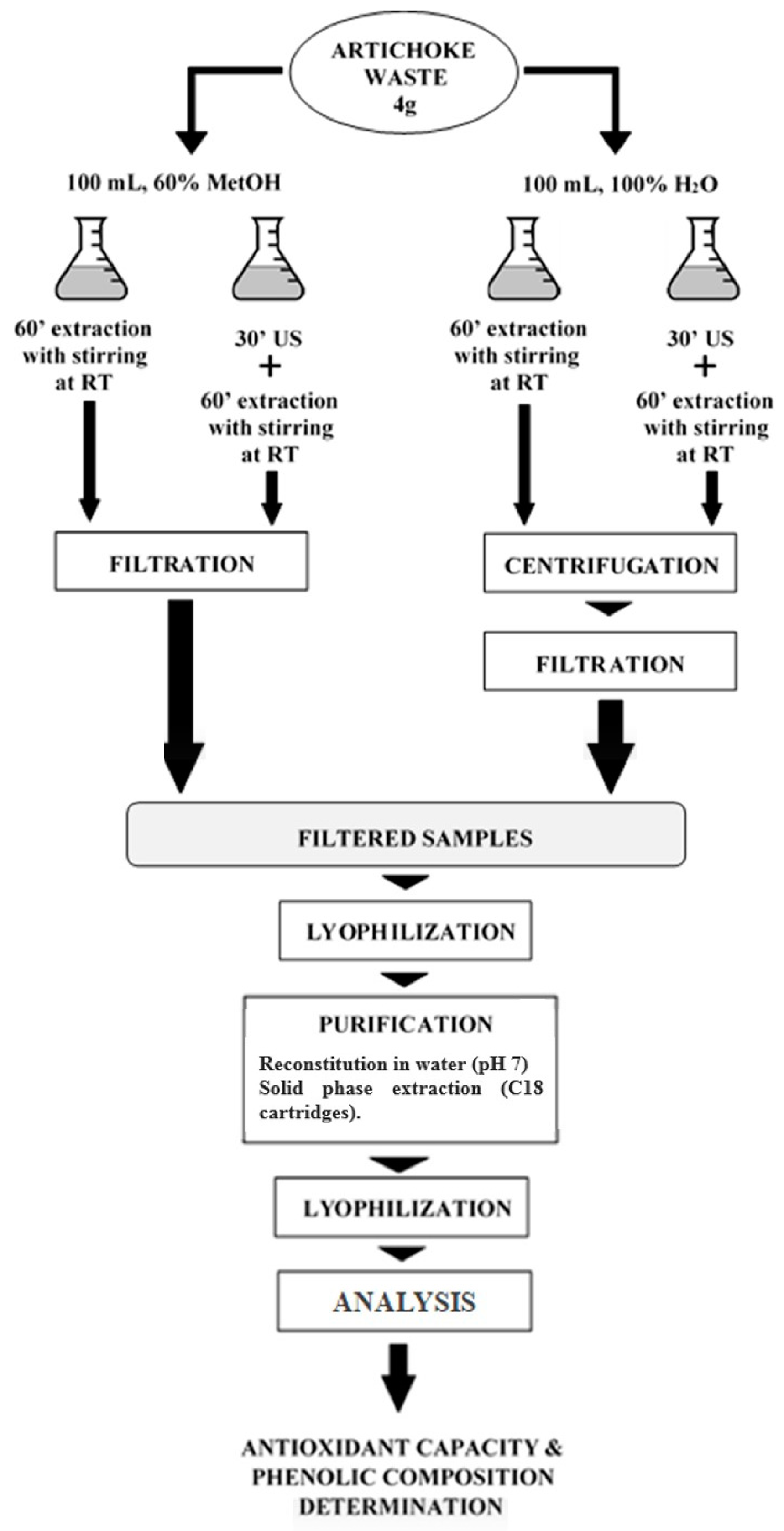
2.1. Raw Material and Extraction Process
2.2. Determination of Phenol Compounds of the Extracts by High Performance Liquid Chromatography
2.3. Antioxidant Capacity and Total Phenol Content of the Extracts Obtained from Artichoke Waste
2.4. Biological Assays
2.4.1. Cell Culture
2.4.2. Measurement of Cell Proliferation
2.4.3. Measurement of Intracellular Reactive Oxygen Species Levels
2.5. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Composition of the Extracts Obtained from Artichoke Waste
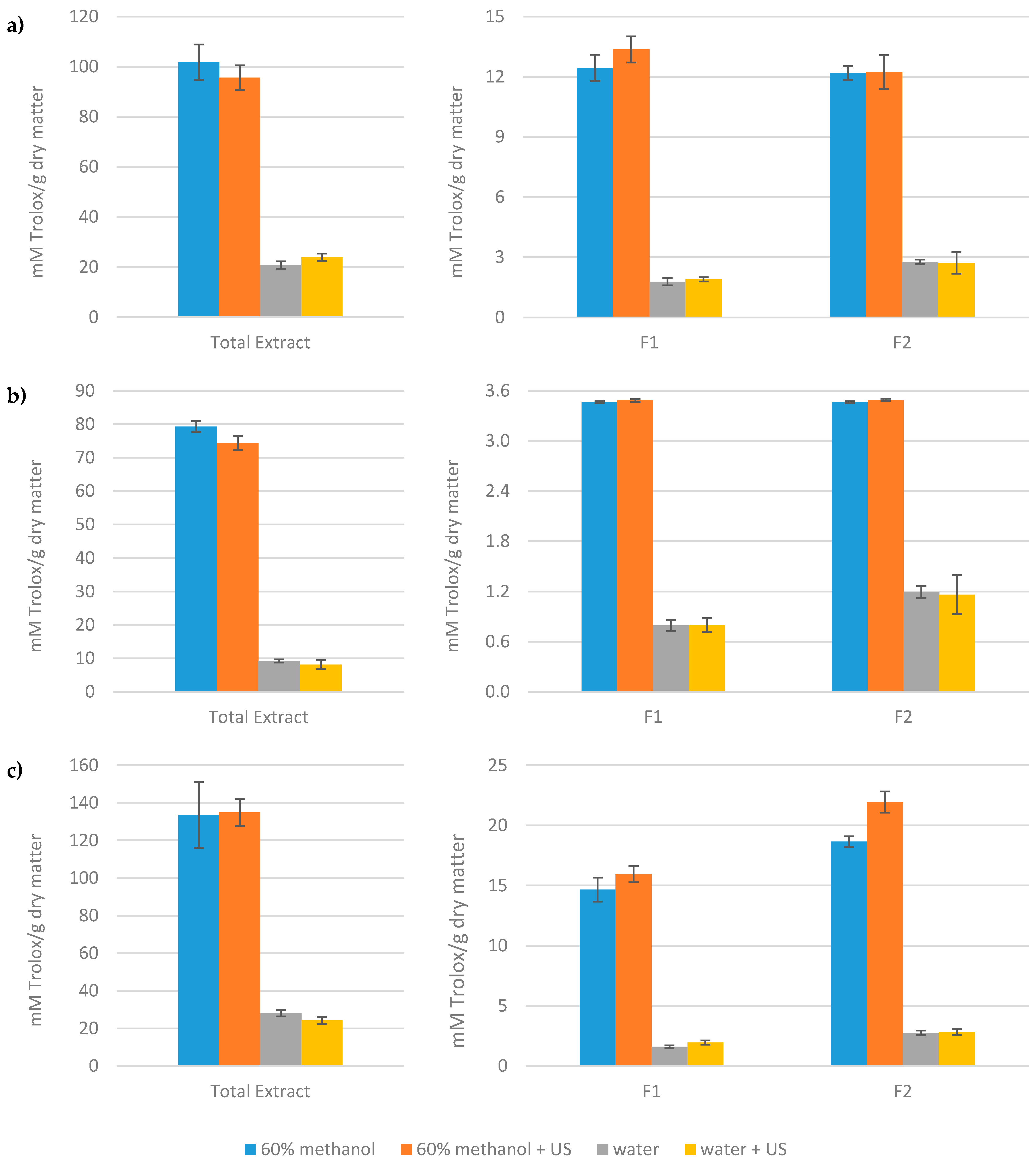
3.2. Antioxidant Capacity and Total Phenol Content of the Extracts Obtained from Artichoke Waste
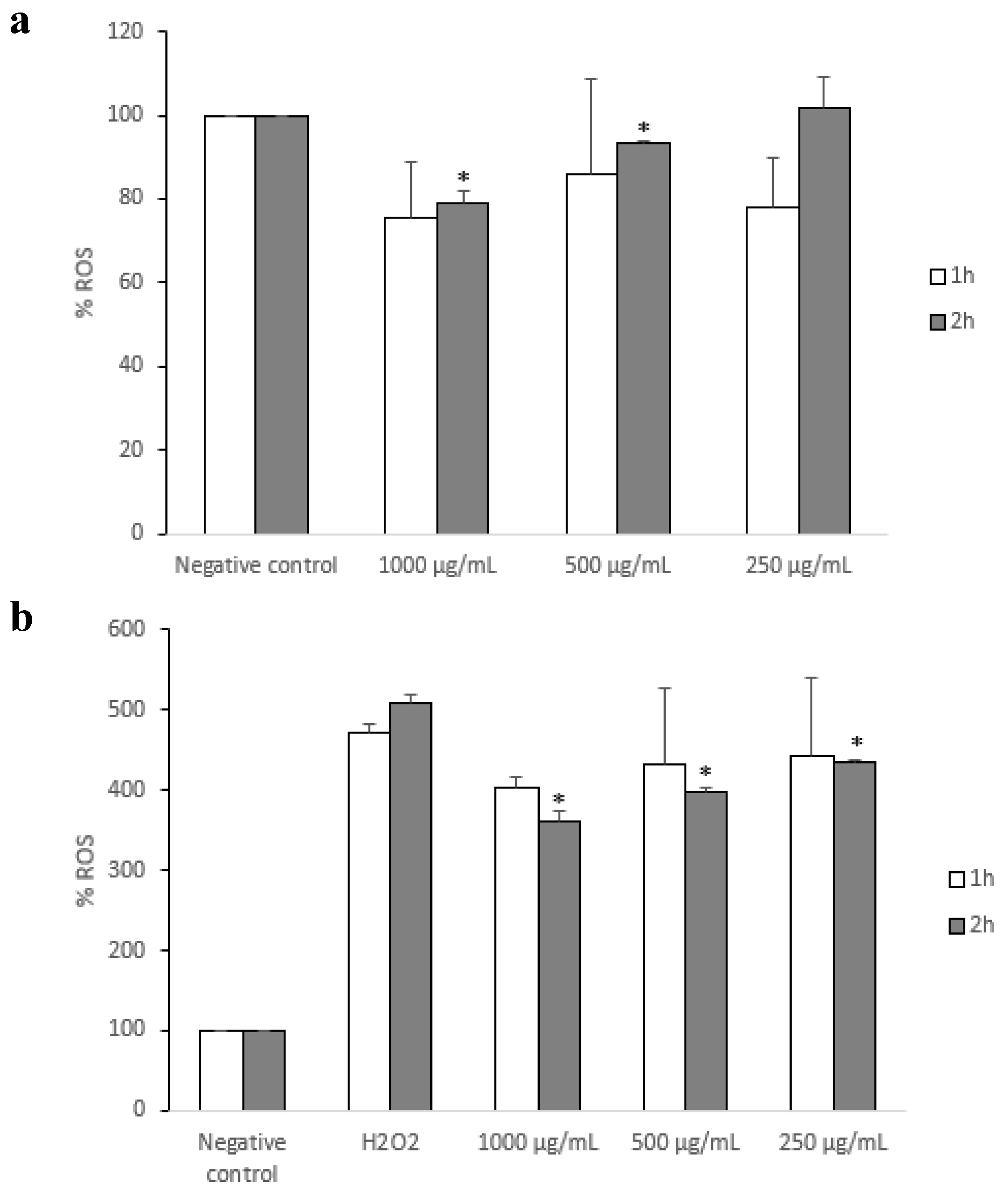
3.3. Antioxidant Capacity on a Model Intestinal Barrier
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Gascón, S.; Jiménez-Moreno, N.; Jiménez, S.; Quero, J.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Nutraceutical composition of three pine bark extracts and their antiproliferative effect on Caco-2 cells. J. Funct. Food 2018, 48, 420–429. [Google Scholar] [CrossRef]
- Jiménez, S.; Jiménez-Moreno, N.; Luquin, A.; Laguna, M.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Chemical composition of rosehips from different Rosa species: An alternative source of antioxidants for the food industry. Food Addit. Contam. Part A Chem. 2017, 34, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-De-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M. Therapeutic applications of rose hips from different Rosa species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Quero, J.; Jiménez-Moreno, N.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. A systematic review of the potential uses of pine bark in food industry and health care. Trends Food Sci. Technol. 2019, 88, 558–566. [Google Scholar] [CrossRef]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F.J. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- López-Molina, D.; Navarro-Martínez, M.D.; Melgarejo, F.R.; Hiner, A.N.P.; Chazarra, S.; Rodríguez-López, J.N. Molecular properties and prebiotic effect of inulin obtained from artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar]
- Orlovskaya, T.V.; Luneva, I.L.; Chelombit’ko, V.A. Chemical composition of Cynara scolymus leaves. Chem. Nat. Compd. 2007, 43, 239–240. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Globe artichoke leaves and floral stems as a source of bioactive compounds. Ind. Crop. Prod. 2013, 44, 44–49. [Google Scholar] [CrossRef]
- Sonnante, G.; de Paolis, A.; Lattanzio, V.; Perrino, P. Genetic variation in wild and cultivated artichoke revealed by RAPD markers. Genet. Resour. Crop. Evol. 2002, 49, 247–252. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Food 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Negro, D.; Montesano, V.; Grieco, S.; Crupi, P.; Sarli, G.; De Lisi, A.; Sonnante, G. Polyphenol compounds in artichoke plant tissues and varieties. J. Food Sci. 2012, 77, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Lombardo, S.; Mauro, R.P.; Mauromicale, G. Variation in polyphenol profile and head morphology among clones of globe artichoke selected from a landrace. Sci. Hortic. 2012, 138, 259–265. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Ntatsi, G.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and chemical composition of Greek artichoke genotypes. Food Chem. 2018, 267, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Tucci, M.; de Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Vázquez-Olivo, G.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Osuna-Enciso, T.; León-Félix, J.; Basilio-Heredia, J. Cellular antioxidant activity and in vitro intestinal permeability of phenolic compounds from four varieties of mango bark (Magnifera indica L.). J. Sci. Food Agric. 2019, 99, 3481–3489. [Google Scholar] [CrossRef]
- Jiménez, S.; Gascón, S.; Luquin, A.; Laguna, M.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Rosa canina extracts have antiproliferative and antioxidant effects on Caco-2 human colon cancer. PLoS ONE 2016, 11, e0159136. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A. Increase of antioxidant activity of tomato juice upon functionalisation with vegetable byproduct extracts. LWT Food Sci. Technol. 2002, 35, 532–542. [Google Scholar] [CrossRef]
- Llorach, R.; Tomás-Barberán, F.A.; Ferreres, F. Functionalisation of commercial chicken soup with enriched polyphenol extract from vegetable by-products. Eur. Food Res. Technol. 2005, 220, 31–36. [Google Scholar] [CrossRef]
- Pasqualone, A.; Punzi, R.; Trani, A.; Summo, C.; Paradiso, V.M.; Caponio, F.; Gambacorta, G. Enrichment of fresh pasta with antioxidant extracts obtained from artichoke canning by-products by ultrasound-assisted technology and quality characterisation of the end product. Int. J. Food Sci. Technol. 2017, 52, 2078–2087. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knödler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Sánchez-de-Diego, C.; Mármol, I.; Pérez, R.; Gascón, S.; Rodríguez-Yoldi, M.J.; Cerrada, E. The anticancer effect related to disturbances in redox balance on Caco-2 cells caused by an alkynyl gold (I) complex. J. Inorg. Biochem. 2017, 166, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Kammerer, D.; Carle, R.; Schieber, A. Identification and quantification of caffeoylquinic acids and flavonoids from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MS. J. Agric. Food Chem. 2004, 52, 4090–4096. [Google Scholar]
- Prasad, N.R.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Kampa, M.; Alexaki, V.I.; Notas, G.; Nifli, A.P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, E.; Kouimtzoglou, E.; Blekas, G.; Boskou, D.; et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004, 6, 63–74. [Google Scholar] [CrossRef]
- Jackson, K.M.P.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Structural constraints and importance of caffeic acid moiety for anti-hyperglycemic effects of caffeoylquinic acids from chicory. Mol. Nutr. Food Res. 2017, 61, 1–9. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Comp. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Gebhardt, R.; Fausel, M. Antioxidant and hepatoprotective effects of artichoke extracts and constituents in cultured rat hepatocytes. Toxicol. Vitro 1997, 11, 669–672. [Google Scholar] [CrossRef]
- Pandino, G.; Courts, F.L.; Lombardo, S.; Mauromicale, G.; Williamson, G. Caffeoylquinic acids and flavonoids in the immature inflorescence of globe artichoke, wild cardoon, and cultivated cardoon. J. Agric. Food Chem. 2010, 58, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Effect of solvent type and extraction conditions on the recovery of phenolic compounds from artichoke waste. Chem. Eng. Trans. 2014, 39, 463–468. [Google Scholar] [CrossRef]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Muks, E.; Carle, R.; Schieber, A. Quantitative determination of phenolic compounds in artichoke-based dietary supplements and pharmaceuticals by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 8812–8817. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef]
- Speroni, E.; Cervellati, R.; Govoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M.C. Efficacy of different Cynara scolymus preparations on liver complaints. J. Ethnopharmacol. 2003, 86, 203–311. [Google Scholar] [CrossRef]
- Punzi, R.; Paradiso, A.; Fasciano, C.; Trani, A.; Faccia, M.; de Pinto, M.C.; Gambacorta, G. Phenols and antioxidant activity in vitro and in vivo of aqueous extracts obtained by ultrasound-assisted extraction from artichoke by-products. Nat. Prod. Commun. 2014, 9, 1315–1318. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Machado, M.T.C.; Martínez, J.; Hubinger, M.D. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J. Food Eng. 2016, 178, 170–180. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Fritsche, J.; Beindorff, C.M.; Dachtler, M.; Zhang, H.; Lammers, J.G. Isolation, characterization and determination of minor artichoke (Cynara scolymus L.) leaf extract compounds. Eur. Food Res. Technol. 2002, 215, 149–157. [Google Scholar] [CrossRef]
- Pérez-García, F.; Adzet, T.; Cañigueral, S. Activity of artichoke leaf extract on reactive oxygen species in human leukocytes. Free Radic. Res. 2000, 33, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Brown, J.E.; Rice-Evans, C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998, 29, 247–255. [Google Scholar] [CrossRef]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant evaluation protocols: Food quality or health effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Food 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef] [PubMed]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; de Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Zeller, P.; Bricks, T.; Vidal, G.; Jacques, S.; Anton, P.M.; Leclerc, E. Multiparametric temporal analysis of the Caco-2/TC7 demonstrated functional and differentiated monolayers as early as 14 days of culture. Eur. J. Pharm. Sci. 2011, 72, 1–11. [Google Scholar] [CrossRef]
- Kolacek, M.; Muchova, J.; Dvorakova, M.; Paduchova, Z.; Zitnanova, I.; Cierna, I.; Országhová, A.; Székyova, D.; Jajcaiová-Zednícková, N.; Kovács, L.; et al. Effect of natural polyphenols (Pycnogenol) on oxidative stress markers in children suffering from Crohn’s disease—A pilot study. Free Radic. Res. 2013, 47, 624–634. [Google Scholar] [CrossRef]
- Almeer, R.S.; Mahmoud, S.M.; Amin, H.K.; Abdel Moneim, A.E. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem. Toxicol. 2018, 115, 49–62. [Google Scholar] [CrossRef]
- Kumar, V.L.; Pandey, A.; Verma, S.; Das, P. Protection afforded by methanol extract of Calotropis procera latex in experimental model of colitis is mediated through inhibition of oxidative stress and pro-inflammatory signaling. Biomed. Pharm. 2019, 109, 1602–1609. [Google Scholar] [CrossRef]
- Shah, N.; Singh, R.; Sarangi, U.; Saxena, N.; Chaudhary, A.; Kaur, G.; Kaul, S.C.; Wadhwa, R. Combinations of Ashwagandha leaf extracts protect brain-derived cells against oxidative stress and induce differentiation. PLoS ONE 2015, 10, e0120554. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M.; Lenehan, C.E.; Hughes, R.R.; Sanderson, B.J. Extracts from Calendula officinalis offer in vitro protection against H2O2 induced oxidative stress cell killing of human skin cells. Phytother. Res. 2015, 29, 120–124. [Google Scholar] [CrossRef]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic. Biol. Med. 2000, 29, 51–60. [Google Scholar] [CrossRef]
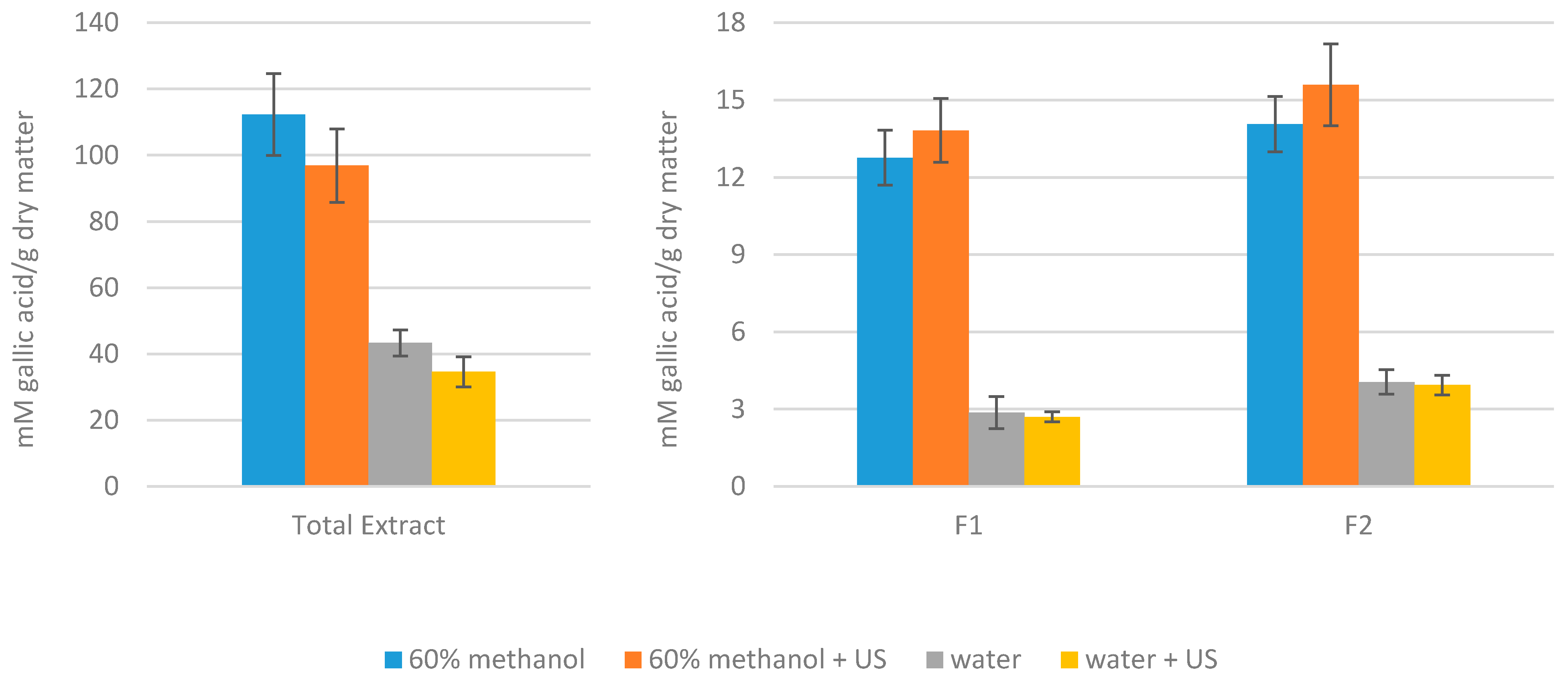
| Name | Chemical Structure | Retention Time (min) | Detection Wavelength (λ, nm) | |
|---|---|---|---|---|
| Hydroxycinnamic Acids | Caffeic acid |  | 17.8 | 320 |
| Chlorogenic acid |  | 15.4 | 320 | |
| Cynarin |  | 29.7 | 320 | |
| Flavonoids | Luteolin |  | 86.9 | 350 |
| Luteolin-7-O-glucoside | 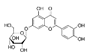 | 59.6 | 350 | |
| Luteolin-7-O-rutinoside | 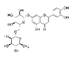 | 59.1 | 350 | |
| Apigenin | 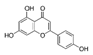 | 88.8 | 330 | |
| Apigenin-7-O-glucoside |  | 73.6 | 330 | |
| Apigenin-7-O-rutinoside |  | 70.9 | 330 | |
| Naringenin-7-O-glucoside | 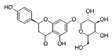 | 64.5 | 280 | |
| Narirutin |  | 62.3 | 280 |
| 60% Methanol (60′ Extraction) | 60% Methanol (60′ Extraction + 30′ Ultrasound) | 100% Water (60′ Extraction) | 100% Water (60′ Extraction + 30′ Ultrasound) | |
|---|---|---|---|---|
| Hydroxycinnamic Acids | ||||
| Caffeic acid | nd | nd | nd | nd |
| Chlorogenic acid | 815 ± 50 | 1006 ± 113 | 10 ± 1 | 8 ± 1 |
| Cynarin | 9.8 ± 0.7 | 12 ± 2 | nd | nd |
| Total hycroxycynamic acids | 825 | 1018 | 10 | 8 |
| Flavonoids | ||||
| Luteolin | 5.2 ± 0.3 | 4.5 ± 0.9 | 2.4 ± 0.4 | 2.4 ± 0.3 |
| Luteolin-7-O-glucoside | 442 ± 14 | 469 ± 6 | 2.7 ± 0.1 | 2.9 ± 0.6 |
| Luteolin-7-O-rutinoside | 684 ± 66 | 1034 ± 20 | 17 ± 2 | 10 ± 2 |
| Apigenin | 2.46 ± 0.01 | 2.49 ± 0.01 | 3.3 ± 0.4 | 4.1 ± 0.6 |
| Apigenin-7-O-glucoside | 7.3 ± 0.1 | 7.2 ± 0.3 | nd | nd |
| Apigenin-7-O-rutinoside | 20.9 ± 0.6 | 20.2 ± 0.9 | 5.5 ± 0.3 | 4.6 ± 0.3 |
| Naringenin-7-O-glucoside | 2.96 ± 0.02 | 2.9 ± 0.2 | 2.2 ± 0.03 | 2.19 ± 0.05 |
| Narirutin | nd | nd | 2.14 ± 0.02 | 2.11 ± 0.02 |
| Total flavonoid content | 1165 | 1540 | 35 | 28 |
| Total phenolic content | 1990 | 2558 | 45 | 36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients 2019, 11, 1723. https://doi.org/10.3390/nu11081723
Jiménez-Moreno N, Cimminelli MJ, Volpe F, Ansó R, Esparza I, Mármol I, Rodríguez-Yoldi MJ, Ancín-Azpilicueta C. Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients. 2019; 11(8):1723. https://doi.org/10.3390/nu11081723
Chicago/Turabian StyleJiménez-Moreno, Nerea, María José Cimminelli, Francesca Volpe, Raul Ansó, Irene Esparza, Inés Mármol, María Jesús Rodríguez-Yoldi, and Carmen Ancín-Azpilicueta. 2019. "Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells" Nutrients 11, no. 8: 1723. https://doi.org/10.3390/nu11081723
APA StyleJiménez-Moreno, N., Cimminelli, M. J., Volpe, F., Ansó, R., Esparza, I., Mármol, I., Rodríguez-Yoldi, M. J., & Ancín-Azpilicueta, C. (2019). Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients, 11(8), 1723. https://doi.org/10.3390/nu11081723










