Congruency of Genetic Predisposition to Lactase Persistence and Lactose Breath Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Lactose Breath Test (LBT)
2.3. Genetic Test
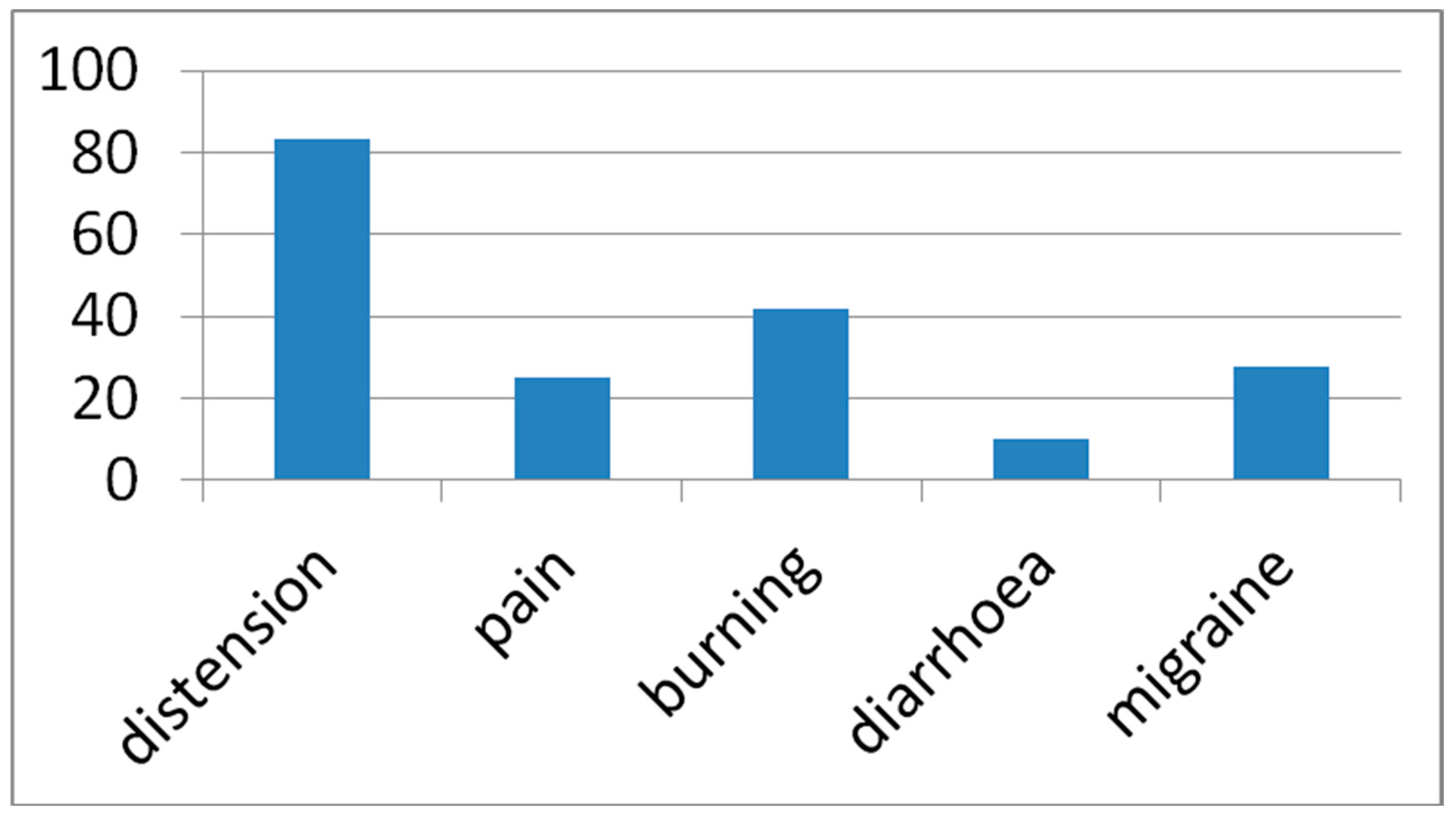
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Troelsen, J.T. Adult-type hypolactasia and regulation of lactase expression. Biochim. Biophys. Acta 2005, 1723, 19–32. [Google Scholar] [CrossRef]
- Enattah, N.S.; Sahi, T.; Savilahti, E.; Terwilliger, J.D.; Peltonen, L.; Jarvela, I. Identification of a variant associated with adult-type hypolactasia. Nat. Genet. 2002, 30, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Fassio, F.; Facioni, M.S.; Guagnini, F. Lactose Maldigestion, Malabsorption, and Intolerance: A Comprehensive Review with a Focus on Current Management and Future Perspectives. Nutrients 2018, 10, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose intolerance in adults: Biological mechanism and dietary management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef] [PubMed]
- Bayless, T.M.; Rothfeld, B.; Massa, C.; Wise, L.; Page, D.; Bedline, M.S. Lactose and milk intolerance: Clinical implications. N. Engl. J. Med. 1975, 292, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Law, D.; Conklin, J.; Pimentel, M. Lactose intolerance and the role of the lactose breath test. Am. J. Gastroenterol. 2010, 105, 1726–1728. [Google Scholar] [CrossRef]
- Lomer, M.C.E.; Parkes, G.C.; Sanderson, J.D. Lactose intolerance in clinical practice. Aliment. Pharmacol. Ther. 2008, 27, 93–103. [Google Scholar] [CrossRef]
- Heyman, M.B. Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics 2006, 118, 1279–1286. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Perman, J.A. Current concepts in lactose malabsorption and intolerance. Ann. Rev. Nutr. 1989, 9, 475–502. [Google Scholar] [CrossRef]
- Di Costanzo, M.; Berni Canani, R. Lactose Intolerance: Common Misunderstandings. Ann. Nutr. Metab. 2018, 73, 30–37. [Google Scholar] [CrossRef]
- Svedlund, J.; Sjodin, I.; Dotevall, G. GSRS-a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig. Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; de Leone, A.; Di Gregorio, G.; Giaquinto, S.; de Magistris, L.; Ferrieri, A.; Riegler, G. Breath test for differential diagnosis between small intestinal bacterial overgrowth and irritable bowel disease: An observation on non-absorbable antibiotics. World J. Gastroenterol. 2007, 13, 6016–6021. [Google Scholar] [CrossRef]
- Troelsen, J.T.; Olsen, J.; Moller, J.; Sjostrom, H. An upstream polymorphism associated with lactase persistence has increased enhancer activity. Gastroenterology 2003, 125, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Olds, L.C.; Sibley, E. Lactase persistence DNA variant enhances lactase promoter activity in vitro: Functional role as a cis regulatory element. Hum. Mol. Genet. 2003, 12, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Linee Guida Della Associazione Italiana Latto-Intolleranti (AILI). Available online: http://www.associazioneaili.it/cosa-e-lintolleranza-al-lattosio/ (accessed on 1 April 2019).
- Cavalli-Sforza, L.T.; Strata, A.; Barone, A.; Cucurachi, L. Primary adult lactose malabsorption in Italy: Regional differences in prevalence and relationship to lactose intolerance and milk consumption. Am. J. Clin. Nutr. 1987, 45, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Mottes, M.; Belpinati, F.; Milani, M.; Saccomandi, D.; Petrelli, E.; Calacoci, M.; Chierici, R.; Pignatti, P.F.; Borgna-Pignatti, C. Genetic testing for adult-type hypolactasia in Italian families. Clin. Chem. Lab. Med. 2008, 46, 980–984. [Google Scholar] [CrossRef]
- Flatz, G. The genetics of lactose digestion in humans. Adv. Hum. Genet. 1987, 16, 1–77. [Google Scholar]
- Holden, C.; Mace, R. Phylogenetic analysis of the evolution of lactose digestion in adults. Hum. Biol. 1997, 69, 605–628. [Google Scholar] [CrossRef]
- De Fanti, S.; Sazzini, M.; Giuliani, C.; Frazzoni, F.; Sarno, S.; Boattini, A.; Marasco, E.; Mantovani, V.; Franceschi, C.; Moral, P.; et al. Inferring the Genetic History of Lactase Persistence Along the Italian Peninsula from a Large Genomic Interval Surrounding the LCT Gene. Am. J. Phys. Anthropol. 2015, 158, 708–718. [Google Scholar] [CrossRef]
- Obinu, D.A.; Enattah, N.S.; Pedroni, A.; Peltonen, L.; Cavalli-Sforza, L.; Dore, M.P. Prevalence of lactase persistence and the performance of a non-invasive genetic test in adult Sardinian patients. Eur. J. Clin. Nutr. Metab. 2010, 5, e1–e5. [Google Scholar] [CrossRef][Green Version]
- Zadro, C.; Dipresa, S.; Zorzetti, G.; Pediroda, A.; Menegoni, F. Lactase non-persitent genotype distribution in Italy. Minerva Gastroenterol. Dietol. 2017, 63, 264–269. [Google Scholar] [PubMed]
- Brüssow, H. Nutrition, population growth and disease: A short history of lactose. Environ. Microbiol. 2013, 15, 2154–2161. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Levitt, M.D.; Taylor, B.C.; MacDonald, R.; Shamliyan, T.A.; Kane, R.L.; Wilt, T.J. Systematic review: Effective management strategies for lactose intolerance. Ann. Int. Med. 2010, 152, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Gugatschka, M.; Dobnig, H.; Fahrleitner-Pammer, A.; Pietschamann, P.; Kudlacek, S.; Strele, A.; Obermayer-Pietsch, B. Molecularly-defined lactose malabsorption, milk consumption and anthropometric differences in adult males. QIM 2005, 98, 857–863. [Google Scholar] [CrossRef]
- Enko, D.; Rezanka, E.; Stolba, R.; Halwachs-Baumann, G. Lactose Malabsorption Testing in Daily Clinical Practice: A Critical Retrospective Analysis and Comparison of the Hydrogen/Methane Breath Test and Genetic Test (C/T−13910 Polymorphism) Results. Gastroenterol. Res. Pract. 2014, 2014, 464382. [Google Scholar] [CrossRef]
- Di Stefano, M.; Veneto, G.; Malservisi, S.; Strocchi, A.; Corazza, G. Lactose malabsorption and intolerance in the elderly. Scand. J. Gastroenterol. 2001, 36, 1274–1278. [Google Scholar] [CrossRef]
- Pohl, D.; Savarino, E.; Hersberger, M.; Behlis, Z.; Stutz, B.; Goetze, O.; Eckardstein, A.; Fried, M.; Tutuian, R. Excellent agreement between genetic and hydrogen breath tests for lactase deficiency and the role of extended symptom assessment. Br. J. Nutr. 2010, 104, 900–907. [Google Scholar] [CrossRef]
- Kuokkanen, M.; Enattah, N.S.; Oksanen, A.; Savilahti, E.; Orpana, A.; Jarvela, I. Transcriptional regulation of the lactasephlorizin-hydrolase gene by polymorphisms associated with adult-type hypolactasia. Gut 2003, 52, 647–652. [Google Scholar] [CrossRef]
- Di Rienzo, T.; D’Angelo, G.; D’Aversa, F.; Campanale, M.C.; Cesario, V.; Montalto, M.; Gasbarrini, A.; Ojetti, V. Lactose intolerance: From diagnosis to correct management. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 18–25. [Google Scholar]
- Bond, J.H.; Levitt, M.D. Quantitative measurements of lactose absorption. Gastroenterology 1976, 70, 1058–1062. [Google Scholar] [CrossRef]
- Di Stefano, M.; Missanelli, A.; Miceli, E.; Strocchi, A.; Corazza, G.R. Hydrogen breath test in the diagnosis of lactose malabsorption: Accuracy of new versus conventional criteria. J. Lab. Clin. Med. 2004, 144, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Furnari, M.; Bonfanti, D.; Parodi, A.; Franzè, J.; Savarino, E.; Bruzzone, L.; Moscatelli, A.; Di Mario, F.; Dulbecco, P.; Savarino, V. A comparison between lactose breath test and quick test on duodenal biopsies for diagnosing lactase deficiency in patients with self-reported lactose intolerance. J. Clin. Gastroenterol. 2013, 47, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Strocchi, A.; Corazza, G.; Ellis, C.J.; Gasbarrini, G.; Levitt, M.D. Detection of malabsorption of low doses of carbohydrate: Accuracy of various breath H2 criteria. Gastroenterology 1993, 105, 1404–1410. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Corazza, G.R.; Gasbarrini, G.; Montalto, M.; Di Stefano, M.; Basilisco, G.; Parodi, A.; Satta, P.U.; Vernia, P.; Anania, C.; et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: The Rome Consensus Conference. Aliment. Pharmacol. Ther. 2009, 29, 1–49. [Google Scholar] [PubMed]
- Mattar, R.; do Socorro Monteiro, M.; Villares, C.B.; dos Santos, A.F.; Carrilho, F.J. Single nucleotide polymorphism C/T-13910, located upstream of lactase gene, associated with adult-type hypolactasia: Validation for clinical practice. Clin. Biochem. 2008, 41, 628–630. [Google Scholar] [CrossRef]
- Bernardes-Silva, C.F.; Pereira, A.C.; da Mota, G.F.A.; Krieger, J.E.; Laudanna, A.A. Lactase persistence/non-persistence variants, C/T 13910 and G/A 22018, as a diagnostic tool for lactose intolerance in IBS patients. Clin. Chim. Acta 2007, 386, 7–11. [Google Scholar] [CrossRef]
- Di Stefano, M.; Terulla, V.; Tana, P.; Mazzocchi, S.; Romero, E.; Corazza, G.R. Genetic test for lactase non-persistence and hydrogen breath test: Is genotype better than phenotype to diagnose lactose malabsorption? Dig. Liver Dis. 2009, 41, 474–479. [Google Scholar] [CrossRef]
- Santonocito, C.; Scapaticci, M.; Guarino, D.; Annicchiarico, E.B.; Lisci, R.; Penitente, R.; Gasbarrini, A.; Zuppi, C.; Capoluongo, E. Lactose intolerance genetic testing: Is it useful as routine screening? Results on 1426 south–central Italy patients. Clin. Chim. Acta 2015, 439, 14–17. [Google Scholar] [CrossRef]
- Itan, Y.; Jones, B.L.; Ingram, C.J.E.; Swallow, D.M.; Thomas, M.G. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol. Biol. 2010, 10, 36–40. [Google Scholar] [CrossRef]
| N°/Name | Sex/Age | BMI | Genetics | Basal Breath H2/CH4 (ppm) | Peak Value ∆ppm | Peak Time |
|---|---|---|---|---|---|---|
| 26 AV | F 51 | 21.4 | C/T | 4 CH4 2 H2 | 29 CH4 4 H2 | 180 |
| 46 NAN | F 18 | 19.5 | C/T | 5 | 77 | 120 |
| 59 PR | F 69 | 22.9 | C/T | 15 | 153 | 90 |
| 157 SB | F 26 | 19.6 | C/T | 30 | 76 | 120 |
| N°/Name | Sex/Age | BMI | Genetics | Basal Breath H2/CH4 (ppm) | Max Registered Value |
|---|---|---|---|---|---|
| 2 PC | M 31 | 20.0 | C/C | 3 | 4 |
| 20 MF | M 28 | 33.0 | C/C | 6 | 6 |
| 28 VL | F 42 | 27.0 | C/C | 15 | 20 |
| 32 MS | F 22 | 32.5 | C/C | 5 | 14 |
| 38 CA | F 19 | 29.0 | C/C | 12 | 17 |
| 75 VL | M 20 | 23.1 | C/C | 2 | 2 |
| 76 DM | F 24 | 16.6 | C/C | 3 | 2 |
| 82 DCA | M 22 | 26.7 | C/C | 5 | 22 |
| 104 AC | M 57 | 28.4 | C/C | 8 | 8 |
| 124 PS | M 72 | 29.7 | C/C | 12 | 15 |
| 134 NA | M 29 | 24.8 | C/C | 2 | 11 |
| 142 SV | M 23 | 28.3 | C/C | 14 | 18 |
| 149 RG | F 33 | 36.3 | C/C | 1 | 2 |
| 151 DNL | F 38 | 41.1 | C/C | 4 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluccia, E.; Iardino, P.; Pappalardo, D.; Brigida, A.L.; Formicola, V.; De Felice, B.; Guerra, C.; Pucciarelli, A.; Amato, M.R.; Riegler, G.; et al. Congruency of Genetic Predisposition to Lactase Persistence and Lactose Breath Test. Nutrients 2019, 11, 1383. https://doi.org/10.3390/nu11061383
Coluccia E, Iardino P, Pappalardo D, Brigida AL, Formicola V, De Felice B, Guerra C, Pucciarelli A, Amato MR, Riegler G, et al. Congruency of Genetic Predisposition to Lactase Persistence and Lactose Breath Test. Nutrients. 2019; 11(6):1383. https://doi.org/10.3390/nu11061383
Chicago/Turabian StyleColuccia, Enza, Patrizia Iardino, Diego Pappalardo, Anna Lisa Brigida, Vincenzo Formicola, Bruna De Felice, Claudia Guerra, Alessia Pucciarelli, Maria Rosaria Amato, Gabriele Riegler, and et al. 2019. "Congruency of Genetic Predisposition to Lactase Persistence and Lactose Breath Test" Nutrients 11, no. 6: 1383. https://doi.org/10.3390/nu11061383
APA StyleColuccia, E., Iardino, P., Pappalardo, D., Brigida, A. L., Formicola, V., De Felice, B., Guerra, C., Pucciarelli, A., Amato, M. R., Riegler, G., & De Magistris, L. (2019). Congruency of Genetic Predisposition to Lactase Persistence and Lactose Breath Test. Nutrients, 11(6), 1383. https://doi.org/10.3390/nu11061383




