Acute Effect of a Single Dose of Tomato Sofrito on Plasmatic Inflammatory Biomarkers in Healthy Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sofrito Samples
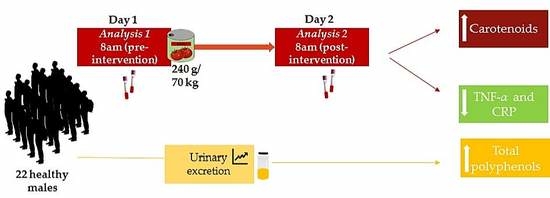
2.2. Participants
2.3. Intervention
2.4. Dietary and Physical Activity Assessments
2.5. Extraction of Biological Samples
2.6. Clinical and Biochemical Evaluations
2.7. Analysis of Total Polyphenol Excretion in Urine
2.8. Quantitative Analysis of Carotenoids in Plasma
2.9. Determination of Plasmatic Inflammatory Biomarkers
2.10. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Clinical Measures
3.3. Phenolic Excretion in Urine
3.4. Quantification of Carotenoids in Plasma
3.5. Inflammatory Biomarkers
3.6. Correlations between Inflammatory Biomarkers and Bioactive Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. PREDIMED Study Investigators, for the P. S. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Willcox, J.K.; Catignani, G.L.; Lazarus, S. Tomatoes and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2003, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Jacob, K.; Periago, M.J.; Böhm, V.; Berruezo, G.R. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br. J. Nutr. 2008, 99, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.; Talbot, J.; Park, E.; Krishnankutty, S.; Edirisinghe, I. Protective activity of processed tomato products on postprandial oxidation and inflammation: A clinical trial in healthy weight men and women. Mol. Nutr. Food Res. 2012, 56, 622–631. [Google Scholar] [CrossRef]
- Valderas-Martinez, P.; Chiva-Blanch, G.; Casas, R.; Arranz, S.; Martínez-Huélamo, M.; Urpi-Sarda, M.; Torrado, X.; Corella, D.; Lamuela-Raventós, R.; Estruch, R. Tomato Sauce Enriched with Olive Oil Exerts Greater Effects on Cardiovascular Disease Risk Factors than Raw Tomato and Tomato Sauce: A Randomized Trial. Nutrients 2016, 8, 170. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; de Alvarenga, J.F.R.; Estruch, R.; Lamuela-Raventos, R.M. Bioactive compounds present in the Mediterranean sofrito. Food Chem. 2013, 141, 3365–3372. [Google Scholar] [CrossRef] [PubMed]
- Boileau, T.W.-M.; Boileau, A.C.; Erdman, J.W. Bioavailability of all-trans and cis–Isomers of Lycopene. Exp. Biol. Med. 2002, 227, 914–919. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Uptake of Lycopene and Its Geometrical Isomers Is Greater from Heat-Processed than from Unprocessed Tomato Juice in Humans. J. Nutr. 1992, 122, 2161–2166. [Google Scholar] [CrossRef]
- Gärtner, C.; Stahl, W.; Sies, H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am. J. Clin. Nutr. 1997, 66, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Arranz, S.; Martínez-Huélamo, M.; Vallverdu-Queralt, A.; Valderas-Martinez, P.; Illán, M.; Sacanella, E.; Escribano, E.; Estruch, R.; Lamuela-Raventos, R.M. Influence of olive oil on carotenoid absorption from tomato juice and effects on postprandial lipemia. Food Chem. 2015, 168, 203–210. [Google Scholar] [CrossRef]
- Van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G.A.J. Dietary Factors That Affect the Bioavailability of Carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef]
- Priyadarshani, A.M.B. Critical Reviews in Food Science and Nutrition A review on factors influencing bioaccessibility and bioefficacy of carotenoids a review on factors influencing bioaccessibility and bioefficacy of carotenoids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1710–1717. [Google Scholar] [CrossRef]
- Rinaldi de Alvarenga, J.F.; Tran, C.; Hurtado-Barroso, S.; Martinez-Huélamo, M.; Illan, M.; Lamuela-Raventos, R.M. Home cooking and ingredient synergism improve lycopene isomer production in Sofrito. Food Res. Int. 2017, 99, 851–861. [Google Scholar] [CrossRef]
- Gahler, S.; Otto, K.; Böhm, V. Alterations of Vitamin C, Total Phenolics, and Antioxidant Capacity as Affected by Processing Tomatoes to Different Products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef]
- Tulipani, S.; Martinez Huelamo, M.; Rotches Ribalta, M.; Estruch, R.; Ferrer, E.E.; Andres-Lacueva, C.; Illan, M.; Lamuela-Raventós, R.M. Oil matrix effects on plasma exposure and urinary excretion of phenolic compounds from tomato sauces: Evidence from a human pilot study. Food Chem. 2012, 130, 581–590. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Tulipani, S.; Estruch, R.; Escribano, E.; Illán, M.; Corella, D.; Lamuela-Raventós, R.M. The tomato sauce making process affects the bioaccessibility and bioavailability of tomato phenolics: A pharmacokinetic study. Food Chem. 2015, 173, 864–872. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Di Lecce, G.; Valderas-Martínez, P.; Tulipani, S.; Jáuregui, O.; Escribano-Ferrer, E.; Estruch, R.; Illan, M.; Lamuela-Raventós, R.M. Bioavailability of tomato polyphenols is enhanced by processing and fat addition: Evidence from a randomized feeding trial. Mol. Nutr. Food Res. 2016, 60, 1578–1589. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of Antioxidant Lycopene in Cancer and Heart Disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gaziano, J.M.; Norkus, E.P.; Buring, J.E.; Sesso, H.D. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Am. J. Clin. Nutr. 2008, 88, 747–754. [Google Scholar] [CrossRef]
- Wang, M.-X.; Jiao, J.-H.; Li, Z.-Y.; Liu, R.-R.; Shi, Q.; Ma, L. Lutein supplementation reduces plasma lipid peroxidation and C-reactive protein in healthy nonsmokers. Atherosclerosis 2013, 227, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C.; Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; You, T.; Pahor, M. Behavioural treatments for chronic systemic inflammation: Effects of dietary weight loss and exercise training. CMAJ 2005, 172, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Jilma, B.; Dirnberger, E.; Löscher, I.; Rumplmayr, A.; Hildebrandt, J.; Eichler, H.G.; Kapiotis, S.; Wagner, O.F. Menstrual cycle-associated changes in blood levels of interleukin-6, alpha1 acid glycoprotein, and C-reactive protein. J. Lab. Clin. Med. 1997, 130, 69–75. [Google Scholar] [CrossRef]
- Wander, K.; Brindle, E.; O’Connor, K.A. C-reactive protein across the menstrual cycle. Am. J. Phys. Anthropol. 2008, 136, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Gornicsar, K.; Mózes, T.; Grósz, A.; Bíro, E.; Ládi, S.; Clayton, P. TNFα variation during the menstrual cycle and thereafter: A new explanation for gender-based disparities in ICU admission rates, trauma outcomes, and general mortality. Shock 2017, 47, 416–421. [Google Scholar] [CrossRef]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Barrionuevo-González, A.; Zamora-Ros, R.; Andres-Lacueva, C.; Estruch, R.; Martínez-González, M.-Á.; Diez-Espino, J.; Lamuela-Raventos, R.M. Rapid Folin–Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal. Chim. Acta 2009, 634, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Colmán-Martínez, M.; Martínez-Huélamo, M.; Miralles, E.; Estruch, R.; Lamuela-Raventós, R.M. A New Method to Simultaneously Quantify the Antioxidants: Carotenes, Xanthophylls, and Vitamin A in Human Plasma. Oxid. Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- rmcorr: Repeated Measures Correlation. Available online: https://cran.r-project.org/web/packages/rmcorr/index.html (accessed on 29 January 2019).
- Orrego-Lagarón, N.; Martínez-Huélamo, M.; Quifer-Rada, P.; Lamuela-Raventos, R.M.; Escribano-Ferrer, E. Absorption and disposition of naringenin and quercetin after simultaneous administration via intestinal perfusion in mice. Food Funct. 2016, 7, 3880–3889. [Google Scholar] [CrossRef] [PubMed]
- Ghavipour, M.; Saedisomeolia, A.; Djalali, M.; Sotoudeh, G.; Eshraghyan, M.R.; Moghadam, A.M.; Wood, L.G. Tomato juice consumption reduces systemic inflammation in overweight and obese females. Br. J. Nutr. 2013, 109, 2031–2035. [Google Scholar] [CrossRef]
- Biddle, M.J.; Lennie, T.A.; Bricker, G.V.; Kopec, R.E.; Schwartz, S.J.; Moser, D.K. Lycopene Dietary Intervention: A pilot study in patients with heart failure. J. Cardiovasc. Nurs. 2015, 30, 205–212. [Google Scholar] [CrossRef]
- Cartier, A.; Côté, M.; Lemieux, I.; Pérusse, L.; Tremblay, A.; Bouchard, C.; Després, J.-P. Sex differences in inflammatory markers: What is the contribution of visceral adiposity? Am. J. Clin. Nutr. 2009, 89, 1307–1314. [Google Scholar] [CrossRef]
- Garcia, V.P.; Rocha, H.N.M.; Sales, A.R.K.; Rocha, N.G.; da Nóbrega, A.C.L. Sex Differences in High Sensitivity C-Reactive Protein in Subjects with Risk Factors of Metabolic Syndrome. Arq. Bras. Cardiol. 2016, 106, 182–187. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; López-Sobaler, A.M.; Navia, B.; Andrés, P.; Jiménez-Ortega, A.I.; Ortega, R.M. β-Carotene Concentration and Its Association with Inflammatory Biomarkers in Spanish Schoolchildren. Ann. Nutr. Metab. 2017, 71, 80–87. [Google Scholar] [CrossRef]
- Muzáková, V.; Kand’ár, R.; Meloun, M.; Skalický, J.; Královec, K.; Záková, P.; Vojtísek, P. Inverse Correlation Between Plasma Beta-Carotene and Interleukin-6 in Patients with Advanced Coronary Artery Disease. Int. J. Vitam. Nutr. Res. 2010, 80, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Upritchard, J.E.; Sutherland, W.H.; Mann, J.I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 2000, 23, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Blum, A.; Monir, M.; Khazim, K.; Peleg, A.; Blum, N. Tomato-rich (Mediterranean) diet does not modify inflammatory markers. Clin. Investig. Med. 2007, 30, E70–E74. [Google Scholar] [CrossRef]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [CrossRef]
- Tsitsimpikou, C.; Tsarouhas, K.; Kioukia-Fougia, N.; Skondra, C.; Fragkiadaki, P.; Papalexis, P.; Stamatopoulos, P.; Kaplanis, I.; Hayes, A.W.; Tsatsakis, A.; et al. Dietary supplementation with tomato-juice in patients with metabolic syndrome: A suggestion to alleviate detrimental clinical factors. Food Chem. Toxicol. 2014, 74, 9–13. [Google Scholar] [CrossRef]
- Gajendragadkar, P.R.; Hubsch, A.; Mäki-Petäjä, K.M.; Serg, M.; Wilkinson, I.B.; Cheriyan, J. Effects of Oral Lycopene Supplementation on Vascular Function in Patients with Cardiovascular Disease and Healthy Volunteers: A Randomised Controlled Trial. PLoS ONE 2014, 9, e99070. [Google Scholar] [CrossRef] [PubMed]
- Colmán-Martínez, M.; Martínez-Huélamo, M.; Valderas-Martínez, P.; Arranz-Martínez, S.; Almanza-Aguilera, E.; Corella, D.; Estruch, R.; Lamuela-Raventós, R.M. trans-Lycopene from tomato juice attenuates inflammatory biomarkers in human plasma samples: An intervention trial. Mol. Nutr. Food Res. 2017, 61, 1600993. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | |
|---|---|
| Age (years) | 23.64 ± 0.86 |
| BMI (kg/m2) | 24.91 ± 0.79 |
| WHR | 0.84 ± 0.01 |
| MedDiet adherence (score) 1 | 8.5 ± 0.4 |
| Physical activity in leisure time (METs/d) | 746 ± 71 |
| Energy (kcal/day) | 2393 ± 129 |
| Total fats (g/day) | 106 ± 9 |
| Saturated fats (g/day) | 32 ± 3 |
| Monounsaturated (g/day) | 47 ± 4 |
| Polyunsaturated (g/day) | 19 ± 2 |
| Cholesterol (mg/day) | 301 ± 30 |
| Carbohydrate (g/day) | 256 ± 15 |
| Protein (g/day) | 100 ± 6 |
| Fiber (g/day) | 30 ± 2 |
| Measures | Baseline | ACS | P |
|---|---|---|---|
| DBP (mmHg) # | 76 ± 2 | 71 ± 2 | 0.006 * |
| SBP (mmHg) # | 123 ± 2 | 124 ± 2 | 0.550 |
| HR (bpm) # | 66 ± 21 | 62 ± 2 | 0.061 |
| Total cholesterol (mmol/L) # | 3.83 ± 0.12 | 3.71 ± 0.12 | 0.005 * |
| HDL (mmol/L) | 1.37 ± 0.06 | 1.32 ± 0.06 | 0.015 * |
| LDL (mmol/L) # | 2.04 ± 0.11 | 1.98 ± 0.11 | 0.130 |
| Triglycerides (mmol/L) # | 0.94 ± 0.08 | 0.89 ± 0.06 | 0.531 |
| Urea (mmol/L) | 5.56 ± 0.30 | 5.08 ± 0.28 | 0.023 * |
| Creatinine (µmol/L) | 76 ± 2 | 74 ± 1 | 0.157 |
| Uric acid (µmol/L) # | 319 ± 11 | 319 ± 10 | 1.000 |
| Total proteins (g/L) # | 73 ± 0.6 | 71± 0.6 | 0.011 * |
| Albumin (g/L) # | 47 ± 0.5 | 46 ± 0.5 | 0.015 * |
| Analyte | Baseline | ACS | p |
|---|---|---|---|
| Lutein (µmol/L) | 1.12 ± 0.02 | 1.69 ± 0.27 | 0.001 * |
| Zeaxanthin (µmol/L) | n.d. | 0.65 ± 0.19 | - |
| Cryptoxanthin (µmol/L) | 1.08 ± 0.12 | 1.34 ± 0.15 | 0.012 * |
| trans-β-carotene (µmol/L) | 3.12 ± 0.58 | 6.64 ± 0.88 | <0.001 * |
| 13-cis-β-carotene (µmol/L) | n.d. | 0.75 ± 0.15 | - |
| 9-cis-β-carotene (µmol/L) | n.d. | 0.95 ± 0.22 | - |
| trans-lycopene (µmol/L) | 2.15 ± 0.30 | 6.33 ± 1.53 | <0.001 * |
| 5-cis-lycopene (µmol/L) | 1.87 ± 0.28 | 7.93 ± 2.73 | <0.001 * |
| 13-cis-lycopene (µmol/L) | 0.21 ± 0.11 | 2.08 ± 0.78 | 0.005 * |
| 9-cis-lycopene (µmol/L) | n.d. | 0.90 ± 0.58 | - |
| Total cis-β-carotene (µmol/L) | n.d. | 1.92 ± 0.33 | - |
| Total β-carotene | 3.45 ± 0.67 | 8.56 ± 1.13 | <0.001 * |
| Total cis-lycopene isomers (µmol/L) | 2.09 ± 0.32 | 10.91 ± 4.00 | <0.001 * |
| Total lycopene (µmol/L) | 4.24 ± 0.59 | 17.23 ± 5.50 | <0.001 * |
| Total carotenoids (µmol/L) | 9.97 ± 0.96 | 29.25 ± 6.45 | <0.001 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurtado-Barroso, S.; Martínez-Huélamo, M.; Rinaldi de Alvarenga, J.F.; Quifer-Rada, P.; Vallverdú-Queralt, A.; Pérez-Fernández, S.; Lamuela-Raventós, R.M. Acute Effect of a Single Dose of Tomato Sofrito on Plasmatic Inflammatory Biomarkers in Healthy Men. Nutrients 2019, 11, 851. https://doi.org/10.3390/nu11040851
Hurtado-Barroso S, Martínez-Huélamo M, Rinaldi de Alvarenga JF, Quifer-Rada P, Vallverdú-Queralt A, Pérez-Fernández S, Lamuela-Raventós RM. Acute Effect of a Single Dose of Tomato Sofrito on Plasmatic Inflammatory Biomarkers in Healthy Men. Nutrients. 2019; 11(4):851. https://doi.org/10.3390/nu11040851
Chicago/Turabian StyleHurtado-Barroso, Sara, Miriam Martínez-Huélamo, Jose Fernando Rinaldi de Alvarenga, Paola Quifer-Rada, Anna Vallverdú-Queralt, Silvia Pérez-Fernández, and Rosa M. Lamuela-Raventós. 2019. "Acute Effect of a Single Dose of Tomato Sofrito on Plasmatic Inflammatory Biomarkers in Healthy Men" Nutrients 11, no. 4: 851. https://doi.org/10.3390/nu11040851