Glycemia Lowering Effect of an Aqueous Extract of Hedychium coronarium Leaves in Diabetic Rodent Models
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation of Aqueous Extract of Hedychium coronarium and SugarOut
2.2. Animal Study
2.2.1. C57BKSdb/db Mice (db/db Model)
2.2.2. STZ-Induced Type 2 Diabetes Model
2.3. Fasting Blood Glucose and Oral Glucose Tolerance Test
2.4. Aldosterone and Insulin Levels
2.5. Histopathological Analysis
2.6. Serum Biochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. HC Improved Fasting Blood Glucose and Glucose Tolerance in Both Diabetic Animal Models After 28 Days of Treatment
3.2. H. coronarium Attenuated STZ-Induced Pancreatic Damage and Ameliorated the Markers of Metabolic Syndrome
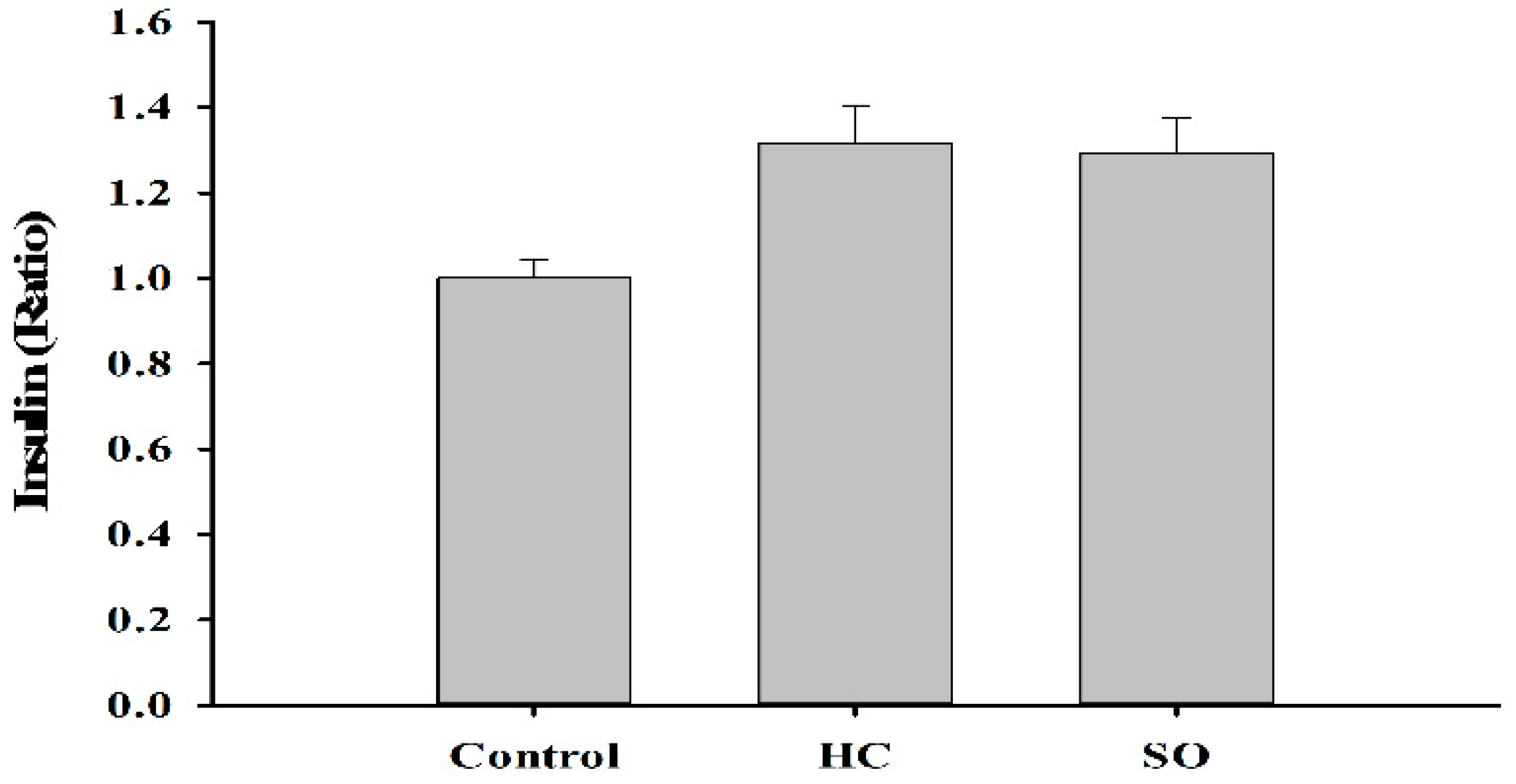
3.3. HC Altered Insulin and Aldosterone Content in Blood
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| T2DM | type 2 diabetes; |
| HDL | high-density lipoproteins cholesterol; |
| LDL | low-density lipoproteins cholesterol; |
| OGTT | oral glucose tolerance test’ |
| FBG | fasting blood glucose |
References
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 24 May 2018).
- Bajaj, H.S.; Zinman, B. Glucose lowering strategies for cardiac benefits: Pathophysiological mechanisms. Physiology (Bethesda) 2018, 33, 197–210. [Google Scholar] [CrossRef]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Green, P.; Chan, A.W.; Alam, U.; Fadavi, H.; Marshall, A.; Asghar, O.; Efron, N.; Tavakoli, M.; Malik, R.A. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PLoS ONE 2015, 10, e0123517. [Google Scholar] [CrossRef] [PubMed]
- Yorek, M.A. Vascular impairment of epineurial arterioles of the sciatic nerve: Implications for diabetic peripheral neuropathy. Rev. Diabet. Stud. 2015, 12, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Alam, U.; Malik, R.A. Treating diabetic neuropathy: Present strategies and emerging solutions. Rev. Diabet. Stud. 2015, 12, 63–83. [Google Scholar] [CrossRef]
- Manski-Nankervis, J.A.; Thuraisingam, S.; Sluggett, J.K.; Kilov, G.; Furler, J.; O’Neal, D.; Jenkins, A. Prescribing of diabetes medications to people with type 2 diabetes and chronic kidney disease: A national cross-sectional study. BMC Fam. Pract. 2019, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, G. Disease management: A proposal for a new definition. Int. J. Integr. Care 2009, 9, e06. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Chacko, E.C.; Pappachan, J.M. Non-pharmacological treatment options in the management of diabetes mellitus. Eur. Endocrinol. 2018, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Diabetes; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Perera, P.K.; Li, Y. Functional herbal food ingredients used in type 2 diabetes mellitus. Pharmacogn. Rev. 2012, 6, 37–45. [Google Scholar] [PubMed] [Green Version]
- Van Thanh, B.; Dai, D.N.; Thang, T.D.; Binh, N.Q.; Anh, L.D.; Ogunwande, I.A. Composition of essential oils of four Hedychium species from Vietnam. Chem. Cent. J. 2014, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, H. Hedychium; Backhuys Publisher: Leiden, The Netherlands, 2001; Volume 2, pp. 290–295. [Google Scholar]
- Ribeiro Rde, A.; de Barros, F.; de Melo, M.M.; Muniz, C.; Chieia, S.; Wanderley M das, G.; Gomes, C.; Trolin, G. Acute diuretic effects in conscious rats produced by some medicinal plants used in the state of São Paulo, Brasil. J. Ethnopharmacol. 1988, 24, 19–29. [Google Scholar] [CrossRef]
- Ribeiro Rde, A.; Fiuza de Melo, M.M.; De Barros, F.; Gomes, C.; Trolin, G. Acute antihypertensive effect in conscious rats produced by some medicinal plants used in the state of São Paulo. J. Ethnopharmacol. 1986, 15, 261–269. [Google Scholar] [CrossRef]
- Bhandary, M.J.; Chandrashekar, K.R.; Kaveriappa, K.M. Medical ethnobotany of the siddis of Uttara-Kannada District, Karnataka, India. J. Ethnopharmacol. 1995, 47, 149–158. [Google Scholar] [CrossRef]
- Zhan, Z.J.; Wen, Y.T.; Ren, F.Y.; Rao, G.W.; Shan, W.G.; Li, C.P. Diterpenoids and a diarylheptanoid from Hedychium coronarium with significant anti-angiogenic and cytotoxic activities. Chem. Biodivers. 2012, 9, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Reuk-ngam, N.; Chimnoi, N.; Khunnawutmanotham, N.; Techasakul, S. Antimicrobial activity of coronarin D and its synergistic potential with antibiotics. Biomed. Res. Int. 2014, 2014, 581985. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Hsieh, M.C.; Lin, S.H.; Lin, C.C.; Hsi, Y.T.; Lo, Y.S.; Chuang, Y.C.; Hsieh, M.J.; Chen, M.K. Coronarin D induces reactive oxygen species-mediated cell death in human nasopharyngeal cancer cells through inhibition of p38 MAPK and activation of JNK. Oncotarget 2017, 8, 108006–108019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, H.; Morikawa, T.; Sakamoto, Y.; Toguchida, I.; Yoshikawa, M. Labdane-type diterpenes with inhibitory effects on increase in vascular permeability and nitric oxide production from Hedychium coronarium. Bioorg. Med. Chem. 2002, 10, 2527–2534. [Google Scholar] [CrossRef]
- Li, F.; Sun, X.Y.; Li, X.W.; Yang, T.; Qi, L.W. Enrichment and separation of quercetin-3-O-beta-d-glucuronide from lotus leaves (nelumbo nucifera gaertn.) and evaluation of its anti-inflammatory effect. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1040, 186–191. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry-Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutic in Adult Healthy Volunteers; Center for Drug Evaluation and Research, Ed.; Food and Drug Administration: Rockville, MD, USA, 2005.
- BKS.Cg-Dock7m +/+ Leprdb/J; The Jackson Laboratory: Bar Harbor, ME, USA, 2019.
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Szkudelski, T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp. Biol. Med. (Maywood) 2012, 237, 481–490. [Google Scholar] [CrossRef]
- Islam, M.S.; Wilson, R.D. Experimentally induced rodent models of type 2 diabetes. Methods Mol. Biol. 2012, 933, 161–174. [Google Scholar] [PubMed]
- Ghasemi, A.; Khalifi, S.; Jedi, S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes. Acta Physiol. Hung. 2014, 101, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.; Rad, B.L. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, J.J.; Echouffo-Tcheugui, J.B.; Kalyani, R.R.; Yeh, H.C.; Bertoni, A.G.; Effoe, V.S.; Casanova, R.; Sims, M.; Correa, A.; Wu, W.C.; et al. Aldosterone, renin, and diabetes mellitus in African Americans: The jackson heart study. J. Clin. Endocrinol. Metab. 2016, 101, 1770–1778. [Google Scholar] [CrossRef]
- Lovshin, J.A.; Lytvyn, Y.; Lovblom, L.E.; Katz, A.; Boulet, G.; Bjornstad, P.; Lai, V.; Cham, L.; Tse, J.; Orszag, A.; et al. Retinopathy and RAAS activation: Results from the Canadian study of longevity in type 1 diabetes. Diabetes Care 2019, 42, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.P.; Islam, M.N.; Blake, L.; Bell, M.; Griffin, M.D.; O’Shea, P.M. Effect of sodium glucose co-transporter-2 inhibition on the aldosterone/renin ratio in type 2 diabetes mellitus. Horm. Metab. Res. 2018, 51, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Mosso, L.M.; Carvajal, C.A.; Maiz, A.; Ortiz, E.H.; Castillo, C.R.; Artigas, R.A.; Fardella, C.E. A possible association between primary aldosteronism and a lower beta-cell function. J. Hypertens. 2007, 25, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Widimsky, J., Jr.; Sindelka, G.; Haas, T.; Prazny, M.; Hilgertova, J.; Skrha, J. Impaired insulin action in primary hyperaldosteronism. Physiol. Res. 2000, 49, 241–244. [Google Scholar] [PubMed]
- Schmidt, B.M. Rapid non-genomic effects of aldosterone on the renal vasculature. Steroids 2008, 73, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Hermidorff, M.M.; de Assis, L.V.; Isoldi, M.C. Genomic and rapid effects of aldosterone: What we know and do not know thus far. Heart Fail. Rev. 2017, 22, 65–89. [Google Scholar] [CrossRef]
- Alzamora, R.; Brown, L.R.; Harvey, B.J. Direct binding and activation of protein kinase C isoforms by aldosterone and 17beta-estradiol. Mol. Endocrinol. 2007, 21, 2637–2650. [Google Scholar] [CrossRef]
- Mihailidou, A.S.; Mardini, M.; Funder, J.W. Rapid, nongenomic effects of aldosterone in the heart mediated by epsilon protein kinase C. Endocrinology 2004, 145, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Shin-ya, H.; Nakai, S.; Yorimoto, A.; Morimoto, T.; Suyama, T.; Sakurai, M. Genomic and non-genomic effects of aldosterone on the individual variation of the sweat Na+ concentration during exercise in trained athletes. Eur. J. Appl. Physiol. 2006, 98, 466–471. [Google Scholar] [CrossRef]
- Bollag, W.B. Regulation of aldosterone synthesis and secretion. Compr. Physiol. 2014, 4, 1017–1055. [Google Scholar] [PubMed]
- Terao, J.; Yamaguchi, S.; Shirai, M.; Miyoshi, M.; Moon, J.H.; Oshima, S.; Inakuma, T.; Tsushida, T.; Kato, Y. Protection by quercetin and quercetin 3-O-beta-d-glucuronide of peroxynitrite-induced antioxidant consumption in human plasma low-density lipoprotein. Free Rad. Res. 2001, 35, 925–931. [Google Scholar] [CrossRef]
- Moon, J.H.; Tsushida, T.; Nakahara, K.; Terao, J. Identification of quercetin 3-O-beta-d-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Rad. Biol. Med. 2001, 30, 1274–1285. [Google Scholar] [CrossRef]
- Liu, K.; Mei, F.; Wang, Y.; Xiao, N.; Yang, L.; Wang, Y.; Li, J.; Huang, F.; Kou, J.; Liu, B.; et al. Quercetin oppositely regulates insulin-mediated glucose disposal in skeletal muscle under normal and inflammatory conditions: The dual roles of AMPK activation. Mol. Nutr. Food Res. 2016, 60, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, K.; Yoshizumi, M.; Kawai, Y.; Terao, J.; Kihira, Y.; Ikeda, Y.; Tomita, S.; Minakuchi, K.; Tsuchiya, K.; Tamaki, T. Pharmacology in health food: Metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J. Pharmacol. Sci. 2011, 115, 466–470. [Google Scholar] [CrossRef]
- Mulrow, P.J. Angiotensin II and aldosterone regulation. Regul. Pept. 1999, 80, 27–32. [Google Scholar] [CrossRef]
- Patel, S. Functional food red yeast rice (RYR) for metabolic syndrome amelioration: A review on pros and cons. World J. Microbiol. Biotechnol. 2016, 32, 87. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, W.; Zhong, Y.; Lu, B.; Shao, J.; Jiang, S.; Gu, P. Xuezhikang attenuated the functional and morphological impairment of pancreatic islets in diabetic mice via the inhibition of oxidative stress. J. Cardiovasc. Pharmacol. 2014, 63, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.-X. Glucose Homeostatis and the Pathogenesis of Diabetes Mellitus, 1st ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]







| AUC | 14 Days | 28 Days |
|---|---|---|
| Sham | 8898.75 ± 1816.12 | 1666.88 ± 3399.66 |
| Control | 23,578.13 ± 9636.76 | 32,130.00 ± 8133.19 |
| H. coronarium leaves (HC) | 11,730.00 ± 5959.70 | 14,979.38 ± 5656.03 * |
| SugarOut (SO) | 10,301.25 ± 5884.57 | 11,945.63 ± 13,782.89 ** |
| Group | Sham | Control | HC | SO | |
|---|---|---|---|---|---|
| Number of Animals | 8 | 8 | 8 | 8 | |
| Cholesterol | mg/dL | 69.28 ± 25.71 | 161.50 ± 63.79 | 76.63 ± 15.30 ** | 111.63 ± 51.48 |
| Triglycerides | mg/dL | 58.13 ± 23.93 | 753.38 ± 434.92 | 105.13 ± 33.31 ** | 480.75 ± 410.14 |
| HDL | mmol/L | 27.13 ± 12.16 | 33.38 ± 13.23 | 42.75 ± 13.10 | 38.50 ± 12.31 |
| LDL | mmol/L | 7.63 ± 1.77 | 37.63 ± 19.83 | 10.50 ± 2.56 ** | 22.25 ± 21.02 |
| Pancreas | Control | HC | SO |
|---|---|---|---|
| Decrease, β-cell, islet, focal | 3.80 ± 0.45 | 3.00 ± 0.71 | 2.60 ± 0.55 |
| Atrophy, acinar cell, diffuse | 4.00 ± 0.00 | 3.40 ± 0.89 | 2.80 ± 1.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse, L.-S.; Liao, P.-L.; Tsai, C.-H.; Li, C.-H.; Liao, J.-W.; Kang, J.-J.; Cheng, Y.-W. Glycemia Lowering Effect of an Aqueous Extract of Hedychium coronarium Leaves in Diabetic Rodent Models. Nutrients 2019, 11, 629. https://doi.org/10.3390/nu11030629
Tse L-S, Liao P-L, Tsai C-H, Li C-H, Liao J-W, Kang J-J, Cheng Y-W. Glycemia Lowering Effect of an Aqueous Extract of Hedychium coronarium Leaves in Diabetic Rodent Models. Nutrients. 2019; 11(3):629. https://doi.org/10.3390/nu11030629
Chicago/Turabian StyleTse, Ling-Shan, Po-Lin Liao, Chi-Hao Tsai, Ching-Hao Li, Jiunn-Wang Liao, Jaw-Jou Kang, and Yu-Wen Cheng. 2019. "Glycemia Lowering Effect of an Aqueous Extract of Hedychium coronarium Leaves in Diabetic Rodent Models" Nutrients 11, no. 3: 629. https://doi.org/10.3390/nu11030629





