Pharmacological Properties of Morus nigra L. (Black Mulberry) as A Promising Nutraceutical Resource
Abstract
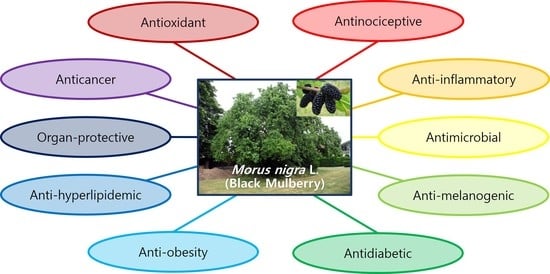
:1. Introduction
2. Antinociceptive Activity
3. Anti-Inflammatory Activity
4. Antimicrobial Activity
5. Anti-Melanogenic (Skin-Whitening) Activity
6. Antidiabetic and Anti-Obesity Activity
7. Anti-Hyperlipidemic and Anti-Atherosclerotic Activity
8. Organ-Protective Activity
8.1. Neuroprotective Effect
8.2. Hepatoprotective Effect
8.3. Renal-Protective Effect
8.4. Gastroprotective Effect
9. Activity on Female Reproductive System
10. Anticancer Activity
11. Antioxidant Activity
12. Other Pharmacological Activities
13. Drug-Food Interaction and Toxicity
| Pharmacological Activity | Study Model | Used Part | SampleType a | Ref. |
|---|---|---|---|---|
| Antinociceptive | Swiss mice | Root bark | C | [10] |
| Male Swiss mice | Leaf | E | [14] | |
| Male Kunming mice | Fruit | E | [15] | |
| Male Kunming mice | Fruit | E,C | [16] | |
| Anti-inflammatory | Kunming male mice; RAW 264.7 cell | Fruit | E | [15] |
| Adult male rats | Leaf | E | [18] | |
| Rat polymorphonuclear leukocytes | Bark | C | [19] | |
| THP-1 human monocytic leukemia cell line | Root | C | [20] | |
| Male C57BL/6 mice | Pulp; leaf | E | [21] | |
| Antimicrobial | In vitro assay | Fruit | E | [16] |
| In vitro assay | Leaf | E,F | [22] | |
| In vitro assay | Leaf | E,F | [23] | |
| In vitro assay | Fruit | J | [24] | |
| In vitro assay | Fruit | E | [25] | |
| In vitro assay | Fruit | E | [26] | |
| In vitro assay | Stem bark; fruit; leaf | E | [27] | |
| In vitro assay | Stem bark; stem wood | E,C | [28] | |
| In vitro assay; THP-1 cell line | Root | E,C | [32] | |
| In vitro assay | Fruit | E | [34] | |
| Anti-melanogenic (Skin-whitening) | In vitro assay; B16 melanoma cells | Stem | C | [38] |
| In vitro assay | Root; twig | C | [39] | |
| In vitro assay | Leaf | E | [40] | |
| In vitro assay | Fruit | E | [41] | |
| Antidiabetic | Male Wistar rats | Leaf | E b | [46] |
| Male albino mice | Leaf | E | [47] | |
| Female albino Fischer rats | Pulp; leaf | E | [49] | |
| PPARγ-transfected HEK293 cells | Twig | C | [50] | |
| In vitro assay | Twig | C | [51] | |
| Anti-obesity | In vitro assay | Fruit | E | [55] |
| Anti-hyperlipidemic | Wistar rats | Leaf | E | [52] |
| Adult male albino Sprague-Dawley rats | Fruit | E | [60] | |
| Male Wistar rats | Leaf | E | [61] | |
| Male Spraque-Dawley rats | Fruit | E | [62] | |
| Organ-protective | Male BALB/c mice | Leaf | E | [63] |
| Male Swiss mice | Leaf | E,C | [64] | |
| HepG2 human hepatocellular carcinoma cell line; male albino rats | Leaf | E | [65] | |
| Male Wistar rats | Leaf | E | [66] | |
| HepG2 cells | Fruit | E | [67] | |
| Adult male Sprague-Dawley rats | Fruit | E | [68] | |
| Male Wistar rats | Fruit | E | [69] | |
| Female Swiss mice | Fruit | E | [70] | |
| Anticancer | Peripheral blood mononuclear cells (PBMCs); peripheral blood T lymphocytes; Jurkat T leukemia cells | Bark | C | [77] |
| HT-29 human colorectal adenocarcinoma cell line | Fruit | E,C | [80] | |
| HeLa human cervical cancer cell line | Leaf | E | [81] | |
| MCF-7 human breast cancer cell line | Fruit | E | [82] | |
| PC-3 human prostate adenocarcinoma cells | Fruit | E | [83] |
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hussain, F.; Rana, Z.; Shafique, H.; Malik, A.; Hussain, Z. Phytopharmacological potential of different species of Morus alba and their bioactive phytochemicals: A review. Asian Pac. J. Trop. Biomed. 2017, 7, 950–956. [Google Scholar] [CrossRef]
- Vijayan, K.; Chauhan, S.; Das, N.K.; Chakraborti, S.P.; Roy, B.N. Leaf yield component combining abilities in mulberry (Morus spp.). Euphytica 1997, 98, 47–52. [Google Scholar] [CrossRef]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Fotirić Akšić, M.M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2014, 171, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, E.M.; Sendra, E.; Carbonell-Barrachina, Á.A.; Martínez, J.J.; Hernández, F. Fatty acids composition of Spanish black (Morus nigra L.) and white (Morus alba L.) mulberries. Food Chem. 2016, 190, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhu, J.J.; Liu, X.Q.; Feng, W.H.; Wang, Z.M.; Yan, L.H. Review of bioactive compounds from root barks of Morus plants (Sang-Bai-Pi) and their pharmacological effects. Cogent. Chem. 2016, 2, 1212320. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, Y.X.; Chen, R.Y.; Kang, J. The latest review on the polyphenols and their bioactivities of Chinese Morus plants. J. Asian Nat. Prod. Res. 2014, 16, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.; Lye, P.Y.; Wong, S.K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef]
- Budiman, A.; Sulastri, A.; Alfauziah, T.Q. Chemical compounds and pharmacological activity of Morus nigra as a potential product of drug: A review. Int. Res. J. Pharm. 2018, 9, 76–81. [Google Scholar] [CrossRef]
- De Souza, M.M.; Bittar, M.; Cechinel-Filho, V.; Yunes, R.A.; Messana, I.; Delle Monache, F.; Ferrari, F. Antinociceptive properties of morusin, a prenylflavonoid isolated from Morus nigra root bark. Z. Naturforsch. C. 2000, 55, 256–260. [Google Scholar] [CrossRef]
- Nomura, T.; Fukai, T.; Yamada, S.; Katayanagi, M. Studies on the constituents of the cultivated mulberry tree. I. Three new prenylflavones from the root bark of Morus alba L. Chem. Pharm. Bull. 1978, 26, 1394–1402. [Google Scholar] [CrossRef]
- Ko, H.H.; Yu, S.M.; Ko, F.N.; Teng, C.M.; Lin, C.N. Bioactive constituents of Morus australis and Broussonetia papyrifera. J. Nat. Prod. 1997, 60, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.B.; Ha, T.J.; Curtis-Long, M.J.; Ryu, H.W.; Gal, S.W.; Park, K.H. Inhibitory effects on mushroom tyrosinase by flavones from the stem barks of Morus lhou (S.) Koidz. J. Enzyme Inhib. Med. Chem. 2008, 23, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Padilha, M.M.; Vilela, F.C.; da Silva, M.J.; dos Santos, M.H.; Alves-da-Silva, G.; Giusti-Paiva, A. Antinociceptive Effect of the extract of Morus nigra leaves in mice. J. Med. Food 2009, 12, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pu, J.; Liu, D.; Yu, W.; Shao, Y.; Yang, G.; Xiang, Z.; He, N. Anti-Inflammatory and Antinociceptive Properties of Flavonoids from the Fruits of Black Mulberry (Morus nigra L.). PLoS ONE 2016, 11, e0153080. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, W.; Chen, G.; Meng, S.; Xiang, Z.; He, N. Antinociceptive and Antibacterial Properties of Anthocyanins and Flavonols from Fruits of Black and Non-Black Mulberries. Molecules 2018, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Chalons, P.; Amor, S.; Courtaut, F.; Cantos-Villar, E.; Richard, T.; Auger, C.; Chabert, P.; Schni-Kerth, V.; Aires, V.; Delmas, D. Study of Potential Anti-Inflammatory Effects of Red Wine Extract and Resveratrol through a Modulation of Interleukin-1-Beta in Macrophages. Nutrients 2018, 10, 1856. [Google Scholar] [CrossRef]
- Padilha, M.M.; Vilela, F.C.; Rocha, C.Q.; Dias, M.J.; Soncini, R.; dos Santos, M.H.; Alves-da-Silva, G.; Giusti-Paiva, A. Antiinflammatory properties of Morus nigra leaves. Phytother. Res. 2010, 24, 1496. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Liu, C.; Chen, R.Y. Three new compounds from Morus nigra L. J. Asian Nat. Prod. Res. 2010, 12, 431–437. [Google Scholar] [CrossRef]
- Zelová, H.; Hanáková, Z.; Čermáková, Z.; Šmejkal, K.; Dalĺ Acqua, S.; Babula, P.; Cvačka, J.; Hošek, J. Evaluation of anti-inflammatory activity of prenylated substances isolated from Morus alba and Morus nigra. J. Nat. Prod. 2014, 77, 1297–1303. [Google Scholar] [CrossRef]
- De Pádua Lúcio, K.; Rabelo, A.C.S.; Araújo, C.M.; Brandão, G.C.; de Souza, G.H.B.; da Silva, R.G.; de Souza, D.M.S.; Talvani, A.; Bezerra, F.S.; Cruz Calsavara, A.J.; et al. Anti-Inflammatory and Antioxidant Properties of Black Mulberry (Morus nigra L.) in a Model of LPS-Induced Sepsis. Oxid. Med. Cell Longev. 2018, 2018, 5048031. [Google Scholar]
- Tahir, L.; Aslam, A.; Ahmed, S. Antibacterial activities of Diospyros blancoi, Phoenix dactylifera and Morus nigra against dental caries causing pathogens: An in vitro study. Pak. J. Pharm. Sci. 2017, 30, 163–169. [Google Scholar] [PubMed]
- Souza, G.R.; Oliveira-Junior, R.G.; Diniz, T.C.; Branco, A.; Lima-Saraiva, S.R.G.; Guimarães, A.L.; Oliveira, A.P.; Pacheco, A.G.M.; Silva, M.G.; Moraes-Filho, M.O.; et al. Assessment of the antibacterial, cytotoxic and antioxidant activities of Morus nigra L. (Moraceae). Braz. J. Biol. 2018, 78, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Fawad, S.A.; Ahmed, I. Antimicrobial activity, phytochemical profile and trace minerals of black mulberry (Morus nigra L.) fresh juice. Pak. J. Bot. 2011, 43, 91–96. [Google Scholar]
- Minhas, M.A.; Begum, A.; Hamid, S.; Babar, M.; Ilyas, R.; Ali, S.; Latif, F.; Andleeb, S. Evaluation of Antibiotic and Antioxidant Activity of Morus nigra (Black Mulberry) Extracts Against Soil Borne, Food Borne and Clinical Human Pathogens. Pak. J. Zool. 2016, 48, 1381–1388. [Google Scholar]
- Budiman, A.; Aulifa, D.L.; Kusuma, A.S.W.; Sulastri, A. Antibacterial and Antioxidant Activity of Black Mulberry (Morus nigra L.) Extract for Acne Treatment. Pharmacogn. J. 2017, 9, 611–614. [Google Scholar] [CrossRef]
- Aulifa, D.L.; Fitriansyah, S.N.; Ardiansyah, S.A.; Wibowo, D.P.; Julata, Y.A.; Christy, D.S. Phytochemical Screening, Antibacterial Activity, and Mode of Action on Morus nigra. Pharmacogn. J. 2018, 10, 167–171. [Google Scholar] [CrossRef]
- Mazimba, O.; Majinda, R.R.T.; Motlhanka, D. Antioxidant and antibacterial constituents from Morus nigra. Afr. J. Pharm. Pharmacol. 2011, 5, 751–754. [Google Scholar] [CrossRef]
- Tuberculosis: WHO Fact Sheet No. 104. Available online: https://www.who.int/en/news-room/fact-sheets/detail/tuberculosis (accessed on 2 January 2019).
- Sajid, A.; Arora, G.; Singhal, A.; Kalia, V.C.; Singh, Y. Protein Phosphatases of Pathogenic Bacteria: Role in Physiology and Virulence. Annu. Rev. Microbiol. 2015, 69, 527–547. [Google Scholar] [CrossRef]
- Mascarello, A.; Chiaradia-Delatorre, L.D.; Mori, M.; Terenzi, H.; Botta, B. Mycobacterium tuberculosis-Secreted Tyrosine Phosphatases as Targets against Tuberculosis: Exploring Natural Sources in Searching for New Drugs. Curr. Pharm. Des. 2016, 22, 1561–1569. [Google Scholar] [CrossRef]
- Mascarello, A.; Orbem Menegatti, A.C.; Calcaterra, A.; Martins, P.G.A.; Chiaradia-Delatorre, L.D.; D’Acquarica, I.; Ferrari, F.; Pau, V.; Sanna, A.; De Logu, A.; et al. Naturally occurring Diels-Alder-type adducts from Morus nigra as potent inhibitors of Mycobacterium tuberculosis protein tyrosinase phosphatase B. Eur. J. Med. Chem. 2018, 144, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Manolakaki, D.; Velmahos, G.; Kourkoumpetis, T.; Chang, Y.; Alam, H.B.; De Moya, M.M.; Mylonakis, E. Candida infection and colonization among trauma patients. Virulence 2010, 1, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Yiğit, N.; Yiğit, D.; Özgen, U.; Aktaş, A.E. Anticandidal activity of black mulberry (Morus nigra L.). Türk. Mikrobiyol. Cem. Derg. 2007, 37, 169–173. [Google Scholar]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Hou, A.; Wang, H. Inhibitory effect of 2,4,2’,4’-tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone on tyrosinase activity and melanin biosynthesis. Biol. Pharm. Bull. 2009, 32, 86–90. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Cheng, K.W.; Zhu, Q.; Wang, X.C.; Lin, Z.X.; Wang, M. Tyrosinase inhibitory constituents from the roots of Morus nigra: A structure-activity relationship study. J. Agric. Food Chem. 2010, 58, 5368–5373. [Google Scholar] [CrossRef]
- De Freitas, M.M.; Fontes, P.R.; Souza, P.M.; William Fagg, C.; Neves Silva Guerra, E.; de Medeiros Nóbrega, Y.K.; Silveira, D.; Fonseca-Bazzo, Y.; Simeoni, L.A.; Homem-de-Mello, M.; et al. Extracts of Morus nigra L. Leaves Standardized in Chlorogenic Acid, Rutin and Isoquercitrin: Tyrosinase Inhibition and Cytotoxicity. PLoS ONE 2016, 11, e0163130. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Demir, S.; Haznedaroglu, M.Z.; Yesil-Celiktas, O. Optimization of microwave assisted extraction of Morus nigra L. fruits maximizing tyrosinase inhibitory activity with isolation of bioactive constituents. Food Chem. 2018, 248, 183–191. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.Y.; Qian, K.; Norris-Natschke, S.L.; Hsu, C.S.; Lee, K.H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012, 29, 580–606. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Wang, Y.; Liu, D. Dietary Flavonoids in the Prevention of T2D: An Overview. Nutrients 2018, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Mawla, A.M.; Mohamed, K.M.; Mostafa, A.M. Induction of Biologically Active Flavonoids in Cell Cultures of Morus nigra and Testing their Hypoglycemic Efficacy. Sci. Pharm. 2011, 79, 951–961. [Google Scholar] [CrossRef] [PubMed]
- AbouZid, S.F.; Ahmed, O.M.; Ahmed, R.R.; Mahmoud, A.; Abdella, E.; Ashour, M.B. Antihyperglycemic effect of crude extracts of some Egyptian plants and algae. J. Med. Food 2014, 17, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Kłysik, A.B.; Naduk-Kik, J.; Hrabec, Z.; Goś, R.; Hrabec, E. Intraocular matrix metalloproteinase 2 and 9 in patients with diabetes mellitus with and without diabetic retinopathy. Arch. Med. Sci. 2010, 6, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.M.; Lúcio Kde, P.; Silva, M.E.; Isoldi, M.C.; de Souza, G.H.; Brandão, G.C.; Schulz, R.; Costa, D.C. Morus nigra leaf extract improves glycemic response and redox profile in the liver of diabetic rats. Food Funct. 2015, 6, 3490–3499. [Google Scholar] [CrossRef]
- Xu, L.J.; Yu, M.H.; Huang, C.Y.; Niu, L.X.; Wang, Y.F.; Wu, C.Z.; Yang, P.M.; Hu, X. Isoprenylated flavonoids from Morus nigra and their PPAR γ agonistic activities. Fitoterapia 2018, 127, 109. [Google Scholar] [CrossRef]
- Xu, L.; Yu, M.; Niu, L.; Huang, C.; Wang, Y.; Wu, C.; Yang, P.; Hu, X. Phenolic compounds isolated from Morus nigra and their α-glucosidase inhibitory activities. Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Volpato, G.T.; Calderson, I.M.P.; Sinzato, S.; Campos, K.E.; Rudge, M.V.C.; Damasceno, D.C. Effect of Morus nigra aqueous extract treatment on the maternal-fetal outcome, oxidative stress status and lipid profile of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2011, 138, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Shimada, Y.; Zang, L.; Terasawa, M.; Nishiura, K.; Matsuda, K.; Toombs, C.; Langdon, C.; Nishimura, N. Novel Anti-Obesity Properties of Palmaria mollis in Zebrafish and Mouse Models. Nutrients 2018, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, N.V.; Ha, C.E. Essentials of Medical Biochemistry, 1st ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 209–223. [Google Scholar]
- Kannel, W.B.; Castelli, W.P.; Gordon, T. Choelsterol in the prediction of atherosclerotic disease: New perspectives based on the Framingham study. Ann. Intern. Med. 1979, 90, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Assmann, G.; Nofer, J.R. Atheroprotective effects of high-density lipoproteins. Annu. Rev. Med. 2003, 54, 321–341. [Google Scholar] [CrossRef]
- Mahmoud, M.Y. Natural antioxidants effect of mulberry fruits (Morus nigra and Morus alba L.) on lipids profile and oxidative stress in hypercholestrolemic rats. Pak. J. Nutr. 2013, 12, 665–672. [Google Scholar] [CrossRef]
- Zeni, A.L.B.; Moreira, T.D.; Dalmagro, A.P.; Camargo, A.; Bini, L.A.; Simionatto, E.L.; Scharf, D.R. Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract. An. Acad. Bras. Cienc. 2017, 89, 2805–2815. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, M.; Nie, W.J.; Yang, X.R.; Zeng, X.C. Effects of the ethanol extract of black mulberry (Morus nigra L.) fruit on experimental atherosclerosis in rats. J. Ethnopharmacol. 2017, 200, 228–235. [Google Scholar] [CrossRef]
- Turgut, N.H.; Mert, D.G.; Kara, H.; Egilmez, H.R.; Arslanbas, E.; Tepe, B.; Gungor, H.; Yilmaz, N.; Tuncel, N.B. Effect of black mulberry (Morus nigra) extract treatment on cognitive impairment and oxidative stress status of D-galactose-induced aging mice. Pharm. Biol. 2016, 54, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Dalmagro, A.P.; Camargo, A.; Zeni, A.L.B. Morus nigra and its major phenolic, syringic acid, have antidepressant-like and neuroprotective effects in mice. Metab. Brain Dis. 2017, 32, 1963–1973. [Google Scholar] [CrossRef]
- Tag, H.M. Hepatoprotective effect of mulberry (Morus nigra) leaves extract against methotrexate induced hepatotoxicity in male albino rat. BMC Complement. Altern. Med. 2015, 15, 252. [Google Scholar] [CrossRef] [PubMed]
- Hassanalilou, T.; Payahoo, L.; Shahabi, P.; Abbasi, M.M.; Jafar-abadi, M.A.; Bishak, Y.K.; Khordadmehr, M.; Esnaashari, S.; Barzegar, A. The protective effects of Morus nigra L. leaves on the kidney function tests and kidney and liver histological structures in streptozotocin-induced diabetic rats. Biomed. Res. 2017, 28, 6113–6118. [Google Scholar]
- Youssef, F.S.; Labib, R.M.; Eldahshan, O.A.; Singab, A.N.B. Synergistic Hepatoprotective and Antioxidant Effect of Artichoke, Fig, Blackberry Herbal Mixture on HepG2 Cells and Their Metabolic Profiling Using NMR Coupled with Chemometrics. Chem. Biodivers. 2017, 14, e1700206. [Google Scholar] [CrossRef] [PubMed]
- Deniz, G.Y.; Laloglu, E.; Koc, K.; Nadaroglu, H.; Geyikoglu, F. The effect of black mulberry (Morus nigra) extract on carbon tetrachloride-induced liver damage. Arch. Biol. Sci. 2018, 70, 371–378. [Google Scholar] [CrossRef]
- Rahimi-Madiseh, M.; Naimi, A.; Heydarian, E.; Rafieian-Kopaei, M. Renal biochemical and histopathological alterations of diabetic rats under treatment with hydro alcoholic Morus nigra extract. J. Renal Inj. Prev. 2016, 6, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Nesello, L.A.N.; Beleza, M.L.M.L.; Mariot, M.; Mariano, L.N.B.; de Souza, P.; Campos, A.; Cechinel-Filho, V.; Andrade, S.F.; da Silva, L.M. Gastroprotective Value of Berries: Evidences from Methanolic Extracts of Morug nigra and Rubus niveus Fruits. Gastroenterol. Res. Pract. 2017, 7089697. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Jung, H.Y. Safety profile of acid suppression with proton pump inhibitors. Korean J. Helicobacter Up. Gastrointest. Res. 2009, 9, 11–17. [Google Scholar]
- De Queiroz, G.T.; Santos, T.R.; Macedo, R.; Peters, V.M.; Leite, M.N.; de Cássia da Silveira e Sá, R.; de Oliveira Guerra, M. Efficacy of Morus nigra L. on reproduction in female Wistar rats. Food Chem. Toxicol. 2012, 50, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, A.; Lins, T.; Santos, J.; Barros, V.; Monte, A.; Barberino, R.S.; Almeida, J.; Matos, M. Supplemented Morus nigra extract-based medium associated with FSH enables the survival and growth of isolated ovine secondary ovarian follicles. Reprod. Domest. Anim. 2018, 53, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.E. Apoptosis in cancer therapy: Crossing the threshold. Cell 1994, 78, 539–542. [Google Scholar] [CrossRef]
- Brown, J.M.; Attardi, L.D. The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer 2005, 5, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Benoist, H.; Culerrier, R.; Poiroux, G.; Ségui, B.; Jauneau, A.; Van Damme, E.J.; Peumans, W.J.; Barre, A.; Rougé, P. Two structurally identical mannose-specific jacalin-related lectins display different effects on human T lymphocyte activation and cell death. J. Leukoc. Biol. 2009, 86, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Takehara, T.; Tatsumi, T.; Suzuki, T.; Jinushi, M.; Kanazawa, Y.; Hiramatsu, N.; Kanto, T.; Tsuji, S.; Hori, M.; et al. Concanavalin a injection activates intrahepatic innate immune cells to provoke an antitumor effect in murine liver. Hepatology 2004, 40, 1190–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, A.R.; Paul, R.K.; Thakur, V.S.; Aqarwal, M.L. A novel role for p73 in the regulation of Akt-Foxo1a-Bim signaling and apoptosis induced by the plant lectin, Concanavalin A. Cancer Res. 2007, 67, 5617–5621. [Google Scholar] [CrossRef]
- Çakıroğlu, E.; Uysal, T.; Çalıbaşı Koçal, G.; Aygenli, F.; Baran, G.; Baskın, Y. The role of Morus nigra extract and its active compounds as drug candidate on human colorectal adenocarcinoma cell line HT-29. Int. J. Clin. Oncol. Cancer Res. 2017, 2, 10–14. [Google Scholar]
- Qadir, M.I.; Ali, M.; Ibrahim, Z. Anticancer activity of Morus nigra leaves extract. Bangladesh J. Pharmacol. 2014, 9, 496–497. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, M.; El-Kholie, E.; El-Garawani, I.; Sherif, N. Anticancer activity of Morus nigra on human breast cancer cell line (MCF-7): The role of fresh and dry fruit extracts. J. Biosci. Appl. Res. 2016, 2, 352–361. [Google Scholar]
- Turan, I.; Demir, S.; Kilinc, K.; Burnaz, N.A.; Yaman, S.O.; Akbulult, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Antiproliferative and apoptotic effect of Morus nigra extract on human prostate cancer cells. Saudi Pharm. J. 2017, 25, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Fry, F.H.; Holme, A.L.; Yiakouvaki, A.; Al-Qenaei, A.; Pourzand, C.; Jacob, C. Towards multifunctional antioxidants: Synthesis, electrochemistry, in vitro and cell culture evaluation of compounds with ligand/catalytic properties. Org. Biomol. Chem. 2005, 3, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Saxena, J.; Pradhan, A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res. 2012, 16, 130–134. [Google Scholar]
- Huyut, Z.; Beydemir, S.; Gülçin, I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 7616791. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef]
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus alba fruits. Sci. Hortic. (Amsterdam) 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Kalkan Yildirim, H. Evaluation of colour parameters and antioxidant activities of fruit wines. Int. J. Food Sci. Nutr. 2006, 57, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Al-Mustafa, A.H.; Al-Thunibat, O.Y. Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pak. J. Biol. Sci. 2008, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Tosun, M.; Duralija, B.; Voća, S.; Sengul, M.; Turan, M. Phytochemical content of some black (Morus nigra L.) and purple (Morus rubra L.) mulberry genotypes. Food Technol. Biotechnol. 2010, 48, 102–106. [Google Scholar]
- El-Khawaga, O.Y.; Abou-Seif, M.A. Biochemical studies on antioxidant and oxidant activities of some plant extracts. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 731–738. [Google Scholar] [PubMed]
- Imran, M.; Khan, H.; Shah, M.; Khan, R.; Khan, F. Chemical composition and antioxidant activity of certain Morus species. J. Zhejiang Univ. Sci. B 2010, 11, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Thabti, I.; Marzougui, N.; Elfalleh, W.; Ferchichi, A. Antioxidant composition and antioxidant activity of white (Morus alba L.), black (Morus nigra L.) and red (Morus rubra L.) mulberry leaves. Acta Bot. Gallica 2011, 158, 205–214. [Google Scholar] [CrossRef]
- Arfan, M.; Khan, R.; Rybarczyk, A.; Amarowicz, R. Antioxidant activity of mulberry fruit extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Sirajuddin; Chan, K.W.; Sarfraz, R.A.; Uddin, K. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): A comparative study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef]
- Kostić, D.A.; Dimitrijević, D.S.; Mitić, S.S.; Mitić, M.N.; Stojanović, G.S.; Živanović, A.V. Phenolic content and antioxidant activities of fruit extracts of Morus nigra L (Moraceae) from Southeast Serbia. Trop. J. Pharm. Res. 2013, 12, 105–110. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Serali, O.; Unal, N.; Capanoglu, E. Antioxidant activity and polyphenol composition of black mulberry (Morus nigra L.) products. J. Berry Res. 2013, 3, 41–51. [Google Scholar]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Hernández, F.; Martínez, J.J. (Poly)phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. J. Funct. Foods 2015, 18, 1039–1046. [Google Scholar] [CrossRef]
- Dimitrova, M.P.; Petkova, N.T.; Denev, P.P.; Aleksieva, I.N. Carbohydrate composition and antioxidant activity of certain Morus species. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 621–627. [Google Scholar]
- Jiang, B.; Mantri, N.; Hu, Y.; Lu, J.; Jiang, W.; Lu, H. Evaluation of bioactive compounds of black mulberry juice after thermal, microwave, ultrasonic processing, and storage at different temperatures. Food Sci. Technol. Int. 2015, 21, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Khattak, K.F.; Rahman, T.R. Effect of geographical distributions on the nutrient composition, phytochemical profile and antioxidant activity of Morus nigra. Pak. J. Pharm. Sci. 2015, 28, 1671–1678. [Google Scholar] [PubMed]
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.; Beekwilder, J.; Capanoglu, E. The effects of juice processing on black mulberry antioxidants. Food Chem. 2015, 186, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Celep, E.; Charehsaz, M.; Akyüz, S.; Acar, E.T.; Yesilada, E. Effect of in vitro gastrointestinal digestion on the bioavailability of phenolic components and the antioxidant potentials of some Turkish fruit wines. Food Res. Int. 2015, 78, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ionica, M.E.; Nour, V.; Trandafir, I. Bioactive compounds and antioxidant capacity of some Morus species. South.-west. J. Hortic. Biol. Environ. 2017, 8, 79–88. [Google Scholar]
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.D.; Beekwilder, J.; Capanoglu, E. Processing black mulberry into jam: Effects on antioxidant potential and in vitro bioaccessibility. J. Sci. Food Agric. 2017, 97, 3106–3113. [Google Scholar] [CrossRef] [PubMed]
- Montenote, M.C.; Wajsman, V.Z.; Konno, Y.T.; Ferreira, P.C.; Silva, R.M.G.; Therezo, A.L.S.; Silva, L.P.; Martins, L.P.A. Antioxidant effect of Morus nigra on Chagas disease progression. Rev. Inst. Med. Trop. Sao. Paulo 2017, 59, e73. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bao, T.; Chen, W. Comparison of the protective effect of black and white mulberry against ethyl carbanate-induced cytotoxicity and oxidative damage. Food Chem. 2018, 243, 65–73. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Bao, X.; Gao, L.; Tao, Y. Extraction of polysaccharides from black mulberry fruit and their effect on enhancing antioxidant activity. Int. J. Biol. Macromol. 2018, 120, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, A.; Martínez-Nicolás, J.J.; Munera-Picazo, S.; Carbonell-Barrachina, A.A.; Legua, P.; Hernández, F. Bioactive compounds and sensory quality of black and white mulberries grown in Spain. Plant. Foods Hum. Nutr. 2013, 68, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.M.; Abdel Bar, F.M.; Baraka, H.N.; Gohar, A.A.; Lahloub, M.F. A new antioxidant stilbene and other constituents from the stem bark of Morus nigra L. Nat. Prod. Res. 2014, 28, 952–959. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Satora, P.; Sroka, P.; Pogoń, P.; Machalica, J. Chaenomeles japonica, Cornus mas, Morus nigra fruits characteristics and their processing potential. J. Food Sci. Technol. 2014, 51, 3934–3941. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, M.; Tunçtürk, M.; Berk, S.; Şekeroğlu, N.; Gezici, S. Antioxidant capacity and bioactive contents of mulberry species from Eastern Anatolia region of Turkey. Indian J. Pharm. Educ. Res. 2018, 52, S96–S101. [Google Scholar] [CrossRef]
- Nikkhah, E.; Khayami, M.; Heidari, R. In vitro screening for antioxidant activity and cancer suppressive effect of blackberry (Morus nigra). Int. J. Cancer Manag. 2008, 1, e80627. [Google Scholar]
- Issa, N.K.; Abd-Aljabar, R.S. Evaluation of antioxidant properties of Morus nigra L. fruit extracts [II]. Jordan J. Biol. Sci. 2013, 6, 258–265. [Google Scholar] [CrossRef]
- Feng, R.Z.; Wang, Q.; Tong, W.Z.; Xiong, J.; Wei, Q.; Zhou, W.H.; Yin, Z.Q.; Yin, X.Y.; Wang, L.Y.; Chen, Y.Q.; et al. Extraction and antioxidant activity of flavonoids of Morus nigra. Int. J. Clin. Exp. Med. 2015, 8, 22328–22336. [Google Scholar]
- Naderi, G.A.; Asgary, S.; Sarraf-Zadegan, N.; Oroojy, H.; Afshin-Nia, F. Antioxidant activity of three extracts of Morus nigra. Phytother. Res. 2004, 18, 365–369. [Google Scholar] [CrossRef]
- Mnaa, S.; Aniess, W.; Olwy, Y.; Shaker, E. Antioxidant activity of white (Morus alba L.) and black (Morus nigra L.) berries against CCl4 hepatotoxic agent. Adv. Tech. Biol. Med. 2014. [Google Scholar] [CrossRef]
- Hassimotto, N.M.; Genovese, M.I.; Lajolo, F.M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005, 53, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.N.H.; Alamgeer; Salma, U.; Qayyum, A.; Samreen, S. Phytochemical analysis and cardiac depressant activity of aqueous methanolic extract of Morus nigra L. fruit. J. Appl. Pharm. Sci. 2012, 2, 39–41. [Google Scholar]
- Akhlaq, A.; Mehmood, M.H.; Rehman, A.; Ashraf, Z.; Syed, S.; Bawany, S.A.; Gilani, A.H.; Ilyas, M.; Siddiqui, B.S. The prokinetic, laxative, and antidiarrheal effects of Morus nigra: Possible muscarinic, Ca2+ channel blocking, and antimuscarinic mechanisms. Phytother. Res. 2016, 30, 1362–1376. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, Z.; Jahromy, M.H. Effects of blackberry (Morus nigra) fruit juice on levodopa-induced dyskinesia in a mice model of Parkinson’s disease. J. Exp. Pharmacol. 2018, 10, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Serpe, L.; Canaparo, R.; Dell’Osso, B.; Galentino, R.; De Michele, S.; Dina, C.Z.; Porta, M.; et al. Food Bioactive Compounds and Their Interference in Drug Pharmacokinetic/Pharmacodynamic Profiles. Pharmaceutics 2018, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoon, Y.J.; Shon, J.H.; Cha, I.J.; Shin, J.G.; Liu, K.H. Inhibitory effects of fruit juices on CYP3A activity. Drug Metab. Dispos. 2006, 34, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [Green Version]
- Drug Interactions Flockhart Table. Available online: https://drug-interactions.medicine.iu.edu/main-table.aspx (assessed on 11 February 2019).
- Figueredo, K.C.; Guex, C.G.; Reginato, F.Z.; Haas da Silva, A.R.; Cassanego, G.B.; Lhamas, C.L.; Boligon, A.A.; Lopes, G.H.H.; de Freitas Bauermann, L. Safety assessment of Morus nigra L. leaves: Acute and subacute oral toxicity studies in Wistar rats. J. Ethnopharmacol. 2018, 224, 290–296. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.H.; Choi, C.-I. Pharmacological Properties of Morus nigra L. (Black Mulberry) as A Promising Nutraceutical Resource. Nutrients 2019, 11, 437. https://doi.org/10.3390/nu11020437
Lim SH, Choi C-I. Pharmacological Properties of Morus nigra L. (Black Mulberry) as A Promising Nutraceutical Resource. Nutrients. 2019; 11(2):437. https://doi.org/10.3390/nu11020437
Chicago/Turabian StyleLim, Sung Ho, and Chang-Ik Choi. 2019. "Pharmacological Properties of Morus nigra L. (Black Mulberry) as A Promising Nutraceutical Resource" Nutrients 11, no. 2: 437. https://doi.org/10.3390/nu11020437