Hydrangenol Isolated from the Leaves of Hydrangea serrata Attenuates Wrinkle Formation and Repairs Skin Moisture in UVB-Irradiated Hairless Mice
Abstract
:1. Introduction
2. Materials and Methods
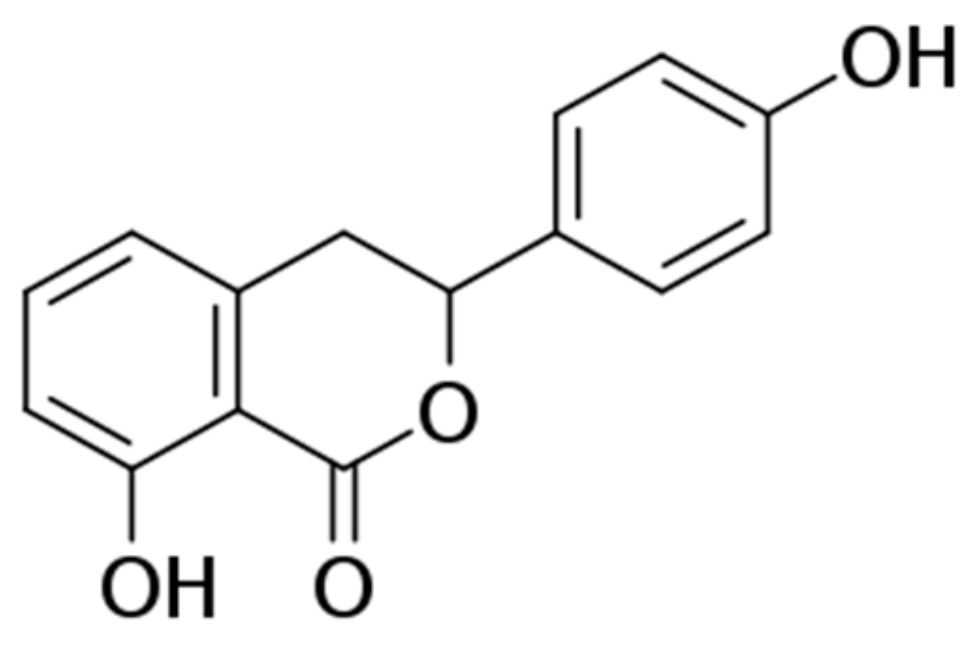
2.1. Preparation of Hydrangenol from the Leaves of Hydrangea serrata
2.2. Animal and Treatment
2.3. UVB Irradiation
2.4. Evaluation of Skin Wrinkle Formation
2.5. Histological Analysis
2.6. Skin Hydration and Skin Transepidermal Water Loss (TEWL) Analysis
2.7. Measurement of Hyaluronic Acid and Procollagen Type I Production
2.8. Western Blot Analysis
2.9. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
2.10. Statistical Analysis
3. Results
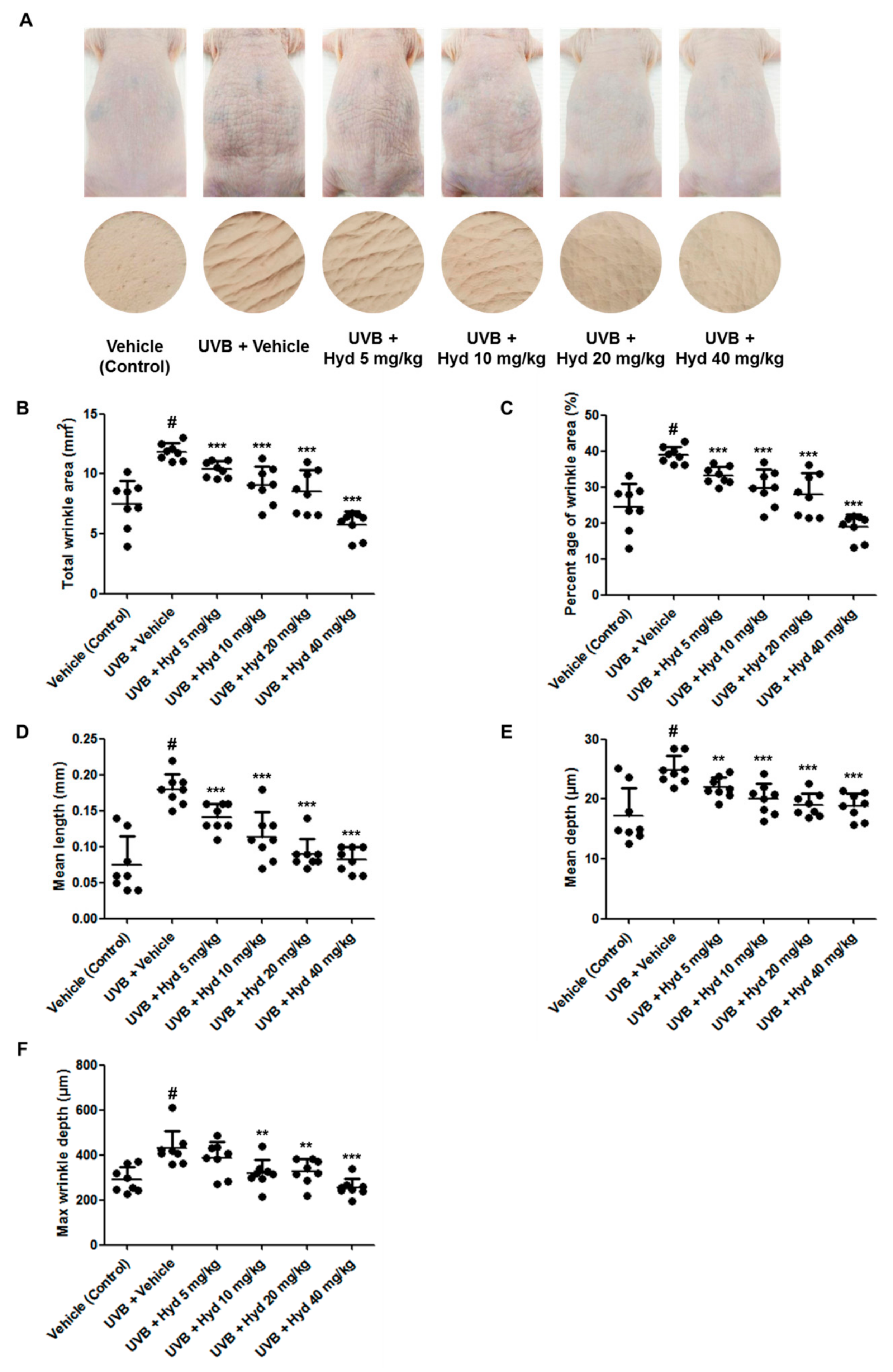
3.1. Hydrangenol Mitigates Wrinkle on the UVB-Irradiated Dorsal Skin of HR-1 Hairless Mice
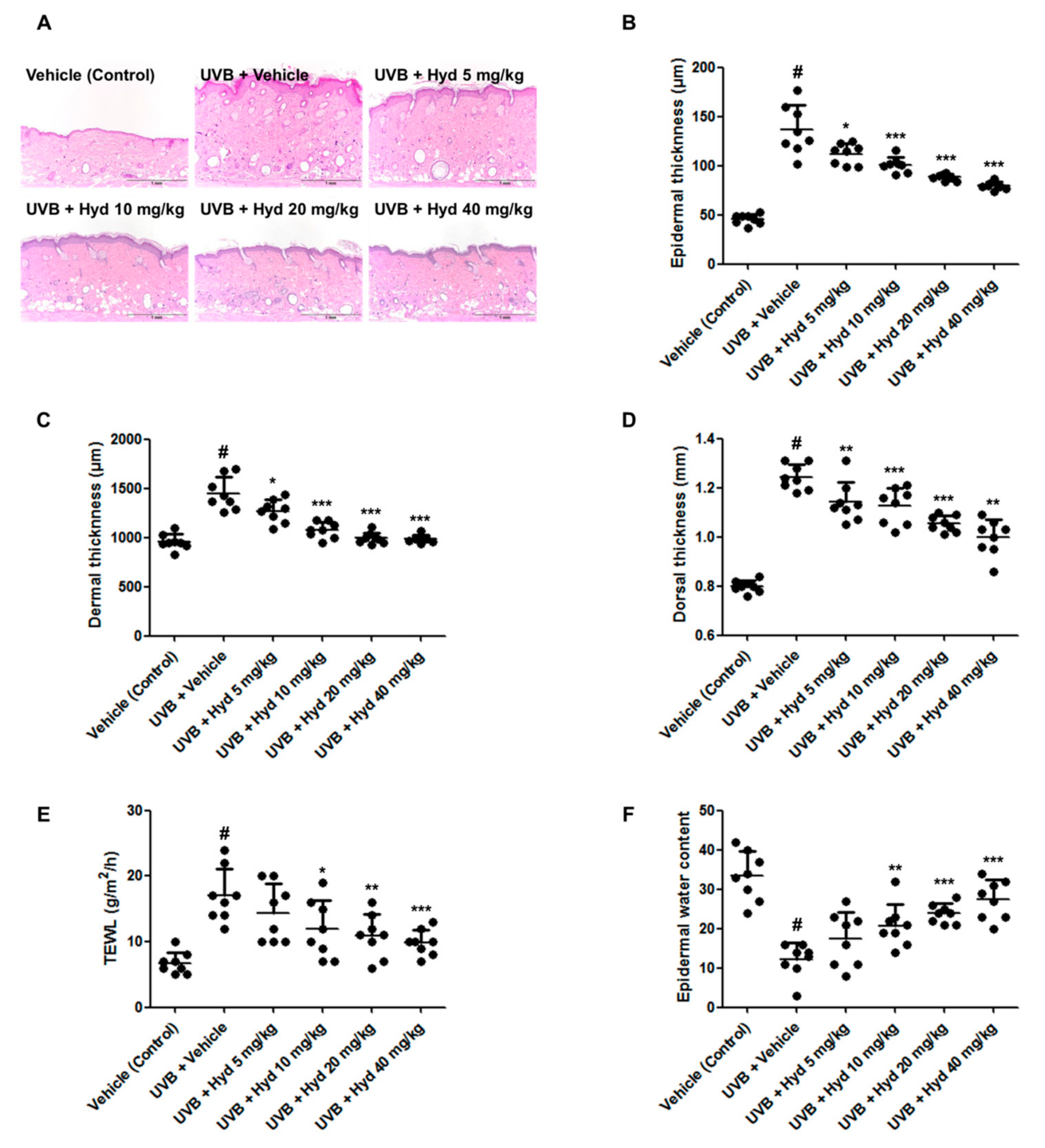
3.2. Hydrangenol Reduces UVB-Induced Epidermis/Dermis Thickness, TEWL, and Skin Dehydration on the Dorsal Skin of HR-1 Hairless Mice
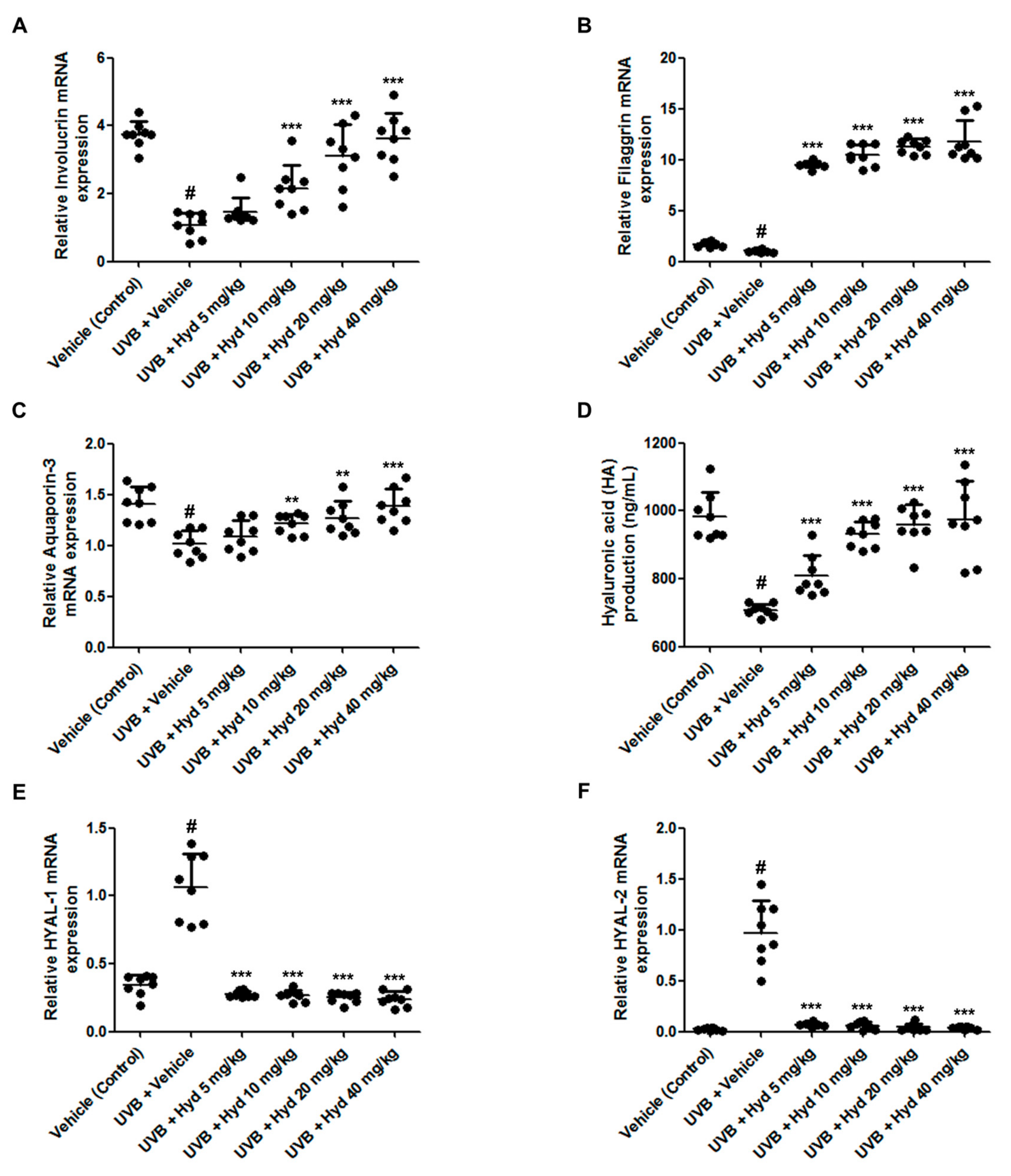
3.3. Hydrangenol Increases Skin Barrier Gene Expression and HA Production Via Downregulating HYAL mRNA Expression in UVB-Irradiated HR-1 Hairless Mice
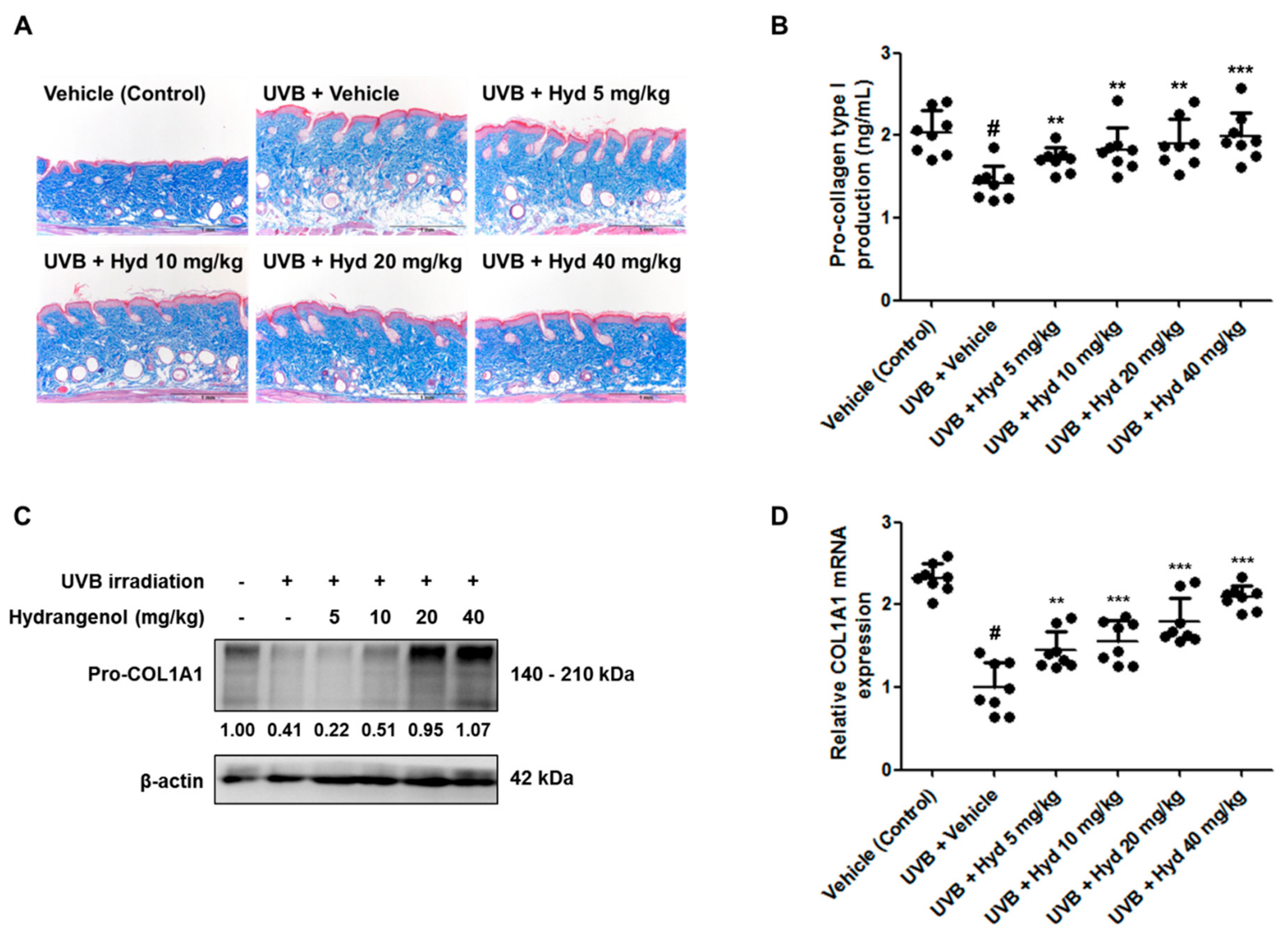
3.4. Hydrangenol Restores Pro-Collagen Type I Production and Pro-COL1A1 Protein and mRNA Expression in UVB-Irradiated HR-1 Hairless Mice
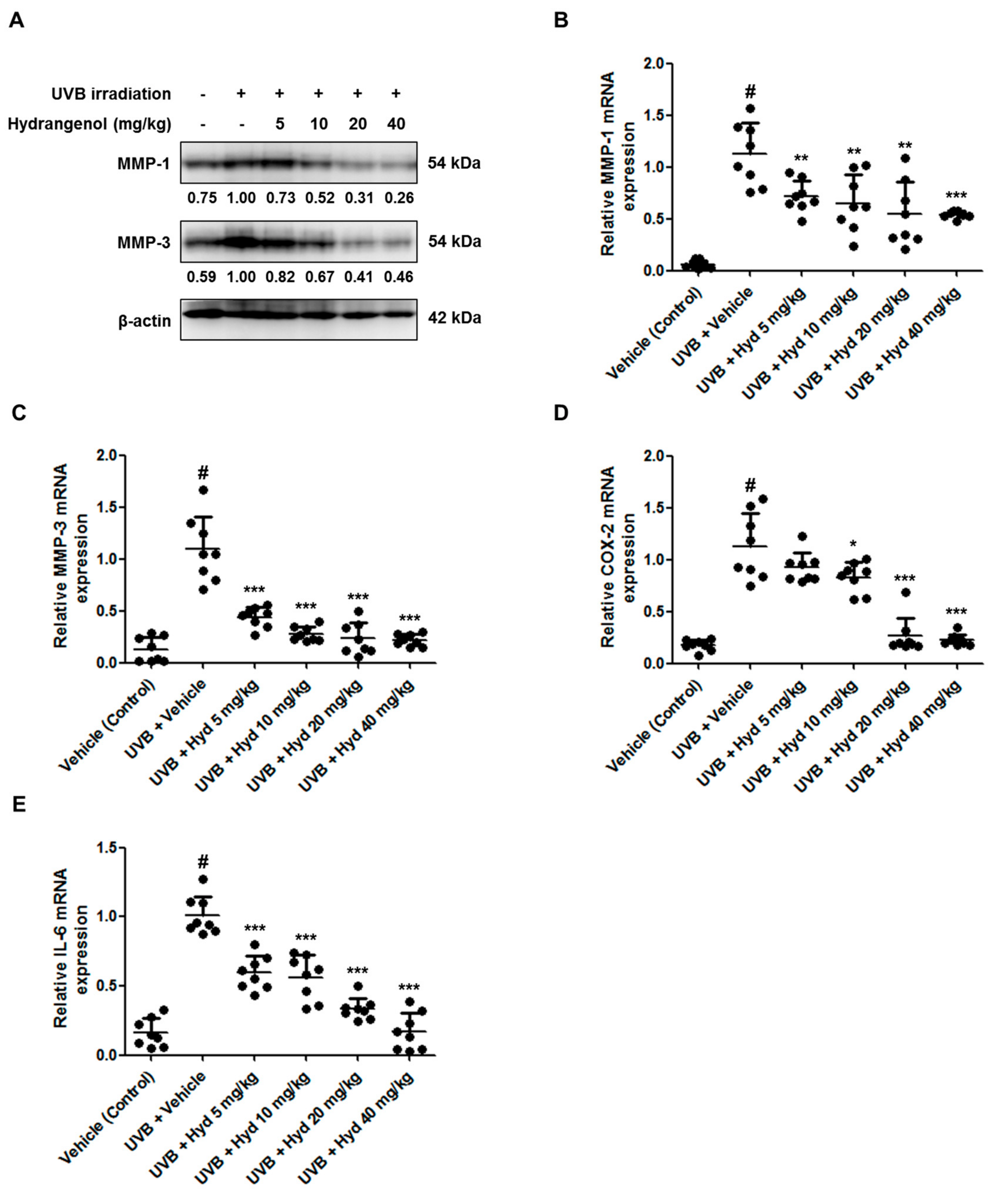
3.5. Hydrangenol Attenuates UVB-Induced Expression of MMP-1/-3, COX-2, and IL-6 in HR-1 Hairless Mice
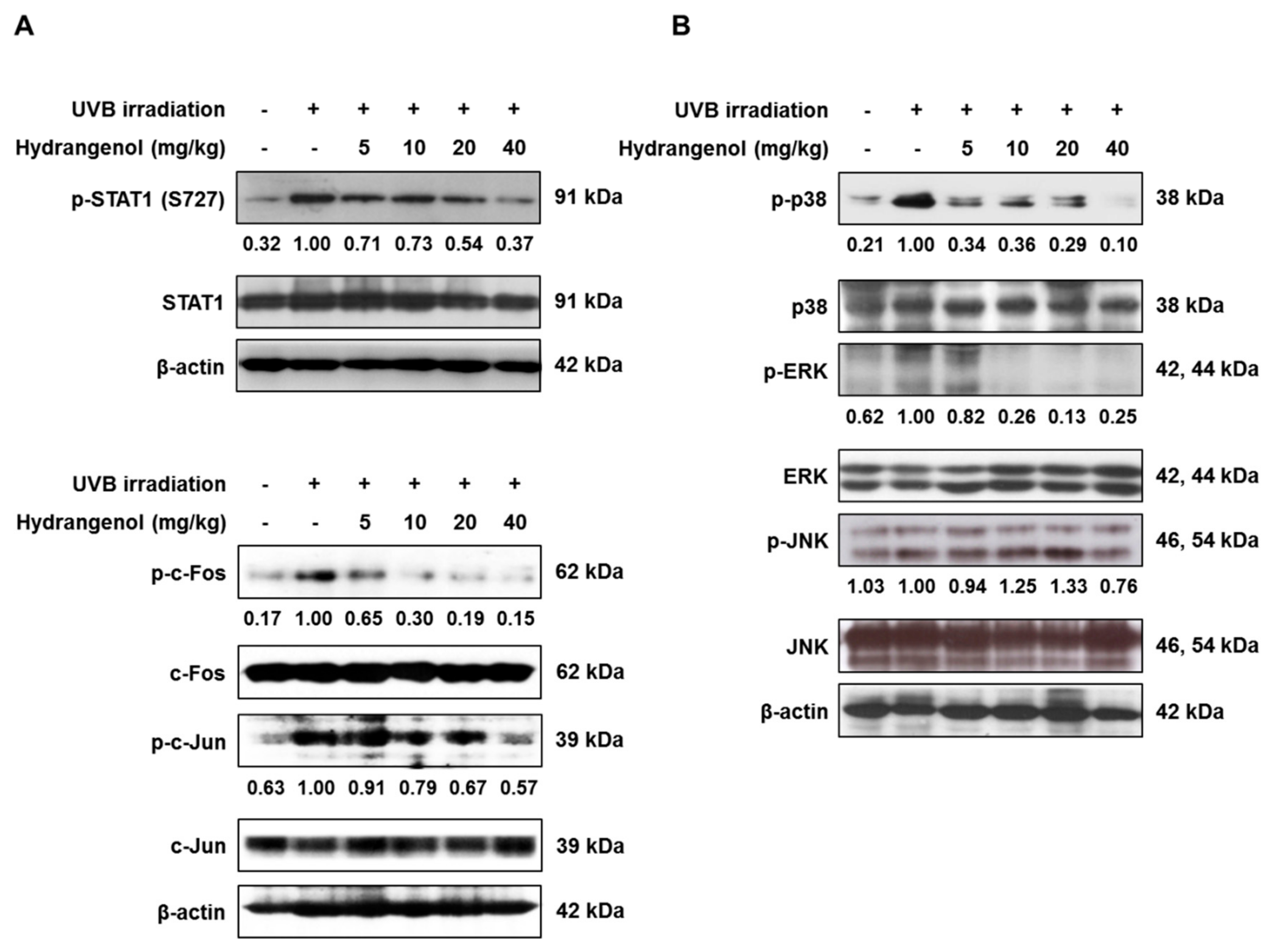
3.6. Hydrangenol Prevents the Activation of STAT1 and AP-1 and the MAPK Signaling Pathway in UVB-Induced HR-1 Hairless Mice
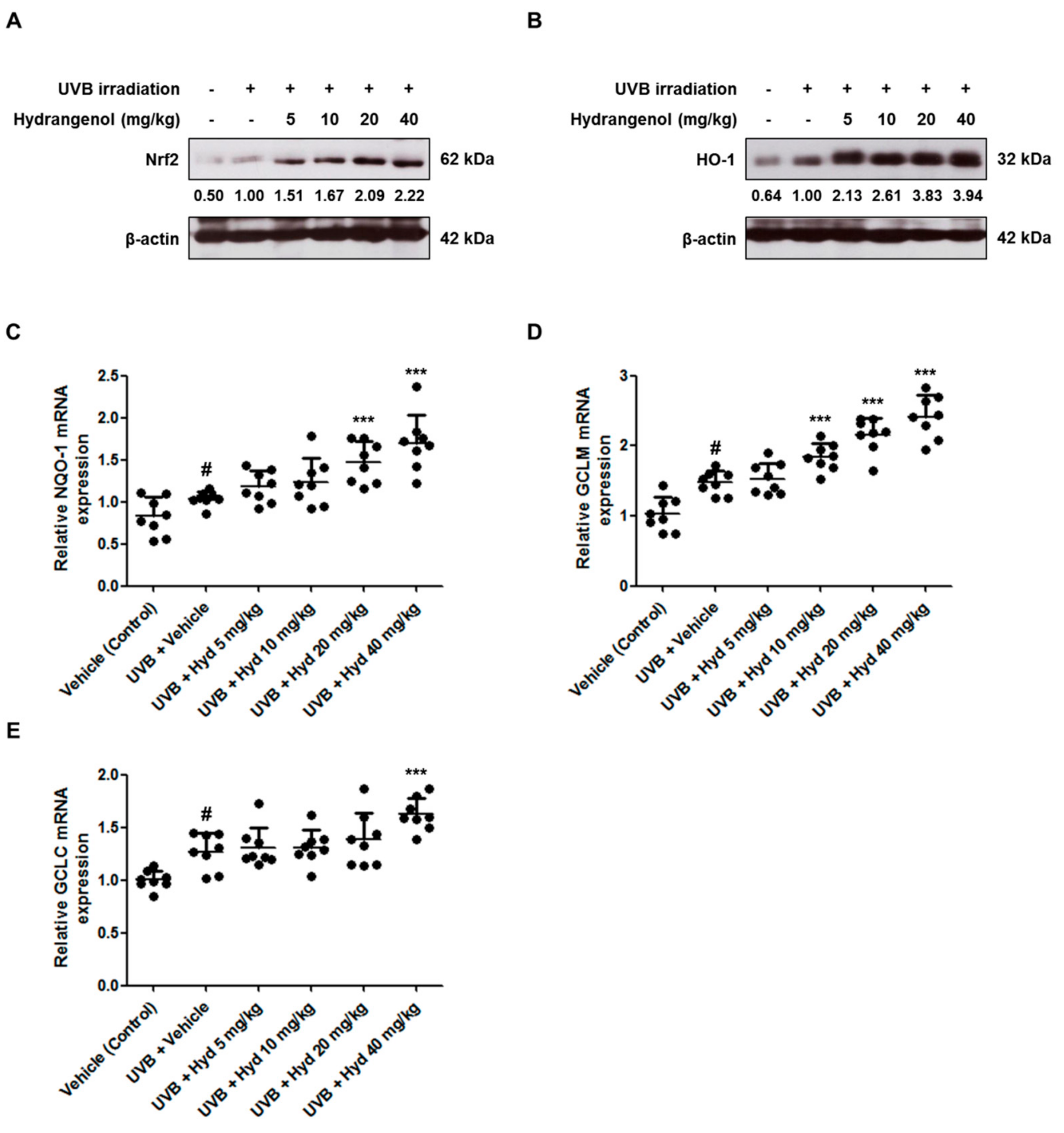
3.7. Hydrangenol Promotes the Nrf2/ARE Signaling Pathway in UVB-Induced HR-1 Hairless Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wan, S.; Jiang, Q.; Amaral, A.; Lu, S.; Hu, G.; Bi, Z.; Kouttab, N.; Chu, W.; Wan, Y. All-trans retinoic acid attenuates ultraviolet radiation-induced down-regulation of aquaporin-3 and water permeability in human keratinocytes. J. Cell. Physiol. 2008, 215, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M.; Marekov, L.N. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J. Biol. Chem. 1995, 270, 17702–17711. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Matts, P.J. Stratum corneum moisturization at the molecular level: An update in relation to the dry skin cycle. J. Investig. Dermatol. 2005, 124, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Chen, W.Y.; Aljuffali, I.A.; Lin, Y.K.; Shih, H.C.; Fang, J.Y. Skin aging modulates percutaneous drug absorption: The impact of ultraviolet irradiation and ovariectomy. Age 2015, 37, 21. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral Intake of Collagen Peptide Attenuates Ultraviolet B Irradiation-Induced Skin Dehydration In Vivo by Regulating Hyaluronic Acid Synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef]
- Del Bino, S.; Vioux, C.; Rossio-Pasquier, P.; Jomard, A.; Demarchez, M.; Asselineau, D.; Bernerd, F. Ultraviolet B induces hyperproliferation and modification of epidermal differentiation in normal human skin grafted on to nude mice. Br. J. Dermatol. 2004, 150, 658–667. [Google Scholar] [CrossRef]
- Boury-Jamot, M.; Daraspe, J.; Bonte, F.; Perrier, E.; Schnebert, S.; Dumas, M.; Verbavatz, J.M. Skin aquaporins: Function in hydration, wound healing, and skin epidermis homeostasis. Handb. Exp. Pharmacol. 2009, 205–217. [Google Scholar] [CrossRef]
- Ji, C.; Cao, C.; Lu, S.; Kivlin, R.; Amaral, A.; Kouttab, N.; Yang, H.; Chu, W.; Bi, Z.; Di, W.; et al. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother. Pharmacol. 2008, 62, 857–865. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Kurdykowski, S.; Mine, S.; Bardey, V.; Danoux, L.; Jeanmaire, C.; Pauly, G.; Brabencova, E.; Wegrowski, Y.; Maquart, F.X. Ultraviolet-B irradiation induces differential regulations of hyaluronidase expression and activity in normal human keratinocytes. Photochem. Photobiol. 2011, 87, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Rittie, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Avila Rodriguez, M.I.; Rodriguez Barroso, L.G.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suarez-Perez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; Gonzalez, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Potapovich, A.I.; Kostyuk, V.A.; Kostyuk, T.V.; de Luca, C.; Korkina, L.G. Effects of pre- and post-treatment with plant polyphenols on human keratinocyte responses to solar UV. Inflamm. Res. 2013, 62, 773–780. [Google Scholar] [CrossRef]
- Marrot, L.; Jones, C.; Perez, P.; Meunier, J.R. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008, 21, 79–88. [Google Scholar] [CrossRef]
- Zhao, P.; Alam, M.B.; Lee, S.H. Protection of UVB-Induced Photoaging by Fuzhuan-Brick Tea Aqueous Extract via MAPKs/Nrf2-Mediated Down-Regulation of MMP-1. Nutrients 2018, 11, 60. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Yamada, M.; Tsuda, Y.; Kawai, K.; Nakajima, S. Antifungal activity of oosponol, oospolactone, phyllodulcin, hydrangenol, and some other related compounds. Chem. Pharm. Bull. 1981, 29, 2689–2691. [Google Scholar] [CrossRef] [PubMed]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Inhibitory effects of hydrangenol derivatives on the activation of hyaluronidase and their antiallergic activities. Planta Med. 1988, 54, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Matsuda, H.; Kumahara, A.; Ito, Y.; Nakamura, S.; Yoshikawa, M. New type of anti-diabetic compounds from the processed leaves of Hydrangea macrophylla var. thunbergii (Hydrangeae Dulcis Folium). Bioorg. Med. Chem. Lett. 2007, 17, 4972–4976. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kang, C.H.; Jayasooriya, R.; Dilshara, M.G.; Lee, S.; Choi, Y.H.; Seo, Y.T.; Kim, G.Y. Hydrangenol inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-kappaB pathway and activating the Nrf2-mediated HO-1 pathway. Int. Immunopharmacol. 2016, 35, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Gho, Y.; Shin, S.S.; Choi, Y.H.; Ko, K.; Kim, W.J.; Moon, S.K. Hydrangenol suppresses VEGF-stimulated angiogenesis by targeting p27KIP1-dependent G1-cell cycle arrest, VEGFR-2-mediated signaling, and MMP-2 expression. Anim. Cells Syst. 2019, 23, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Han, H.S.; Lee, S.B.; Myung, D.B.; Lee, K.; Lee, S.H.; Kim, H.J.; Lee, K.T. Chemical Constituents from Leaves of Hydrangea serrata and Their Anti-photoaging Effects on UVB-Irradiated Human Fibroblasts. Biol. Pharm. Bull. 2019, 42, 424–431. [Google Scholar] [CrossRef]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.J.; McLean, W.H. One remarkable molecule: Filaggrin. J. Investig. Dermatol. 2012, 132, 751–762. [Google Scholar] [CrossRef]
- Schroeder, P.; Haendeler, J.; Krutmann, J. The role of near infrared radiation in photoaging of the skin. Exp. Gerontol. 2008, 43, 629–632. [Google Scholar] [CrossRef]
- Zykova, T.A.; Zhang, Y.; Zhu, F.; Bode, A.M.; Dong, Z. The signal transduction networks required for phosphorylation of STAT1 at Ser727 in mouse epidermal JB6 cells in the UVB response and inhibitory mechanisms of tea polyphenols. Carcinogenesis 2005, 26, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Krajisnik, A.; Zhang, D.D.; Wondrak, G.T. Targeting NRF2 for Improved Skin Barrier Function and Photoprotection: Focus on the Achiote-Derived Apocarotenoid Bixin. Nutrients 2017, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Wu, J.; Zhang, D.; Cheng, B.; Zhang, Y.; Yin, Z.; Wang, Y.; Du, J.; Ling, C. Ginsenoside Rg1 attenuates ultraviolet B-induced glucocortisides resistance in keratinocytes via Nrf2/HDAC2 signalling. Sci. Rep. 2016, 6, 39336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo de la Vega, M.; Zhang, D.D.; Wondrak, G.T. Topical Bixin Confers NRF2-Dependent Protection Against Photodamage and Hair Graying in Mouse Skin. Front. Pharmacol. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Durr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol. Cell 2005, 97, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef]
- De Guzman Strong, C.; Wertz, P.W.; Wang, C.; Yang, F.; Meltzer, P.S.; Andl, T.; Millar, S.E.; Ho, I.C.; Pai, S.Y.; Segre, J.A. Lipid defect underlies selective skin barrier impairment of an epidermal-specific deletion of Gata-3. J. Cell Biol. 2006, 175, 661–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masse, I.; Barbollat-Boutrand, L.; Kharbili, M.E.; Berthier-Vergnes, O.; Aubert, D.; Lamartine, J. GATA3 inhibits proliferation and induces expression of both early and late differentiation markers in keratinocytes of the human epidermis. Arch. Dermatol. Res. 2014, 306, 201–208. [Google Scholar] [CrossRef]
- Son, D.N.; Li, L.; Katsuyama, H.; Komatsu, N.; Saito, M.; Tanii, H.; Saijoh, K. Abundant expression of Kallikrein 1 gene in human keratinocytes was mediated by GATA3. Gene 2009, 436, 121–127. [Google Scholar] [CrossRef]
- Zeitvogel, J.; Jokmin, N.; Rieker, S.; Klug, I.; Brandenberger, C.; Werfel, T. GATA3 regulates FLG and FLG2 expression in human primary keratinocytes. Sci. Rep. 2017, 7, 11847. [Google Scholar] [CrossRef] [PubMed]
- Wiest, L.; Kerscher, M. Native hyaluronic acid in dermatology—Results of an expert meeting. J. Dtsch. Dermatol. Ges. 2008, 6, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Kimata, K. Mammalian hyaluronan synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; Frost, G.I.; Stern, R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001, 20, 499–508. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.Y.; Noh, E.M.; Song, H.K.; Lee, G.S.; Kwon, K.B.; Lee, Y.R. Reversine inhibits MMP-1 and MMP-3 expressions by suppressing of ROS/MAPK/AP-1 activation in UV-stimulated human keratinocytes and dermal fibroblasts. Exp. Dermatol. 2018, 27, 298–301. [Google Scholar] [CrossRef] [Green Version]
- Angel, P.; Szabowski, A.; Schorpp-Kistner, M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene 2001, 20, 2413–2423. [Google Scholar] [CrossRef] [Green Version]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Tan, Q.; Yang, H.; Liu, E.; Wang, H. P38/ERK MAPK signaling pathways are involved in the regulation of filaggrin and involucrin by IL17. Mol. Med. Rep. 2017, 16, 8863–8867. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.K.; Kang, M.K.; Lee, G.T.; Lee, K.K.; Lee, H.S.; Woo, W.H.; Mun, Y.J. EPA attenuates ultraviolet radiation-induced downregulation of aquaporin-3 in human keratinocytes. Arch. Pharm. Res. 2015, 38, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Justiniano, R.; Zhang, D.D.; Wondrak, G.T. The Nrf2-inducers tanshinone I and dihydrotanshinone protect human skin cells and reconstructed human skin against solar simulated UV. Redox Biol. 2013, 1, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic. Res. 2017, 51, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.; Tsen, J.H.; Yen, H.; Yang, T.Y.; Huang, H.C. Extract from Periostracum cicadae Inhibits Oxidative Stress and Inflammation Induced by Ultraviolet B Irradiation on HaCaT Keratinocytes. Evid. Based Complement. Alternat. Med. 2017, 2017, 8325049. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Lee, C.L.; Korivi, M.; Liao, J.W.; Rajendran, P.; Wu, J.J.; Hseu, Y.C. Zerumbone protects human skin keratinocytes against UVA-irradiated damages through Nrf2 induction. Biochem. Pharmacol. 2018, 148, 130–146. [Google Scholar] [CrossRef]
- Ji, C.; Huang, J.W.; Xu, Q.Y.; Zhang, J.; Lin, M.T.; Tu, Y.; He, L.; Bi, Z.G.; Cheng, B. Gremlin inhibits UV-induced skin cell damages via activating VEGFR2-Nrf2 signaling. Oncotarget 2016, 7, 84748–84757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myung, D.-B.; Han, H.-S.; Shin, J.-S.; Park, J.Y.; Hwang, H.J.; Kim, H.J.; Ahn, H.S.; Lee, S.H.; Lee, K.-T. Hydrangenol Isolated from the Leaves of Hydrangea serrata Attenuates Wrinkle Formation and Repairs Skin Moisture in UVB-Irradiated Hairless Mice. Nutrients 2019, 11, 2354. https://doi.org/10.3390/nu11102354
Myung D-B, Han H-S, Shin J-S, Park JY, Hwang HJ, Kim HJ, Ahn HS, Lee SH, Lee K-T. Hydrangenol Isolated from the Leaves of Hydrangea serrata Attenuates Wrinkle Formation and Repairs Skin Moisture in UVB-Irradiated Hairless Mice. Nutrients. 2019; 11(10):2354. https://doi.org/10.3390/nu11102354
Chicago/Turabian StyleMyung, Da-Bin, Hee-Soo Han, Ji-Sun Shin, Ji Yeon Park, Han Jun Hwang, Hyoung Ja Kim, Hye Shin Ahn, Sun Hee Lee, and Kyung-Tae Lee. 2019. "Hydrangenol Isolated from the Leaves of Hydrangea serrata Attenuates Wrinkle Formation and Repairs Skin Moisture in UVB-Irradiated Hairless Mice" Nutrients 11, no. 10: 2354. https://doi.org/10.3390/nu11102354




