Effects of Inflammation on Biomarkers of Vitamin A Status among a Cohort of Bolivian Infants
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Design
2.2. Ethical Approval
2.3. Laboratory Analysis
2.4. Definitions of Anemia, Inflammation, and Vitamin A Deficiency (VAD)
2.5. Adjustment of Vitamin A Biomarkers for Inflammation
2.6. Statistical Methods
3. Results
3.1. Characteristics of the Study Population
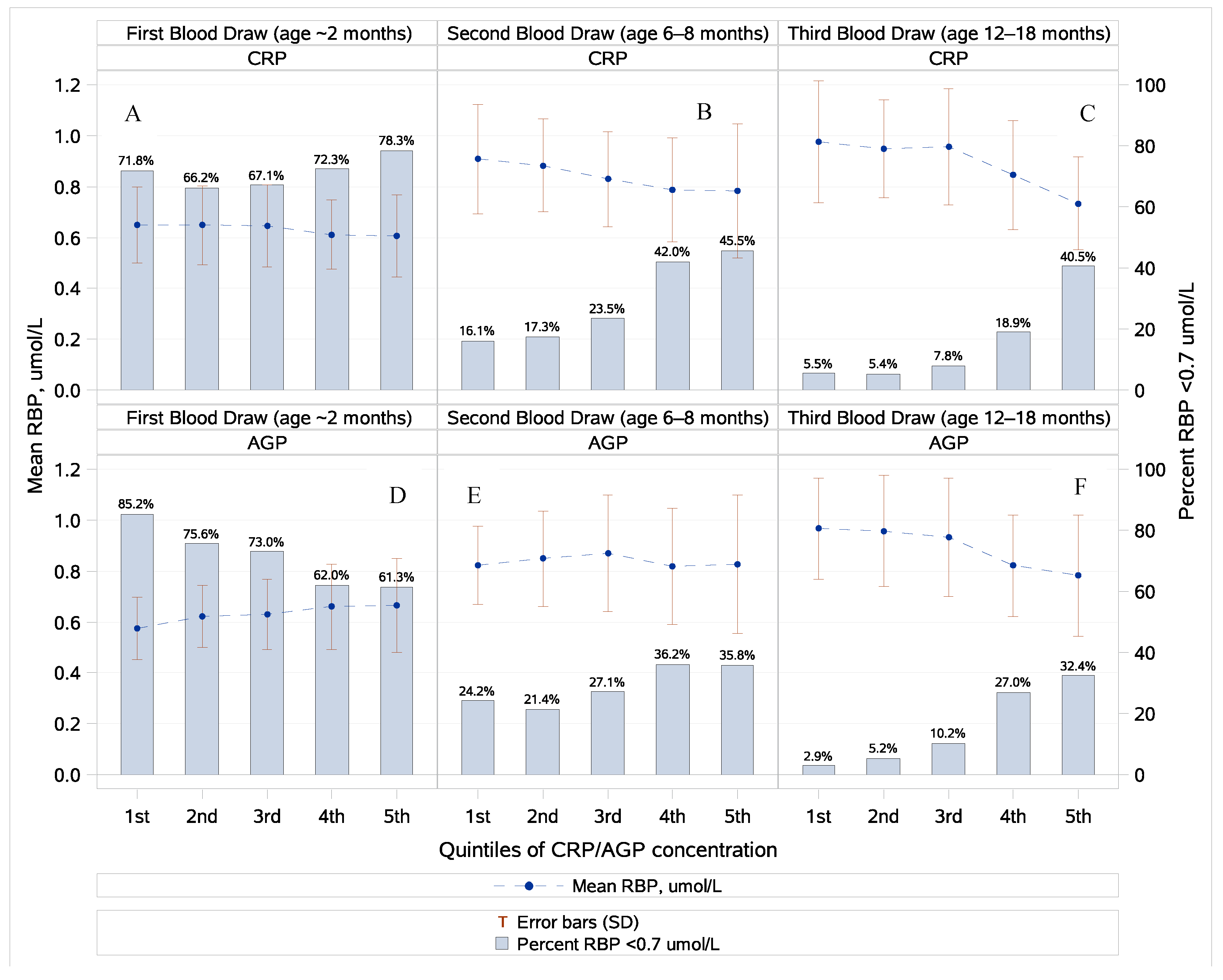
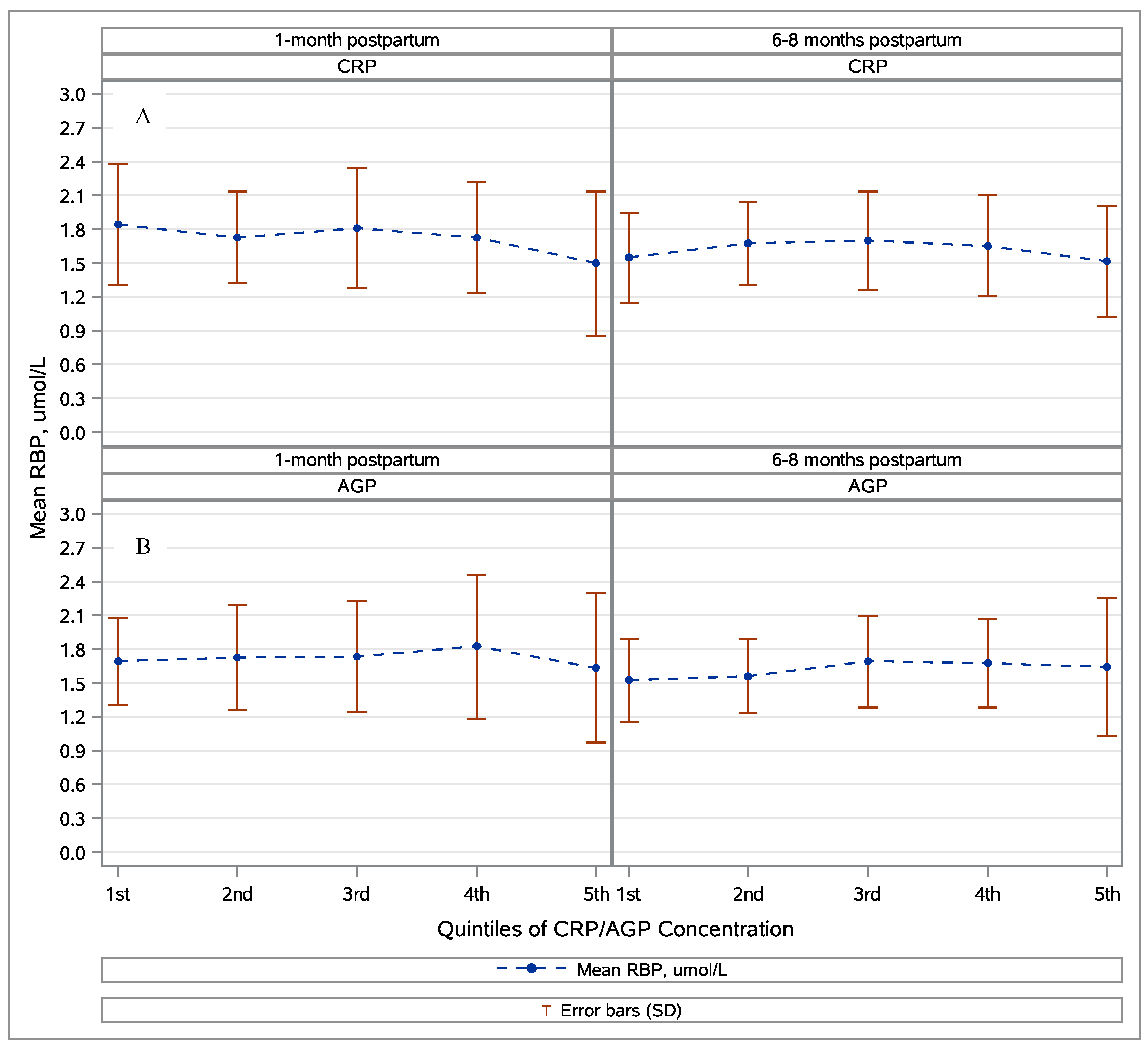
3.2. Retinol, Retinol-Binding Protein, and Inflammation
3.3. Vitamin A Status
3.4. Potential Consequences of Low Vitamin A Status in Early Life
3.5. Potential Correlations with Low Vitamin A Status in Early Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VAD | (vitamin A deficiency) |
| RBP | (retinol-binding protein) |
| CRP | (C-reactive protein) |
| AGP | (alpha(1)-acid glycoprotein) |
| MNP | (multiple micronutrient powder) |
| VAS | (vitamin A supplementation) |
References
- World Health Organization (WHO). Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Cediel, G.; Olivares, M.; Brito, A.; Lopez de Romana, D.; Cori, H.; La Frano, M.R. Interpretation of Serum Retinol Data From Latin America and the Caribbean. Food Nutr. Bull. 2015, 36 (Suppl. 2), S98–S108. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.P.; Petry, N.; Tanumihardjo, S.A.; Rogers, L.M.; McLean, E.; Greig, A.; Garrett, G.S.; Klemm, R.D.W.; Rohner, F. Vitamin A Supplementation Programs and Country-Level Evidence of Vitamin A Deficiency. Nutrients 2017, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- WHO. Priorities in the Assessment of Vitamin A and Iron Status in Populations, Panama City, Panama, 15–17 September 2010; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Tanumihardjo, S.A. Usefulness of vitamin A isotope methods for status assessment: From deficiency through excess. Int. J. Vitam. Nutr. Res. 2014, 84 (Suppl. 1), 16–24. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Erhardt, J.G.; Estes, J.E.; Pfeiffer, C.M.; Biesalski, H.K.; Craft, N.E. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J. Nutr. 2004, 134, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Gorstein, J.L.; Dary, O.; Pongtorn Shell-Duncan, B.; Quick, T.; Wasanwisut, E. Feasibility of using retinol-binding protein from capillary blood specimens to estimate serum retinol concentrations and the prevalence of vitamin A deficiency in low-resource settings. Public Health Nutr. 2008, 11, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Engle-Stone, R.; Haskell, M.J.; Ndjebayi, A.O.; Nankap, M.; Erhardt, J.G.; Gimou, M.M.; Brown, K.H. Plasma retinol-binding protein predicts plasma retinol concentration in both infected and uninfected Cameroonian women and children. J. Nutr. 2011, 141, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; Van Den Schrieck, H.G.; De Nayer, P.; De Visscher, M. The role of retinol-binding protein in protein-calorie malnutrition. Metabolism 1975, 24, 633–641. [Google Scholar] [CrossRef]
- Smith, F.R.; Goodman, D.S.; Arroyave, G.; Viteri, F. Serum vitamin A, retinol-binding protein, and prealbumin concentrations in protein-calorie malnutrition. II. Treatment including supplemental vitamin A. Am. J. Clin. Nutr. 1973, 26, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.R.; Goodman, D.S.; Zaklama, M.S.; Gabr, M.K.; el-Maraghy, S.; Patwardhan, V.N. Serum vitamin A, retinol-binding protein, and prealbumin concentrations in protein-calorie malnutrition. I. A functional defect in hepatic retinol release. Am. J. Clin. Nutr. 1973, 26, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Yuniar, Y.; Gamble, M.V.; Natadisastra, G.; Muhilal. Assessment of vitamin A status of preschool children in Indonesia using plasma retinol-binding protein. J. Trop. Pediatr. 2002, 48, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Gamble, M.V.; Ramakrishnan, R.; Palafox, N.A.; Briand, K.; Berglund, L.; Blaner, W.S. Retinol binding protein as a surrogate measure for serum retinol: Studies in vitamin A-deficient children from the Republic of the Marshall Islands. Am. J. Clin. Nutr. 2001, 73, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Almekinder, J.; Manda, W.; Soko, D.; Lan, Y.; Hoover, D.R.; Semba, R.D. Evaluation of plasma retinol-binding protein as a surrogate measure for plasma retinol concentrations. Scand. J. Clin. Lab. Investig. 2000, 60, 199–203. [Google Scholar] [CrossRef]
- Thurnham, D.I.; McCabe, G.P. Influence of Infection and Inflammation on Biomarkers of Nutritional Status with an Emphasis on Vitamin A and Iron; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Thurnham, D.I.; McCabe, G.P.; Northrop-Clewes, C.A.; Nestel, P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: Meta-analysis. Lancet 2003, 362, 2052–2058. [Google Scholar] [CrossRef]
- Larson, L.M.; Namaste, S.M.; Williams, A.M.; Engle-Stone, R.; Addo, O.Y.; Suchdev, P.S.; Wirth, J.P.; Temple, V.; Serdula, M.; Northrop-Clewes, C.A. Adjusting retinol-binding protein concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 390S–401S. [Google Scholar] [PubMed]
- Louw, J.A.; Werbeck, A.; Louw, M.E.; Kotze, T.J.; Cooper, R.; Labadarios, D. Blood vitamin concentrations during the acute-phase response. Crit. Care Med. 1992, 20, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.M.; Guo, J.; Williams, A.M.; Young, M.F.; Ismaily, S.; Addo, O.Y.; Thurnham, D.; Tanumihardjo, S.A.; Suchdev, P.S.; Northrop-Clewes, C.A. Approaches to Assess Vitamin A Status in Settings of Inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Nutrients 2018, 10, 1325S–1333S. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.M.; Addo, O.Y.; Sandalinas, F.; Faigao, K.; Kupka, R.; Flores-Ayala, R.; Suchdev, P.S. Accounting for the influence of inflammation on retinol-binding protein in a population survey of Liberian preschool-age children. Matern Child Nutr. 2016, 13, e12298. [Google Scholar] [CrossRef] [PubMed]
- Azais-Braesco, V.; Pascal, G. Vitamin A in pregnancy: Requirements and safety limits. Am. J. Clin. Nutr. 2000, 71 (Suppl. 5), 1325S–1333S. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.S.; Araujo, K.F.; Azevedo, G.M.; Dimenstein, R. Maternal supplementation with retinyl palmitate during immediate postpartum period: Potential consumption by infants. Rev. Saude Publ. 2009, 43, 572–579. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guideline: Vitamin A Supplementation in Infants and Children 6–59 Months of Age; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Haider, B.A.; Sharma, R.; Bhutta, Z.A. Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries. Cochrane Database Syst. Rev. 2017, 2, CD006980. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Ahmed, Z.; Bhutta, Z.A. Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age. Cochrane Database Syst. Rev. 2016, 9, CD007480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolivia Ministry of Health. Atencion Integrada a las Enfermedades Prevalentes de la Infancia en el Marco de la Meta “Desnutricion Cero”: AIEPI—Nut: Cuadros de Procedimientos. In Documentos Técnico Normativos; Ministerio de Salud y Deportes: La Paz, Bolivia, 2006. [Google Scholar]
- Burke, R.M.; Suchdev, P.S.; Rebolledo, P.A.; de Aceituno, A.M.; Revollo, R.; Iniguez, V.; Klein, M.; Drews-Botsch, C.; Leon, J.S. Predictors of Inflammation in a Cohort of Bolivian Infants and Toddlers. Am. J. Trop. Med. Hyg. 2016, 95, 954–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aceituno, A.M.; Stanhope, K.K.; Rebolledo, P.A.; Burke, R.M.; Revollo, R.; Iniguez, V.; Suchdev, P.S.; Leon, J.S. Using a monitoring and evaluation framework to improve study efficiency and quality during a prospective cohort study in infants receiving rotavirus vaccination in El Alto, Bolivia: The Infant Nutrition, Inflammation, and Diarrheal Illness (NIDI) study. BMC Public Health 2017, 17, 911. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Growth Charts. The WHO Growth Charts; 2010. Available online: http://www.cdc.gov/growthcharts/who_charts.htm (accessed on 29 May 2014).
- Sullivan, K.M.; Mei, Z.; Grummer-Strawn, L.; Parvanta, I. Haemoglobin adjustments to define anaemia. Trop. Med. Int. Health. 2008, 13, 1267–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Thurnham, D.I.; Mburu, A.S.; Mwaniki, D.L.; Muniu, E.M.; Alumasa, F.; de Wagt, A. Using plasma acute-phase protein concentrations to interpret nutritional biomarkers in apparently healthy HIV-1-seropositive Kenyan adults. Br. J. Nutr. 2008, 100, 174–182. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Suchdev, P.S.; Namaste, S.; Aaron, G.J.; Raiten, D.J.; Brown, K.H.; Flores-Ayala, R. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Adv. Nutr. 2016, 7, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engle-Stone, R.; Nankap, M.; Ndjebayi, A.O.; Erhardt, J.G.; Brown, K.H. Plasma ferritin and soluble transferrin receptor concentrations and body iron stores identify similar risk factors for iron deficiency but result in different estimates of the national prevalence of iron deficiency and iron-deficiency anemia among women and children in Cameroon. J. Nutr. 2013, 143, 369–377. [Google Scholar] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Ygberg, S.; Nilsson, A. The developing immune system—From foetus to toddler. Acta Paediatr. 2012, 101, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, B.A.; Lima, L.M.; Moreira, M.E.; Priore, S.E.; Henriques, B.D.; Carlos, C.F.; Sabino, J.S.; Franceschini Sdo, C. Micronutrient supplementation adherence and influence on the prevalences of anemia and iron, zinc and vitamin A deficiencies in preemies with a corrected age of six months. Clinics 2016, 71, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Virmani, D.; Jaipal, M.; Gupta, S.; Toteja, G.S. Investigators of LBWMSG. Vitamin A status of low and normal birth weight infants at birth and in early infancy. Indian Pediatr. 2013, 50, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Diana, A.; Haszard, J.J.; Purnamasari, D.M.; Nurulazmi, I.; Luftimas, D.E.; Rahmania, S.; Nugraha, G.I.; Erhardt, J.; Gibson, R.S.; Houghton, L. Iron, zinc, vitamin A and selenium status in a cohort of Indonesian infants after adjusting for inflammation using several different approaches. Br. J. Nutr. 2017, 118, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, C.; Helland, I.B.; Ronnestad, A.; Gronn, M.; Iversen, P.O.; Drevon, C.A. Fat-soluble vitamins in breast-fed preterm and term infants. Eur J. Clin. Nutr. 2006, 60, 756–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kositamongkol, S.; Suthutvoravut, U.; Chongviriyaphan, N.; Feungpean, B.; Nuntnarumit, P. Vitamin A and E status in very low birth weight infants. J. Perinatol. 2011, 31, 471–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinman, A.R.; Jorge, S.M.; Martins, A.R.; de Assis, M.; Martinez, F.E.; Camelo, J.S., Jr. Assessment of vitamin A nutritional status in newborn preterm infants. Nutrition 2007, 23, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Rohner, F.; Woodruff, B.A.; Aaron, G.J.; Yakes, E.A.; Lebanan, M.A.; Rayco-Solon, P.; Saniel, O.P. Infant and young child feeding practices in urban Philippines and their associations with stunting, anemia, and deficiencies of iron and vitamin A. Food Nutr. Bull. 2013, 34 (Suppl. 2), S17–S34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, F.; Yin, H.; Zhu, X.; Ji, G.; Kong, S.; Song, Q.; Chen, J.; Chu, C.; Li, Z. Breast-feeding, dietary intakes and their associations with subclinical vitamin A deficiency in children in Anhui Province, China. Public Health Nutr. 2007, 10, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Danneskiold-Samsoe, N.; Fisker, A.B.; Jorgensen, M.J.; Ravn, H.; Andersen, A.; Balde, I.D.; Leo-Hansen, C.; Rodrigues, A.; Aaby, P.; Benn, C.S. Determinants of vitamin A deficiency in children between 6 months and 2 years of age in Guinea-Bissau. BMC Public Health 2013, 13, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Instituto Nacional de Estadisticas. Bolivia DHS Report 2008–2009; Ministerio de Salud y Deportes: La Paz, Bolivia, 2009.
- Namaste, S.M.; Aaron, G.J.; Varadhan, R.; Peerson, J.M.; Suchdev, P.S.; Group, B.W. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106 (Suppl. 1), 333S–347S. [Google Scholar] [PubMed]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Zhao, Y.; Soares, M.J. The impact of cholecalciferol supplementation on the systemic inflammatory profile: A systematic review and meta-analysis of high-quality randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C. Genetic Variations Associated with Vitamin A Status and Vitamin A Bioavailability. Nutrients 2017, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Yu, K.; Wheeler, W.; Zhang, H.; Weinstein, S.J.; Major, J.M.; Cornelis, M.C.; Männistö, S.; Hazra, A.; Hsing, A.W. Genome-wide association study of circulating retinol levels. Hum. Mol. Genet. 2011, 20, 4724–4731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| N or Mean ± SD | Frequency (%) | |
|---|---|---|
| Infant Characteristics | ||
| Male | 195 | 53.4 |
| Caesarian section birth | 104 | 28.5 |
| Preterm (<37 weeks) | 67 | 18.8 |
| Low birth weight (<2500 g) | 22 | 6.5 |
| Birth interval < 36 months 1 | 60 | 17.0 |
| Ever breastfed | 362 | 99.2 |
| Maternal Characteristics | ||
| Age (years) | 25.4 ± 6.4 | – |
| Primipara | 218 | 47.9 |
| Overweight or obese (Body Mass Index > 25 kg/m2) | 225 | 57.5 |
| Married or cohabiting with partner | 395 | 86.8 |
| Employed | 116 | 25.6 |
| Maternal Education | ||
| At least some secondary schooling | 99 | 21.9 |
| Completed secondary school | 176 | 38.9 |
| Incomplete secondary education | 178 | 39.3 |
| Household Characteristics | ||
| Number of people in household | 5.1 ± 2.1 | – |
| Refrigerator | 117 | 25.7 |
| High-quality flooring 2 | 148 | 32.5 |
| Water piped inside home | 168 | 36.9 |
| Private toilet | 261 | 57.4 |
| 2 Months † | 6–8 Months | 12–18 Months | ||||
|---|---|---|---|---|---|---|
| N or Mean ± SD | Frequency (%) | N or Mean ± SD | Frequency (%) | N or Mean ± SD | Frequency (%) | |
| Infants and Toddlers | ||||||
| N | 365 | – | 310 | – | 168 | – |
| Age | 2.1 ± 0.3 | – | 6.7 ± 0.9 | – | 14.2 ± 2.3 | – |
| Currently breastfed | 357 ** | 98.3 | 296 | 95.5 | 138 | 82.1 |
| Received any vitamin A supplementation | 0 | 0 | 130 | 41.9 | 165 | 98.2 |
| Stunted (length-for-age Z scores [LAZ] < −2) | 72 | 19.8 | 44 | 14.4 | 35 | 21.1 |
| Overweight (weight-for-length Z score [WFL] > 1) | 123 | 33.8 | 86 | 28.2 | 32 | 19.3 |
| Anemia present ¥ | 261 | 71.7 | 232 | 74.8 | 137 | 82.0 |
| Inflammation present ‡ | 10 | 2.7 | 65 | 21.0 | 27 | 16.1 |
| Mothers | ||||||
| N | 455 | – | 363 | – | – | – |
| Overweight/obese £ | 279 | 61.3 | 198 | 54.7 | – | – |
| Anemia present € | 134 | 29.5 | 53 | 15.1 | ||
| Inflammation present ‡ | 160 | 35.2 | 65 | 17.9 | – | – |
| Retinol-Binding Protein (RBP) | Retinol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Point | N | Crude | Adjusted ‡ | N | Crude | Adjusted ‡ | ||||||
| Continuous RBP (Mean ± SD) | VAD | Continuous RBP (Mean ± SD) | VAD | Continuous Retinol (Mean ± SD) | VAD | Continuous Retinol (Mean ± SD) | VAD | |||||
| Infants | <0.33 μmol/L | <0.7 μmol/L | <0.33 μmol/L | <0.7 μmol/L | <0.7 μmol/L | <0.7 μmol/L | ||||||
| 2 Months | 365 | 0.63 ± 0.15 | 0 (0%) | 259 (71.0%) | – | – | – | 38 | 0.95 ± 0.24 | 7 (18.4%) | – | – |
| 6–8 Months | 310 | 0.84 ± 0.22 | 0 (0%) | 88 (28.4%) | 0.91 ± 0.23 | 0 (0%) | 46 (14.8%) | 29 | 1.21 ± 0.33 | 2 (6.9%) | 1.39 ± 0.37 | 0 (0%) |
| 12–18 Months | 168 | 0.90 ± 0.23 | 0 (0%) | 26 (15.5%) | 0.97 ± 0.24 | 0 (0%) | 13 (7.7%) | 9 | 1.27 ± 0.37 | 0 (0%) | 1.46 ± 0.34 | 0 (0%) |
| Mothers | <0.65 μmol/L | <0.7 μmol/L | <0.65 μmol/L | <0.7 μmol/L | <0.7 μmol/L | <0.7 μmol/L | ||||||
| 1 Month postpartum | 455 | 1.73 ± 0.54 | 3 (0.7%) | 4 (0.9%) | 1.83 ± 0.56 | 3 (0.7%) | 3 (0.7%) | 45 | 1.88 ± 0.46 | 0 (0%) | 2.04 ± 0.49 | 0 (0%) |
| 6–8 Months postpartum | 363 | 1.62 ± 0.44 | 1 (0.3%) | 1 (0.3%) | 1.69 ± 0.44 | 1 (0.3%) | 1 (0.3%) | 37 | 1.96 ± 0.36 | 0 (0%) | 2.09 ± 0.39 | 0 (0%) |
| Crude | Adjusted ** | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | p-Value † | Estimate | 95% CI | p-Value † | |
| Rates (Morbidity episodes per 100 child-months) | ||||||
| Overall population | 7.89 | (7.41, 8.39) | – | – | – | – |
| Non-VAD at 2 months | 8.22 | (7.35, 9.19) | – | 8.65 | (7.01, 10.68) | – |
| VAD at 2 months | 7.75 | (7.20, 8.35) | – | 8.13 | (6.78, 9.76) | – |
| Rate Ratio | 0.94 | (0.82, 1.08) | 0.39 | 0.94 | (0.82, 1.09) | 0.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burke, R.M.; Whitehead, R.D., Jr.; Figueroa, J.; Whelan, D.; Aceituno, A.M.; Rebolledo, P.A.; Revollo, R.; Leon, J.S.; Suchdev, P.S. Effects of Inflammation on Biomarkers of Vitamin A Status among a Cohort of Bolivian Infants. Nutrients 2018, 10, 1240. https://doi.org/10.3390/nu10091240
Burke RM, Whitehead RD Jr., Figueroa J, Whelan D, Aceituno AM, Rebolledo PA, Revollo R, Leon JS, Suchdev PS. Effects of Inflammation on Biomarkers of Vitamin A Status among a Cohort of Bolivian Infants. Nutrients. 2018; 10(9):1240. https://doi.org/10.3390/nu10091240
Chicago/Turabian StyleBurke, Rachel M., Ralph D. Whitehead, Jr., Janet Figueroa, Denis Whelan, Anna M. Aceituno, Paulina A. Rebolledo, Rita Revollo, Juan S. Leon, and Parminder S. Suchdev. 2018. "Effects of Inflammation on Biomarkers of Vitamin A Status among a Cohort of Bolivian Infants" Nutrients 10, no. 9: 1240. https://doi.org/10.3390/nu10091240





