Moderate Beer Intake and Cardiovascular Health in Overweight Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
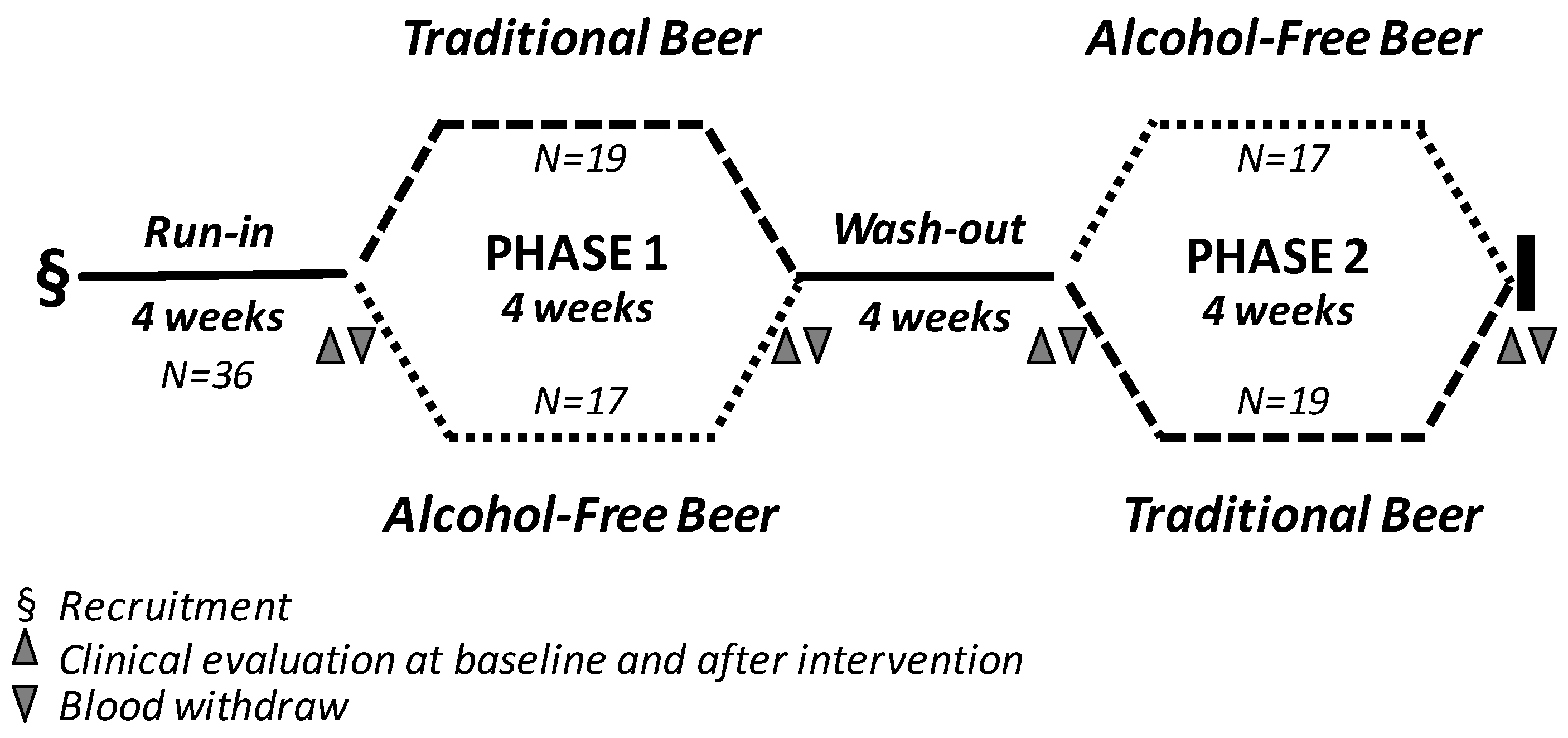
2.2. Study Design and Dietary Monitoring
2.3. Blood Samples
2.4. Anthropometric Data, Blood Pressure, Serum Lipid Profile and Other Biochemical Measurements
2.5. LDL and HDL Sample Preparation, Purity Control and Oxidation Assays
2.5.1. Lipoprotein Preparation
2.5.2. Conjugated Dienes Assay
2.5.3. HDL Antioxidant Potential
2.6. HDL Cholesterol Efflux Capacity Assay
2.7. Vascular Endothelial Function and Arterial Stiffness
2.8. Inflammatory Markers
2.9. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population and Side Effects of Beer Consumption
3.2. Effects of Beer Consumption on Weight Indexes
3.3. Effect of Beer Consumption on Lipid Profile and Lipoprotein Functionality
3.3.1. Lipid Profile
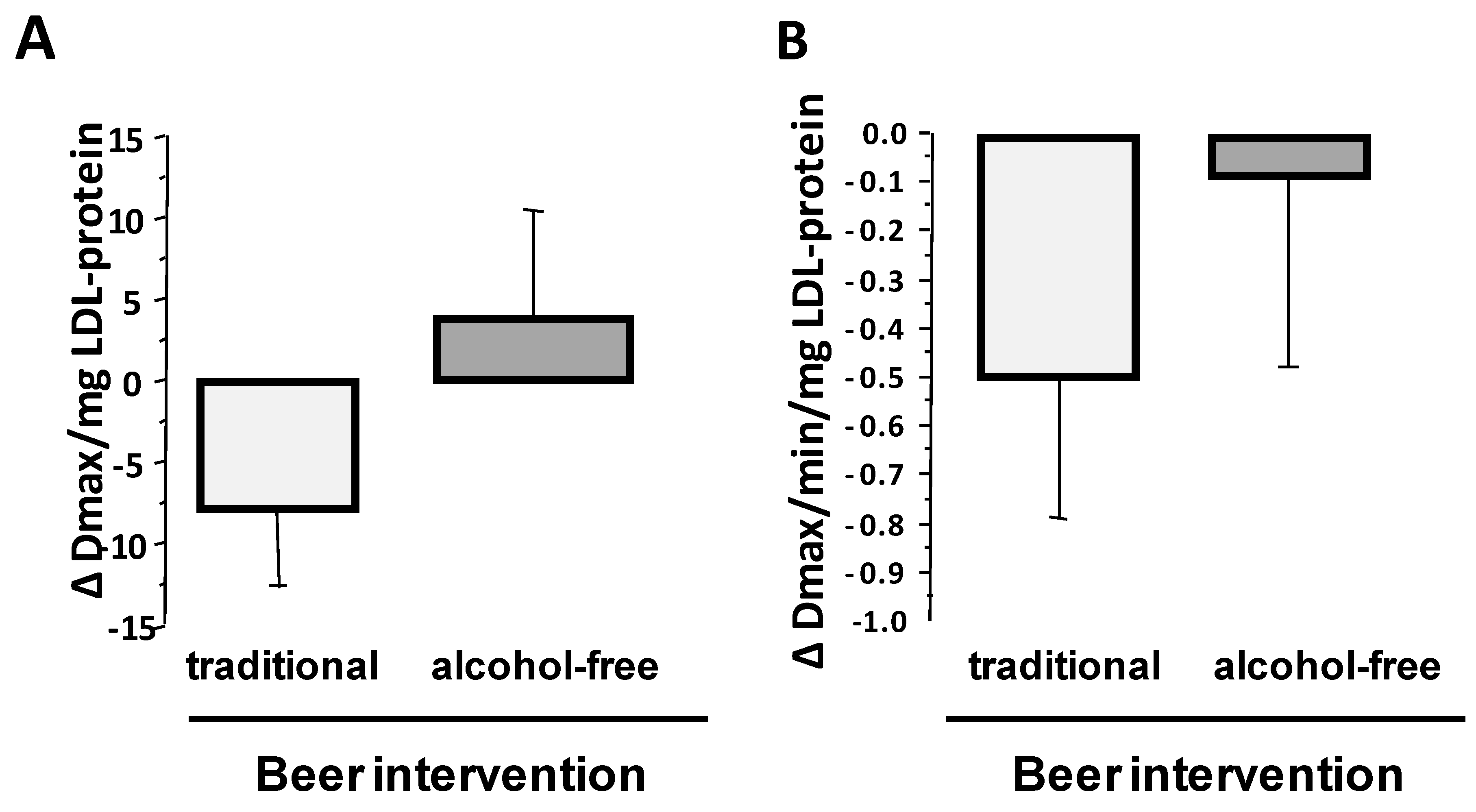
3.3.2. LDL Susceptibility to Oxidation
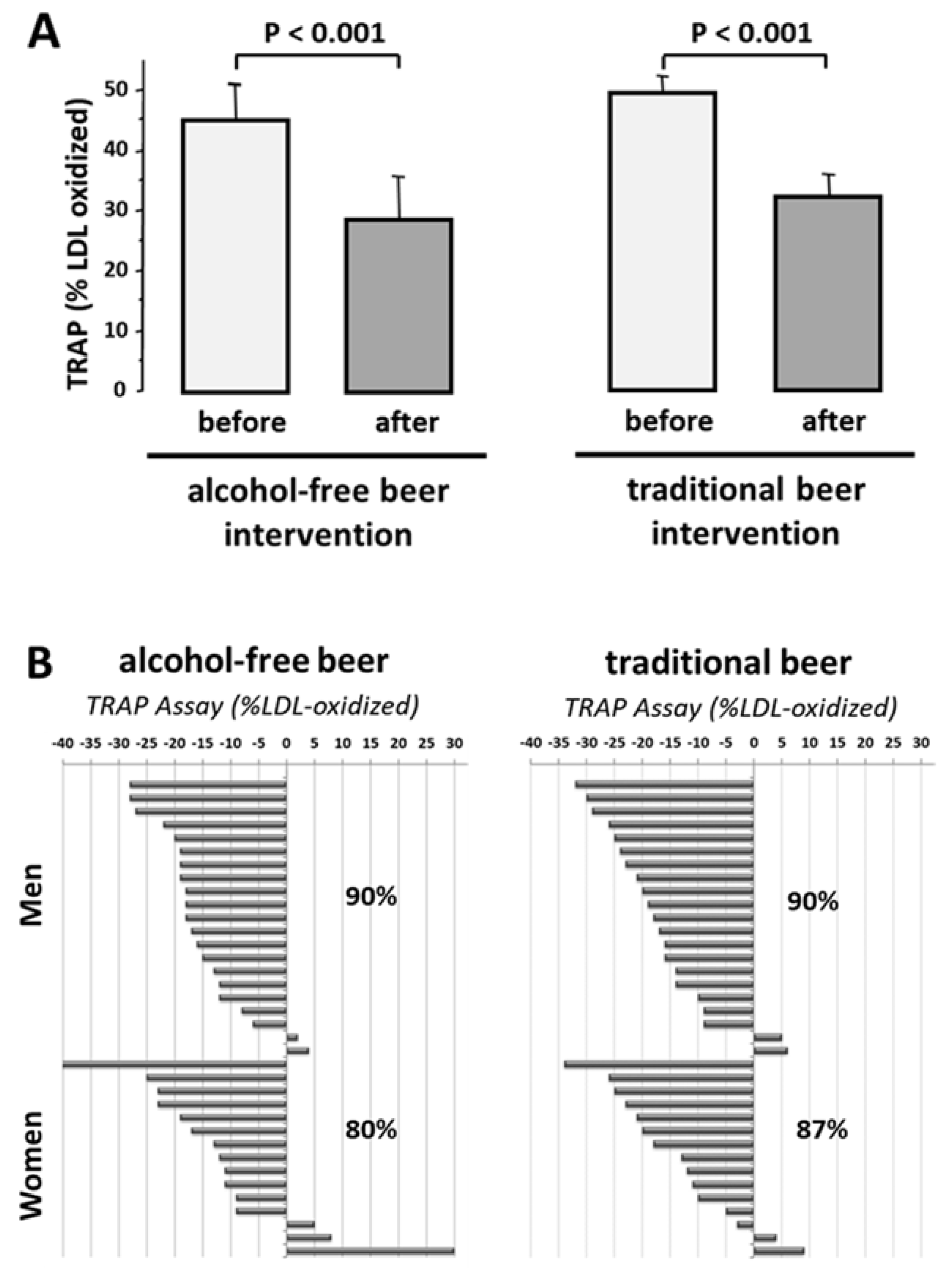
3.3.3. HDL Antioxidant Capacity
3.3.4. Effect of Beer Intake on HDL Cholesterol Efflux Capacity
3.4. Plasma Inflammatory Markers and Beer Intervention
3.5. Vascular Endothelial Function and Arterial Stiffness before and after Intervention
3.6. Beer Consumption and Cardiovascular Risk Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costanzo, S.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Alcohol Consumption and Mortality in Patients With Cardiovascular Disease. A Meta-Analysis. J. Am. Coll. Cardiol. 2010, 55, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bybee, K.A.; Lavie, C.J. Alcohol and Cardiovascular Health. The Razor-Sharp Double-Edged Sword. J. Am. Coll. Cardiol. 2007, 50, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Sharma, A.; Garg, A. Effect of Alcohol Consumption on Cardiovascular Health. Curr. Cardiol. Rep. 2018, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Volcik, K.A.; Ballantyne, C.M.; Fuchs, F.D.; Sharrett, A.R.; Boerwinkle, E. Relationship of Alcohol Consumption and Type of Alcoholic Beverage Consumed With Plasma Lipid Levels: Differences Between Whites and African Americans of the ARIC Study. Ann. Epidemiol. 2008, 18, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiva-Blanch, G.; Magraner, E.; Condines, X.; Valderas-Martínez, P.; Roth, I.; Arranz, S.; Casas, R.; Navarro, M.; Hervas, A.; Sisó, A.; et al. Effects of alcohol and polyphenols from beer on atherosclerotic biomarkers in high cardiovascular risk men: A randomized feeding trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, J.; Shearer, G.C.; Lichtenstein, A.H.; Zheng, X.; Wu, Y.; Jin, C.; Wu, S.; Gao, X. Longitudinal study of alcohol consumption and HDL concentrations: A community-based study. Am. J. Clin. Nutr. 2017, 105, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J.; Conigrave, K.M.; Mittleman, M.A.; Camargo, C.A.; Stampfer, M.J.; Willett, W.C.; Rimm, E.B. Roles of drinking pattern and type of alchohol consumed in coronary heart disease in men. N. Engl. J. Med. 2003, 348, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Smyth, A.; Teo, K.K.; McKee, M.; Rangarajan, S.; Pais, P.; Liu, L.; Anand, S.S.; Yusuf, S. Patterns of alcohol consumption and myocardial infarction risk: Observations from 52 countries in the INTERHEART case-control study. Circulation 2014, 130, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, T.; Zhao, J.; Panwar, S.; Roemer, A.; Naimi, T.; Chikritzhs, T. Do “Moderate” Drinkers Have Reduced Mortality Risk? A Systematic Review and Meta-Analysis of Alcohol Consumption and All-Cause Mortality. J. Stud. Alcohol Drugs 2016, 77, 185–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naimi, T.S.; Brown, D.W.; Brewer, R.D.; Giles, W.H.; Mensah, G.; Serdula, M.K.; Mokdad, A.H.; Hungerford, D.W.; Lando, J.; Naimi, S.; et al. Cardiovascular risk factors and confounders among nondrinking and moderate-drinking U.S. adults. Am. J. Prev. Med. 2005, 28, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Naimi, T.S.; Xuan, Z.; Brown, D.W.; Saitz, R. Confounding and studies of “moderate” alcohol consumption: The case of drinking frequency and implications for low-risk drinking guidelines. Addiction 2013, 108, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Hansel, B.; Thomas, F.; Pannier, B.; Bean, K.; Kontush, A.; Chapman, M.J.; Guize, L.; Bruckert, E. Relationship between alcohol intake, health and social status and cardiovascular risk factors in the urban Paris-Ile-De-France Cohort: Is the cardioprotective action of alcohol a myth. Eur. J. Clin. Nutr. 2010, 64, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Gronbaek, M.; Becker, U.; Johansen, D.; Gottschau, A.; Schnohr, P.; Hein, H.O.; Jensen, G.; Sorensen, T.I.A. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 2000, 133, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; Rotondo, S.; Iacoviello, L.; Donati, M.B.; De Gaetano, G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation 2002, 105, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L.; De Gaetano, G. Wine, beer or spirit drinking in relation to fatal and non-fatal cardiovascular events: A meta-analysis. Eur. J. Epidemiol. 2011, 26, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Global Status Report on Alcohol and Health 2014. Available online: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed on 2 April 2018).
- Pi-Sunyer, F.; Becker, D.; Bouchard, C.; Carletom, R.; Colditz, G.; Dietz, W.; Foreyt, J.; Garrison, R.; Grundy, R.; Hansen, B.; et al. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: Executive Summary. Am. J. Clin. Nutr. 1998, 68, 899–917. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bradgon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- De Juan-Franco, E.; Pérez, A.; Ribas, V.; Sánchez-Hernández, J.A.; Blanco-Vaca, F.; Ordóñez-Llanos, J.; Sánchez-Quesada, J.L. Standardization of a method to evaluate the antioxidant capacity of high-density lipoproteins. Int. J. Biomed. Sci. 2009, 5, 402–410. [Google Scholar] [PubMed]
- Esterbauer, H.; Striegl, G. Continuous Monitoring of in Vitro Oxidation of Human Low Density Lipoprotein. Free Radic. Biol. Med. 1989, 6, 67–75. [Google Scholar]
- Valkonen, M.; Kuusi, T. Spectrophotometric assay for total peroxyl radical-trapping antioxidant potential in human serum. J. Lipid Res. 1997, 38, 823–833. [Google Scholar] [PubMed]
- Aldini, G.; Yeum, K.J.; Russell, R.M.; Krinsky, N.I. A method to measure the oxidizability of both the aqueous and lipid compartments of plasma. Free Radic. Biol. Med. 2001, 31, 1043–1050. [Google Scholar] [CrossRef]
- Escolà-Gil, J.C.; Lee-Rueckert, M.; Santos, D.; Cedó, L.; Blanco-Vaca, F.; Julve, J. Quantification of in vitro macrophage cholesterol efflux and in vivo macrophage-specific reverse cholesterol transport. In Methods in Molecular Biology; Springer Science + Business Media: New York, NY, USA, 2015; Volume 1339, pp. 211–233. ISBN 978-1-4939-2928-3. [Google Scholar]
- Padró, T.; Cubedo, J.; Camino, S.; Béjar, M.T.; Ben-Aicha, S.; Mendieta, G.; Escolà-Gil, J.C.; Escate, R.; Gutiérrez, M.; Casani, L.; et al. Detrimental Effect of Hypercholesterolemia on High-Density Lipoprotein Particle Remodeling in Pigs. J. Am. Coll. Cardiol. 2017, 70, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Klimis-Zacas, D.; Del Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hard Coronary Heart Disease (10-Year Risk). Available online: https://www.framinghamheartstudy.org/fhs-risk-functions/hard-coronary-heart-disease-10-year-risk/ (accessed on 30 July 2018).
- Ronksley, P.E.; Brien, S.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ 2011, 342, 479. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G.; Padro, T. Nutraceuticals and atherosclerosis: Human trials. Cardiovasc. Ther. 2010, 28, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Casani, L.; Guerra, J.M.; Badimon, L. Intake of fermented beverages protect against acute myocardial injury: Target organ cardiac effects and vasculoprotective effects. Basic Res. Cardiol. 2012, 107. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; Daskalopoulou, M.; Rapsomaniki, E.; George, J.; Britton, A.; Bobak, M.; Casas, J.P.; Dale, C.E.; Denaxas, S.; Shah, A.D.; et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: Population based cohort study using linked health records. BMJ 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef]
- Anastasius, M.; Kockx, M.; Jessup, W.; Sullivan, D.; Rye, K.A.; Kritharides, L. Cholesterol efflux capacity: An introduction for clinicians. Am. Heart J. 2016, 180, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Romeo, J.; González-Gross, M.; Wärnberg, J.; Díaz, L.E.; Marcos, A. Effects of moderate beer consumption on blood lipid profile in healthy Spanish adults. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkau, B.; Deanfield, J.E.; Després, J.-P.; Bassand, J.-P.; Fox, K.A.A.; Smith, S.C.; Barter, P.; Tan, C.-E.; Van Gaal, L.; Wittchen, H.-U.; et al. International Day for the Evaluation of Abdominal Obesity (IDEA): A study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation 2007, 116, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-H.; Yim, S.H.; Yu, S.H.; Lee, J.Y.; Kim, J.D.; Seo, M.H.; Jeon, W.S.; Park, S.-E.; Park, C.-Y.; Lee, W.-Y.; et al. The relationship of body composition and coronary artery calcification in apparently healthy Korean adults. Endocrinol. Metab. 2013, 28, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overwight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.M.; Tjønneland, A.; et al. General and Abdominal Adiposity and Risk of Death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bhatti, S.K.; Bajwa, A.; DiNicolantonio, J.J.; Lavie, C.J. Alcohol and cardiovascular health: The dose makes the poison or the remedy. Mayo Clin. Proc. 2014, 89, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Baliunas, D.O.; Taylor, B.J.; Irving, H.; Roerecke, M.; Patra, J.; Mohapatra, S.; Rehm, J. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2009, 32, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, D.Y.; Lee, Y.J.; Park, K.J.; Kim, K.H.; Kim, J.W.; Kim, W.-H. Chronic alcohol consumption potentiates the development of diabetes through pancreatic β-cell dysfunction. World J. Biol. Chem. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Pochareddy, S.; Edenberg, H.J. Chronic Alcohol Exposure Alters Gene Expression in HepG2 Cells. Alcohol. Clin. Exp. Res. 2012, 36, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Alexander, E.T.; Weibel, G.L.; Billheimer, J.; Rothblat, G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009, 50, S189–S194. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, A.E. Reverse cholesterol transport: From classical view to new insights. World J. Gastroenterol. 2010, 16, 5908–5915. [Google Scholar] [PubMed]
- Beulens, J.W.J.; Sierksma, A.; van Tol, A.; Fournier, N.; van Gent, T.; Paul, J.-L.; Hendriks, H.F.J. Moderate alcohol consumption increases cholesterol efflux mediated by ABCA1. J. Lipid Res. 2004, 45, 1716–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, W.S.; Heink, A.; Sexmith, H.; Melchior, J.T.; Gordon, S.M.; Kuklenyik, Z.; Woollett, L.; Barr, J.R.; Jones, J.I.; Toth, C.A.; et al. The effects of apolipoprotein B depletion on HDL subspecies composition and function. J. Lipid Res. 2016, 57, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Stockley, C.S. The relationships between alcohol, wine and cardiovascular diseases—A review. Nutr. Aging 2016, 3, 55–88. [Google Scholar] [CrossRef]
- Vilahur, G.; Casani, L.; Mendieta, G.; Lamuela-Raventos, R.M.; Estruch, R.; Badimon, L. Beer elicits vasculoprotective effects through Akt/eNOS activation. Eur. J. Clin. Investig. 2014, 44, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Hines, L.M. Moderate alcohol consumption and coronary heart disease: A review. Postgrad. Med. J. 2001, 77, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Lamuela-Raventós, R.M. Wine, alcohol, polyphenols and cardiovascular disease. Nutr. Aging 2014, 2, 101–109. [Google Scholar]
- Shaw, J.; Anderson, T. Coronary endothelial dysfunction in non-obstructive coronary artery disease: Risk, pathogenesis, diagnosis and therapy. Vasc. Med. 2016, 21, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Veerasamy, M.; Bagnall, A.; Neely, D.; Allen, J.; Sinclair, H.; Kunadian, V. Endothelial dysfunction and coronary artery disease: A state of the art review. Cardiol. Rev. 2015, 23, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Ntarladimas, I.; Antoniades, C.; Vasiliadou, C.; Tentolouris, C.; Papageorgiou, N.; Latsios, G.; Stefanadis, C. Acute effects of different alcoholic beverages on vascular endothelium, inflammatory markers and thrombosis fibrinolysis system. Clin. Nutr. 2008, 27, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Rontoyanni, V.G.; Protogerou, A.D.; Georgoulia, A.; Xenos, K.; Chrysou, J.; Sfikakis, P.P.; Sidossis, L.S. Acute effects of beer on endothelial function and hemodynamics: Asingle-blind, crossover study in healthy volunteers. Nutrition 2013, 29, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, M.; Kora, N.; Matsumoto, N. Ingesting a small amount of beer reduces arterial stiffness in healthy humans. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
| After Run-In Period | After Wash-Out Period | p-Value | |
|---|---|---|---|
| Anthropometric parameters | |||
| Sex (Men/Women) | 21/15 | 21/15 | |
| Weight (kg) | 87.9 ± 2.3 | 88.2 ± 2.0 | 0.92 |
| BMI (kg/m2) | 30.5 ± 0.5 | 30.6 ± 0.5 | 0.88 |
| Waist circumference (cm) | 100.4 ± 1.7 | 101.5 ± 1.5 | 0.63 |
| Hemodynamic control | |||
| Systolic blood pressure (mmHg) | 127.1 ± 1.8 | 125.6 ± 1.8 | 0.57 |
| Diastolic blood pressure (mmHg) | 75.8 ± 1.5 | 75.1 ± 1.3 | 0.71 |
| Cardiac Frequency (beats/min) | 65.3 ± 1.6 | 64.9 ± 1.4 | 0.85 |
| Biochemical parameters | |||
| Glucose (mg/dL) | 88.5 ± 1.6 | 89.2 ± 1.7 | 0.77 |
| Creatinine (mg/dL) | 0.77 ± 0.02 | 0.77 ± 0.02 | 0.75 |
| Urea (mg/dL) | 14.0 ± 0.6 | 14.8 ± 0.5 | 0.28 |
| AST (U/L) | 16.8 ± 0.7 | 16.9 ± 0.7 | 0.85 |
| GGT (U/L) | 20.5 ± 1.8 | 22.5 ± 1.9 | 0.46 |
| Lipid parameters | |||
| TC (mg/dL) | 188.9 ± 4.5 | 191.4 ± 4.5 | 0.67 |
| HDLc (mg/dL) | 48.3 ± 1.7 | 48.8 ± 1.6 | 0.82 |
| Non-HDLc (mg/dL) | 140.5 ± 4.1 | 142.6 ± 4.3 | 0.71 |
| LDLc (mg/dL) | 124.1 ± 3.8 | 125. 4 ± 3.9 | 0.80 |
| VLDLc (mg/dL | 16.4 ± 1.0 | 16.9 ± 1.0 | 0.58 |
| TGL (mg/dL) | 81.5 ± 4.8 | 85.6 ± 5.0 | 0.58 |
| Alcohol-Free Beer | Traditional Beer | |||||
|---|---|---|---|---|---|---|
| Before Intervention | After Intervention | p-Value | Before Intervention | After Intervention | p-Value | |
| Anthropometric parameters | ||||||
| Weight (kg) | 87.7 ± 2.3 | 88.1 ± 2.3 | 0.02 | 87.7 ± 2.3 | 88.1 ± 2.3 | 0.08 |
| BMI (kg/m2) | 30.4 ± 0.5 | 30.5 ± 0.5 | 0.01 | 30.4 ± 0.5 | 30.4 ± 0.5 | 0.11 |
| Waist circumference (cm) | 99.7 ± 1.9 | 101.1 ± 1.5 | 0.11 | 100.5 ± 1.4 | 102.1 ± 1.3 | 0.06 |
| Hemodynamic control | ||||||
| Systolic blood pressure (mmHg) | 125.4 ± 1.9 | 125.8 ± 1.8 | 0.76 | 125.3 ± 2.0 | 125.7 ± 2.0 | 0.82 |
| Diastolic blood pressure (mmHg) | 75.1 ± 1.6 | 75.2 ± 1.5 | 0.93 | 75.9 ± 1.5 | 74.9 ± 1.4 | 0.36 |
| Cardiac Frequency (beats/min) | 65.4 ± 1.7 | 63.9 ± 1.7 | 0.25 | 64.8 ± 1.5 | 66.0 ± 1.8 | 0.25 |
| Biochemical parameters | ||||||
| Glucose (mg/dL) | 88.0 ± 1.6 | 88.8 ± 1.7 | 0.43 | 88.2 ± 2.0 | 90.1 ± 1.7 | 0.04 |
| Creatinine (mg/dL) | 0.76 ± 0.02 | 0.78 ± 0.13 | 0.03 | 0.78 ± 0.02 | 0.78 ± 0.02 | 0.84 |
| Urea (mg/dL) | 14.9 ± 0.6 | 15.0 ± 0.5 | 0.75 | 14.9 ± 0.6 | 15.6 ± 0.6 | 0.21 |
| AST (U/L) | 16.6 ± 0.7 | 16.3 ± 0.6 | 0.49 | 16.6 ± 0.6 | 17.2 ± 0.7 | 0.14 |
| GGT (U/L) | 21.5 ± 2.0 | 21.7 ± 1.9 | 0.82 | 20.6 ± 1.9 | 23.8 ± 2.0 | 0.00 |
| Hemogram | ||||||
| RBC (106 mm) | 4.3 ± 0.1 | 4.3 ± 0.1 | 0.09 | 4.4 ± 0.1 | 4.3 ± 0.1 | 0.14 |
| HCT (%) | 36.7 ± 0.6 | 36.2 ± 0.6 | 0.14 | 37.2 ± 0.7 | 36.5 ± 0.5 | 0.21 |
| PLT (103 mm3) | 198.9 ± 5.5 | 204.1 ± 6.7 | 0.17 | 200.9 ± 6.4 | 205.4 ± 6.2 | 0.26 |
| MPV (Um3) | 8.4 ± 0.1 | 8.4 ± 0.1 | 0.74 | 8.4 ± 0.1 | 8.4 ± 0.1 | 0.41 |
| WBC (103 mm3) | 5.8 ± 0.2 | 5.9 ± 0.2 | 0.27 | 5.9 ± 0.2 | 6.1 ± 0.2 | 0.36 |
| Beer Intervention | p-Value | ||||
|---|---|---|---|---|---|
| Week-0 | Week-4 | Week-8 | Week-12 | ||
| Men (N = 21) | |||||
| Weight (kg) | 94.6 ± 2.5 [89.4–99.8] | 94.9 ± 2.6 [89.6–100.3] | 94.3 ± 2.4 [89.2–99.4] | 94.8 ± 2.5 [89.6–100.1] | 0.99 |
| BMI (kg/m2) | 30.2 ± 0.5 [29.1–31.3] | 30.4 ± 0.5 [29.3–31.5] | 30.5 ± 0.5 [29.3–31.6] | 30.4 ± 0.5 [29.3–31.5] | 0.99 |
| Waist-circumference (cm) | 103.3 ± 2.2 [98.7–108.0] | 103.7 ± 1.9 [99.8–107.6] | 102.4 ± 1.6 [99.2–105.7] | 103.9 ± 1.5 [100.8–107.1] | 0.88 |
| Women (N = 15) | |||||
| Weight (kg) | 78.6 ± 2.2 [72.5–84.6] | 78.8 ± 3.0 [72.5–85.1] | 77.9 ± 3.1 [71.3–84.5] | 78.4 ± 3.0 [71.9–84.9] | 0.99 |
| BMI (kg/m2) | 30.8 ± 0.9 [28.8–32.7] | 30.8 ± 0.9 [28.8–32.8] | 30.5 ± 1.0 [28.4–32.6] | 30.6 ± 1.0 [28.6–32.7] | 0.44 |
| Waist-circumference (cm) | 96.3 ± 2.5 [91.0–101.7] | 98.4 ± 2.3 [93.5–103.3] | 97.4 ± 2.5 [92.1–102.7] | 98.3 ± 1.9 [94.2–102.4] | 0.94 |
| Alcohol-Free Beer | Traditional Beer | |||||
|---|---|---|---|---|---|---|
| Before Intervetion | After Intervention | p-Value | Before Intervetion | After Intervention | p-Value | |
| TC (mg/dL) | 189.3 ± 4.5 | 191.0 ± 4.7 | 0.63 | 189.9 ± 5.0 | 193.1 ± 4.5 | 0.33 |
| HDLc (mg/dL) | 47.7 ± 1.6 | 48.0 ± 1.7 | 0.69 | 48.2 ± 1.6 | 48.8 ± 1.5 | 0.41 |
| Non-HDLc (mg/dL) | 141.6 ± 4.0 | 143.0 ± 4.4 | 0.65 | 141.7 ± 4.5 | 144.2 ± 4.4 | 0.39 |
| LDLc (mg/dL) | 124.8 ± 3.8 | 125.6 ± 4.0 | 0.78 | 125.7 ± 4.3 | 126.1 ± 3.9 | 0.88 |
| VLDLc (mg/dL) | 16.8 ± 1.1 | 17.4 ± 1.2 | 0.43 | 16.0 ± 0.9 | 18.1 ± 1.4 | 0.06 |
| TG (mg/dL) | 83.5 ± 5.5 | 86.4 ± 6.0 | 0.43 | 79.5 ± 4.4 | 90.1 ± 6.9 | 0.06 |
| Alcohol-Free Beer Intervention | Traditional Beer Intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Δ | p-Value | Before | After | Δ | p-Value | |
| Serum lipids-subjects with LDL < 130 mg/dL | ||||||||
| CT (mg/dL) | 170.5 ± 3.5 | 176.5 ± 4.0 | +6.0 | 0.14 | 170.6 ± 2.5 | 179.1 ± 3.8 | +8.6 | 0.04 |
| HDLc (mg/dL) | 46.3 ± 2.2 | 47.0 ± 2.2 | +0.7 | 0.34 | 46.6 ± 1.7 | 48.8 ± 1.7 | +2.2 | 0.01 |
| Non-HDLc (mg/dL) | 124.2 ± 3.1 | 129.5 ± 4.3 | +5.2 | 0.17 | 124.9 ± 2.6 | 130.5 ± 3.7 | +5.6 | 0.12 |
| LDLc (mg/dL) | 108.8 ± 2.9 | 112.4 ± 3.5 | +3.6 | 0.35 | 109.9 ± 2.2 | 114.2 ± 3.4 | +4.3 | 0.18 |
| VLDLc (mg/dL) | 15.4 ± 1.4 | 17.1 ± 1.6 | +1.6 | 0.08 | 15.0 ± 1.1 | 16.3 ± 1.3 | +1.3 | 0.13 |
| TG (mg/dL) | 76.8 ± 6.8 | 84.9 ± 8.1 | +8.2 | 0.08 | 74.7 ± 5.3 | 81.2 ± 6.7 | +6.6 | 0.13 |
| Serum lipids-subjects with LDL > 130 mg/dL | ||||||||
| CT (mg/dL) | 215.6 ± 3.7 | 211.3 ± 6.9 | −4.3 | 0.49 | 226.1 ± 5.5 | 221.4 ± 4.6 | −4.7 | 0.22 |
| HDLc (mg/dL) | 49.7 ± 2.5 | 49.3 ± 2.6 | −0.3 | 0.82 | 50.9 ± 3.5 | 49.7 ± 3.0 | −1.1 | 0.46 |
| Non-HDLc (mg/dL) | 165.9 ± 2.6 | 161.9 ± 5.7 | −4.0 | 0.43 | 175.2 ± 3.9 | 171.6 ± 4.8 | −3.5 | 0.52 |
| LDLc (mg/dL) | 147.2 ± 2.5 | 144.1 ± 5.3 | −3.1 | 0.51 | 157.2 ± 4.8 | 149.9 ± 4.7 | −7.3 | 0.16 |
| VLDLc (mg/dL) | 18.7 ± 1.7 | 17.8 ± 1.9 | −0.9 | 0.45 | 17.9 ± 1.5 | 21.7 ± 3.0 | +3.7 | 0.22 |
| TG (mg/dL) | 92.9 ± 8.7 | 88.4 ± 9.3 | −4.5 | 0.45 | 89.2 ± 7.6 | 107.8 ± 14.9 | +18.6 | 0.22 |
| Cholesterol Efflux (%) | Alcohol-Free Beer | Traditional Beer | ||||
|---|---|---|---|---|---|---|
| Before Intervention | After Intervention | p-Value | Before Intervention | After Intervention | p-Value | |
| Total population (N = 36) | 16.4 ± 0.4 | 16.6 ± 0.4 | 0.16 | 16.6 ± 0.4 | 17.2 ± 0.4 | 0.02 |
| Men (N = 21) | 16.9 ± 0.4 | 17.1 ± 0.5 | 0.47 | 16.9 ± 0.5 | 17.6 ± 0.5 | 0.09 |
| Women (N = 15) | 15.6 ± 0.7 | 16.0 ± 0.6 | 0.21 | 16.4 ± 0.7 | 16.8 ± 0.7 | 0.45 |
| Inflammatory Markers | Alcohol-Free Beer | Traditional Beer | ||||
|---|---|---|---|---|---|---|
| Before Intervention | After Intervention | p-Value | Before Intervention | After Intervention | p-Value | |
| PCR (ng/mL) TNFα (ng/mL) IL-6 (ng/mL) | 3.4 ± 0.6 83.1 ± 0.96 0.03 ± 0.00 | 4.1 ± 0.8 2.0 ± 0.1 0.03 ± 0.01 | 0.06 0.25 0.20 | 4.8 ± 1.2 1.8 ± 0.1 0.03 ± 0.01 | 4.4 ± 0.7 2.0 ± 0.2 0.04 ± 0.01 | 0.63 0.06 0.58 |
| Alcohol-Free Beer | Traditional Beer | |||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | 10-Year Risk | p-Value | Before | After | 10-Year Risk | p-Value | |
| Total population (N = 36) | 7.9 ± 0.5 | 7.8 ± 0.5 | <1% | 0.50 | 7.9 ± 0.5 | 7.8 ± 0.5 | <1% | 0.50 |
| Men (N = 21) | 7.3 ± 0.5 | 7.2 ± 0.5 | 3% | 0.54 | 7.3 ± 0.5 | 7.2 ± 0.5 | 3% | 0.54 |
| Women (N = 15) | 8.7 ± 1.1 | 8.7 ± 1.1 | <1% | 0.75 | 8.7 ± 1.1 | 8.7 ± 1.1 | <1% | 0.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padro, T.; Muñoz-García, N.; Vilahur, G.; Chagas, P.; Deyà, A.; Antonijoan, R.M.; Badimon, L. Moderate Beer Intake and Cardiovascular Health in Overweight Individuals. Nutrients 2018, 10, 1237. https://doi.org/10.3390/nu10091237
Padro T, Muñoz-García N, Vilahur G, Chagas P, Deyà A, Antonijoan RM, Badimon L. Moderate Beer Intake and Cardiovascular Health in Overweight Individuals. Nutrients. 2018; 10(9):1237. https://doi.org/10.3390/nu10091237
Chicago/Turabian StylePadro, Teresa, Natàlia Muñoz-García, Gemma Vilahur, Patricia Chagas, Alba Deyà, Rosa Maria Antonijoan, and Lina Badimon. 2018. "Moderate Beer Intake and Cardiovascular Health in Overweight Individuals" Nutrients 10, no. 9: 1237. https://doi.org/10.3390/nu10091237






