Effects of Beetroot Juice Supplementation on Performance and Fatigue in a 30-s All-Out Sprint Exercise: A Randomized, Double-Blind Cross-Over Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
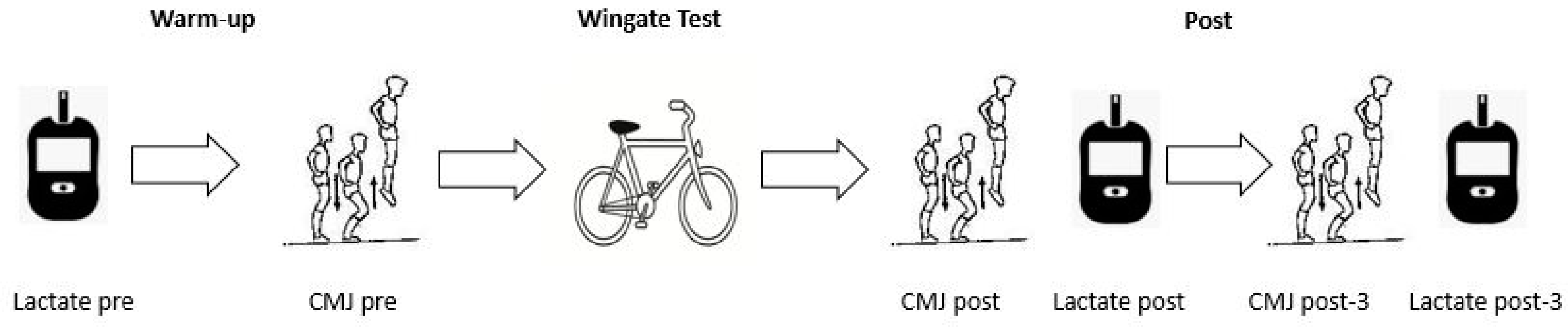
2.2. Experimental Design
2.3. Placebo vs. BJ Ingestion
2.4. Sprint Performance Variables
2.5. Neuromuscular Fatigue
2.6. Blood Lactate
2.7. Statistical Analysis
3. Results
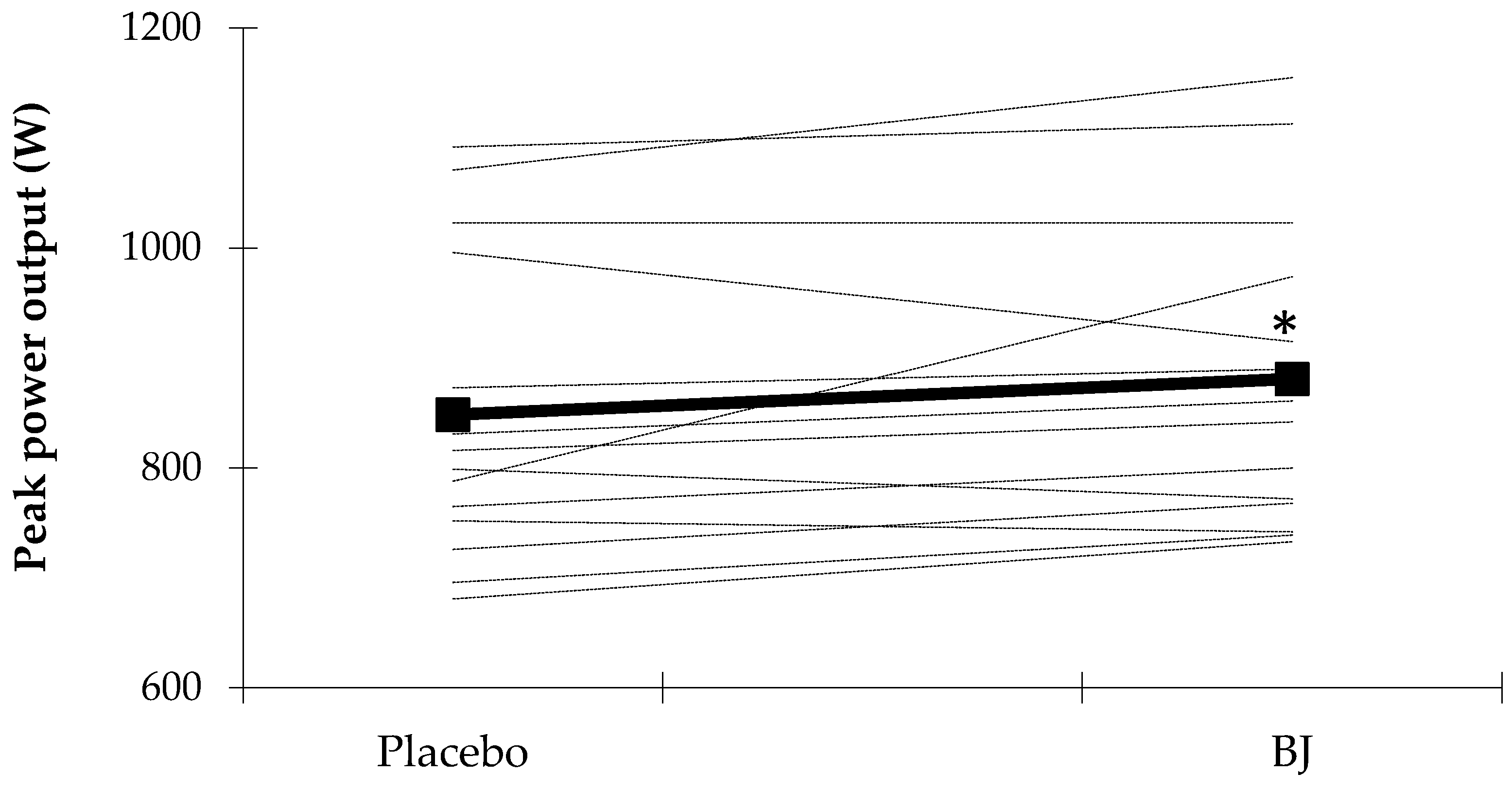
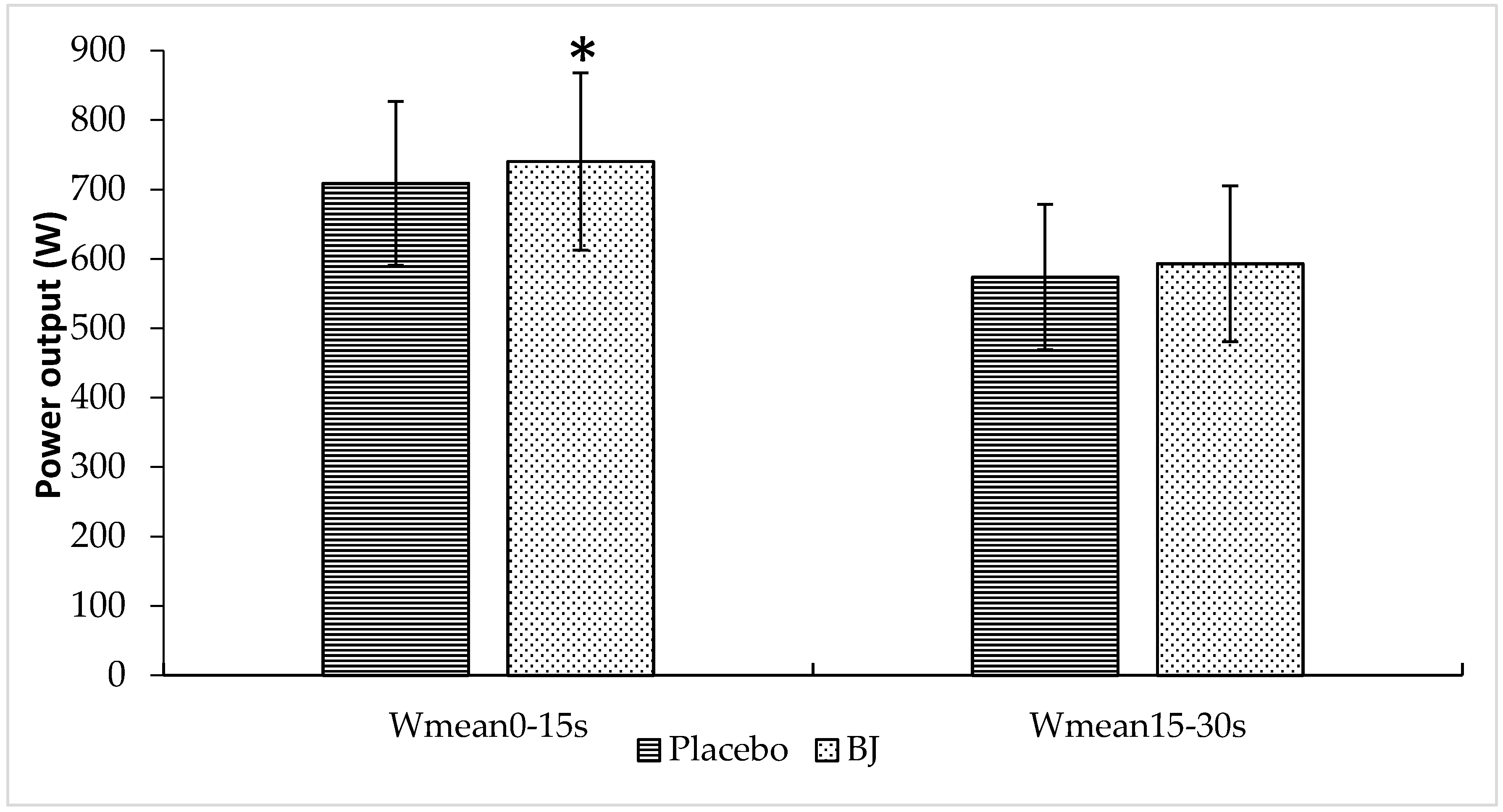
3.1. Sprint Performance Variables
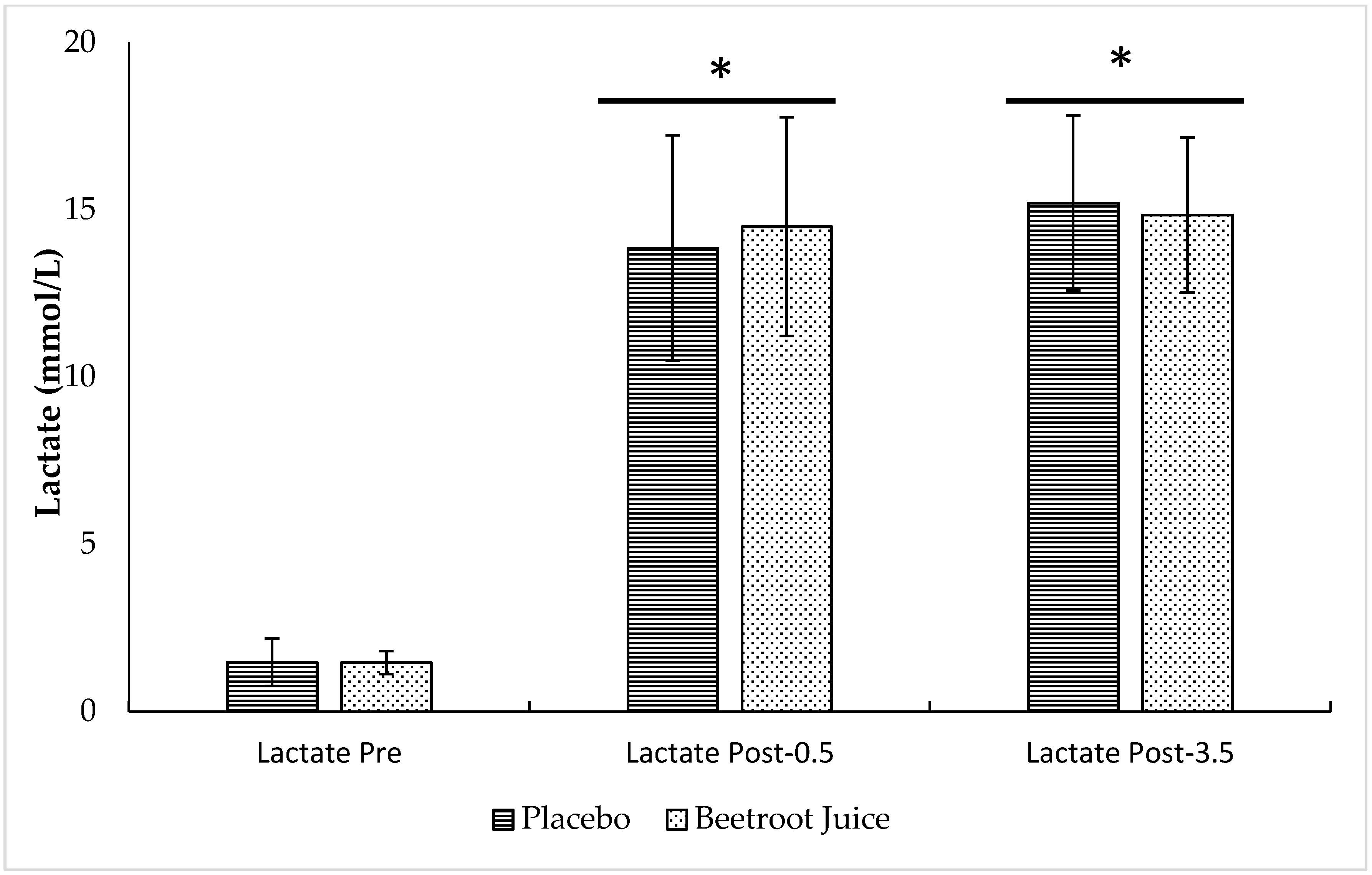
3.2. Neuromuscular Fatigue and Blood Lactate Concentrations
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haider, G.; Folland, G.P. Nitrate Supplementation Enhances the Contractile Properties of Human Skeletal Muscle. Med. Sci. Sports Exerc. 2014, 46, 2234–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coggan, A.R.; Leibowitz, J.L.; Kadkhodayan, A.; Thomas, D.P.; Ramamurthy, S.; Spearie, C.A.; Waller, S.; Farmer, M.; Peterson, L.R. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide 2015, 48, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, J.; Ludzki, A.; Heigenhauser, G.; Senden, S.; Verdijk, L.; Van, L.; Spriet, L.L.; Holloway, G.P. Beetroot Juice Supplementation Reduces Whole Body Oxygen Consumption But Does Not Improve Indices Of Mitochondrial Efficiency in Human Skeletal Muscle. J. Physiol. 2016, 594, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Vanhatalo, A.; Fulford, J.; Carter, J.; Nyman, L.; Bailey, S.J.; Jones, A.M. Dietary nitrate supplementation improves sprint and high-intensity intermittent running performance. Nitric Oxide 2016, 61, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, C.; Wylie, L.J.; Blackwell, J.R.; Fulford, J.; Black, M.I.; Kelly, J.; McDonagh, S.T.; Carter, J.; Bailey, S.J.; Vanhatalo, A.; et al. Influence of dietary nitrate supplementation on physiological and muscle metabolic adaptations to sprint interval training. J. Appl. Physiol. 2018, 18, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Nyakayiru, J.; Van Dicj, J.W.; Maase, K.; Ballak, S.B.; Senden, J.M.G.; Van Loon, L.J.C.; Verdjik, L.B. Repeated-sprint performance and plasma responses following beetroot juice supplementation do not differ between recreational, competitive and elite sprint athletes. Eur. J. Sport Sci. 2018, 18, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.M.; Wu, Z.F.; Pang, B.X.; Jin, L.Y.; Qin, L.Z.; Wang, S.L. From nitrate to nitric oxide: The role of salivary glands and oral bacteria. J. Dent. Res. 2016, 95, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Tiso, M.; Schechter, A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Erzurum, S.C.; Ghosh, S.; Janocha, A.J.; Xu, W.; Bauer, S.; Bryan, N.S.; Tejero, J.; Hermann, C.; Hille, R.; Stuehr, D.J.; et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc. Natl. Acad. Sci. USA 2007, 104, 17593–17598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejam, A.; Hunter, C.; Schechter, A.; Gladwin, M. Emerging role of nitrite in human biology. Blood. Cells Mol. Dis. 2004, 32, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Pinna, M.; Roberto, S.; Milia, R.; Maronquiu, E.; Olla, S.; Loi, A.; Migliaccio, G.M.; Padulo, J.; Orlandi, C.; Tocco, F.; et al. Effect of beetroot juice supplementation on aerobic response during swimming. Nutrients 2014, 6, 605–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peri, L.; Pietraforte, D.; Scorza, G.; Napolitano, A.; Fogliano, V.; Minetti, M. Apples increase nitric oxide production by human saliva at the acidic pH of the stomach: A new biological function for polyphenols with a catechol group? Free Radic. Biol. Med. 2005, 39, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Vanhatalo, A.; Kadach, S.; Wylie, L.J.; Fulford, J.; Ferguson, S.K.; Blackwell, J.R.; Bailey, S.J.; Jones, A.M. Discrete physiological effects of beetroot juice and potassium nitrate supplementation following 4 weeks sprint interval training. J. Appl. Physiol. 2018, 124, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.C.; Herreros, P.V.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Nutrients 2017, 9, 43. [Google Scholar]
- Hernández, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fasttwitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Ferguson, S.K.; Bailey, S.J.; Vanhatalo, A.; Poole, D.C. Fiber-type specific effects of dietary nitrate. Exerc. Sci. Sports Rev. 2016, 44, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Garnacho-Castaño, M.V.; Cuenca, E.; García-Fernández, P.; Muñoz-González, A.; de Jesús, F.; Lozano-Estevan, M.C.; Fernandes da Silva, S.; Veiga-Herreros, P.; Maté-Muñoz, J.L. Effects of beetroot juice supplementation on a 30-s high-intensity inertial cycle ergometer test. Nutrients 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; Fernandes da Silva, S.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J. Int. Soc. Sports Nutr. 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoon, M.W.; Johnson, N.A.; Chapman, P.G.; Burke, L.M. The Effect of Nitrate Supplementation on Exercise Performance in Healthy Individuals: A Systematic Review and Meta-Analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Rimer, E.G.; Peterson, L.R.; Coggan, A.R.; Martin, J.C. Increase in Maximal Cycling Power with Acute Dietary Nitrate Supplementation. Int. J. Sports Physiol. Perform. 2016, 11, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.J.; Baur, D.A.; Spicer, M.T.; Vukovich, M.D.; Ormsbee, M.J. The effect of six days of dietary nitrate supplementation on performance in trained CrossFit athletes. J. Int. Soc. Sports Nutr. 2016, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, M.F.; van Soest, A.J. Why do people jump the way they do? Exerc. Sport Sci. Rev. 2001, 29, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Van Hooren, B.; Zolotarjova, J. The difference between countermovement and squat jump performance: A review of underlying mechanisms with practical applications. J. Strength Cond. Res. 2017, 31, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Rodacki, A.L.; Fowler, N.E.; Bennett, S.J. Vertical jump coordination: Fatigue effects. Med. Sci. Sports Exerc. 2002, 34, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Maté-Muñoz, J.L.; Lougedo, J.H.; Barba, M.; García-Fernández, P.; Garnacho-Castaño, M.V.; Domínguez, R. Muscular fatigue in response to different modalities of CrossFit sessions. PLoS ONE 2017, 12, 0181855. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Castaño, M.V.; Domínguez, R.; Maté-Muñoz, J.L. Understanding the meaning of the lactate threshold in resistance exercises. Int. J. Sports Med. 2015, 36, 371–377. [Google Scholar] [PubMed]
- Garnacho-Castaño, M.V.; Domínguez, R.; Ruiz-Solano, P.; Maté-Muñoz, J.L. Acute physiological and mechanical responses during resistance exercise executed at the lactate threshold workload. J. Strength Cond. Res. 2015, 29, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Broadstreet, S.R.; Mikhalkova, D.; Bole, I.; Leibowitz, J.L.; Kadkhodayan, A.; Park, S.; Thomas, D.P.; Thies, D.; Peterson, L.R. Dietary nitrate-induced increases in human muscle power: High versus low responders. Physiol. Rev. 2018, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Leibowitz, J.L.; Spearie, C.A.; Kadkhodayan, A.; Thomas, D.P.; Ramamurthy, S.; Mahmood, K.; Park, S.; Waller, S.; Farmer, M.; et al. Acute dietary nitrate intake improves muscle contractile function in patients with heart failure: A double-blind, placebo-controlled, randomized trial. Circ. Heart Fail. 2015, 8, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Brown, N.A.; Anderson, F.C.; Spirduso, W.W. A governing relationship for repetitive muscular contraction. J. Biomech. 2000, 33, 969–974. [Google Scholar] [CrossRef]
- Beelen, A.; Sargeant, A.J. Effect of prior exercise at different pedalling frequencies on maximal power in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 66, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.A.; Malomouzh, A.L.; Kovyazina, I.V.; Kejci, E.; Nikitashina, A.D.; Prokurina, S.E.; Zobov, V.V.; Nikolsky, E.E. Regulation of acetylcholinesterase activity by nitric oxide in rat neuromuscular junction via N-methyl-d-aspartate receptor activation. Eur. J. Neurosci. 2013, 37, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.O.; Kalnins, V.I.; Zubrycka, E.; Maclennan, D.H. Assembly of the sarcoplasmic reticulum. Localization by immunofluorescence of sarcoplasmic reticulum proteins in differentiating rat skeletal muscle cell cultures. J. Cell Biol. 1977, 74, 287–298. [Google Scholar] [PubMed]
- Marechal, G.; Gailly, P. Effects of nitric oxide on the contraction of skeletal muscle. Cell. Mol. Life Sci. 1999, 55, 1088–1102. [Google Scholar] [CrossRef] [PubMed]
- Lockie, R.G.; Murphy, A.J.; Knight, T.J.; Janse de Jonge, X.A. Factors that differentiate acceleration ability in field sport athletes. J. Strength Cond. Res. 2011, 25, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Komi, P.V.; Gregor, R.J. Biomechanics of sprint running. A review. Sports Med. 1992, 13, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; Chavarren, J.; Dorado, C. Fractional use of anaerobic capacity during a 30- and 45-s Wingate test. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; De Paz, J.A.; Garatachea, N.; Cabeza de Vaca, S.; Chavarren, J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J. Appl. Physiol. 2003, 94, 668–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaitanos, G.C.; Williams, C.; Boobis, L.H.; Brooks, S. Human muscle metabolism during intermittent maximal exercise. J. Appl. Physiol. 1993, 75, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolin, M.L.; Chesley, A.; Matsos, M.P.; Spriet, L.L.; Jones, N.L.; Heigenhauser, G.J. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am. J. Physiol. 1999, 277, 890–900. [Google Scholar] [CrossRef]
- Kerley, C.P. Dietary nitrate as modulator of physical performance and cardiovascular health. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Wylie, L.J.; Mohr, M.; Krustrup, P.; Jackman, S.R.; Ermιdis, G.; Kelly, J.; Black, M.I.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur. J. Appl. Physiol. 2013, 113, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Bosco, C.; Montanari, G.; Ribacchi, R.; Giovenali, P.; Latteri, F.; Iachelli, G.; Faina, M.; Colli, R.; Dal Monte, A.; La Rosa, M. Relationship between the efficiency of muscular work during jumping and the energetics of running. Eur. J. Appl. Physiol. Occup. 1987, 56, 138–143. [Google Scholar] [CrossRef]
- Bosco, C.; Tihanyi, J.; Komi, P.V.; Fekete, G.; Apor, P. Store and recoil of elastic energy in slow and fast types of human skeletal muscles. Acta Physiol. Scand. 1982, 116, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; McBridge, J.M.; McCaulley, G.O. Power-time, force-time, and velocity-time curve analysis of the countermovement jump: Impact of training. J. Strength Cond. Res. 2009, 23, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Claudino, J.G.; Cronin, J.; Mezêncio, B.; McMaster, D.T.; McGuigan, M.; Tricoli, V.; Amadio, A.C.; Serrao, J.C. The countermovement jump to monitor neuromuscular status: A meta-analysis. J. Sci. Med. Sport 2017, 20, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Skare, O.C.; Skadberg, O.; Wisnes, A.R. Creatine supplementation improves sprint performance in male sprinters. Scand. J. Med. Sci. Sports 2001, 11, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, D.L.; Wenger, H.A. The Relationship between Aerobic Fitness and Recovery from High Intensity Intermittent Exercise. Sports Med. 2001, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Dousset, E.; Avela, J.; Kyrolainen, H.; Kallio, J.; Linnamo, V.; Komi, P.V. Changes in the soleus muscle architecture after exhausting stretch-shortening cycle exercise in humans. Eur. J. Appl. Physiol. 2016, 97, 298–306. [Google Scholar] [CrossRef] [PubMed]

| Variable | Placebo | BJ | p-Value |
|---|---|---|---|
| Wpeak (W) | 848 ± 134 | 881 ± 135 | 0.049 |
| Time to Wpeak (s) | 8.9 ± 1.4 | 7.3 ± 0.9 | 0.003 |
| Wmean (W) | 641 ± 91 | 666 ± 100 | 0.023 |
| Wmin (W) | 453 ± 64 | 472 ± 72 | 0.064 |
| Fatigue index (FI) (%) | 46 ± 8 | 46 ± 7 | 0.914 |
| Variable | Placebo | BJ | Suppl. | Time | Suppl. × Time | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Post-3 | Pre | Post | Post-3 | ||||
| CMJheight (cm) | 30.8 ± 4.6 | 19.5 ± 5.1 a | 25.0 ± 4.3 a,b | 31.5 ± 3.4 | 19.0 ± 4.2 a | 25.3 ± 4.2 a,b | 0.863 | <0.001 | 0.864 |
| CMJWpeak (W) | 50.5 ± 4.7 | 36.9 ± 5.9 a | 45.2 ± 4.6 a,b | 51.1 ± 3.6 | 36.6 ± 4.9 a | 44.7 ± 4.5 a,b | 0.947 | <0.001 | 0.850 |
| CMJWmean (W) | 27.3 ± 3.6 | 20.0 ± 4.2 a | 24.0 ± 3.8 a,b | 27.9 ± 3.3 | 19.6 ± 3.5 a | 23.8 ± 3.6 a,b | 0.994 | <0.001 | 0.850 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuenca, E.; Jodra, P.; Pérez-López, A.; González-Rodríguez, L.G.; Fernandes da Silva, S.; Veiga-Herreros, P.; Domínguez, R. Effects of Beetroot Juice Supplementation on Performance and Fatigue in a 30-s All-Out Sprint Exercise: A Randomized, Double-Blind Cross-Over Study. Nutrients 2018, 10, 1222. https://doi.org/10.3390/nu10091222
Cuenca E, Jodra P, Pérez-López A, González-Rodríguez LG, Fernandes da Silva S, Veiga-Herreros P, Domínguez R. Effects of Beetroot Juice Supplementation on Performance and Fatigue in a 30-s All-Out Sprint Exercise: A Randomized, Double-Blind Cross-Over Study. Nutrients. 2018; 10(9):1222. https://doi.org/10.3390/nu10091222
Chicago/Turabian StyleCuenca, Eduardo, Pablo Jodra, Alberto Pérez-López, Liliana G. González-Rodríguez, Sandro Fernandes da Silva, Pablo Veiga-Herreros, and Raúl Domínguez. 2018. "Effects of Beetroot Juice Supplementation on Performance and Fatigue in a 30-s All-Out Sprint Exercise: A Randomized, Double-Blind Cross-Over Study" Nutrients 10, no. 9: 1222. https://doi.org/10.3390/nu10091222





