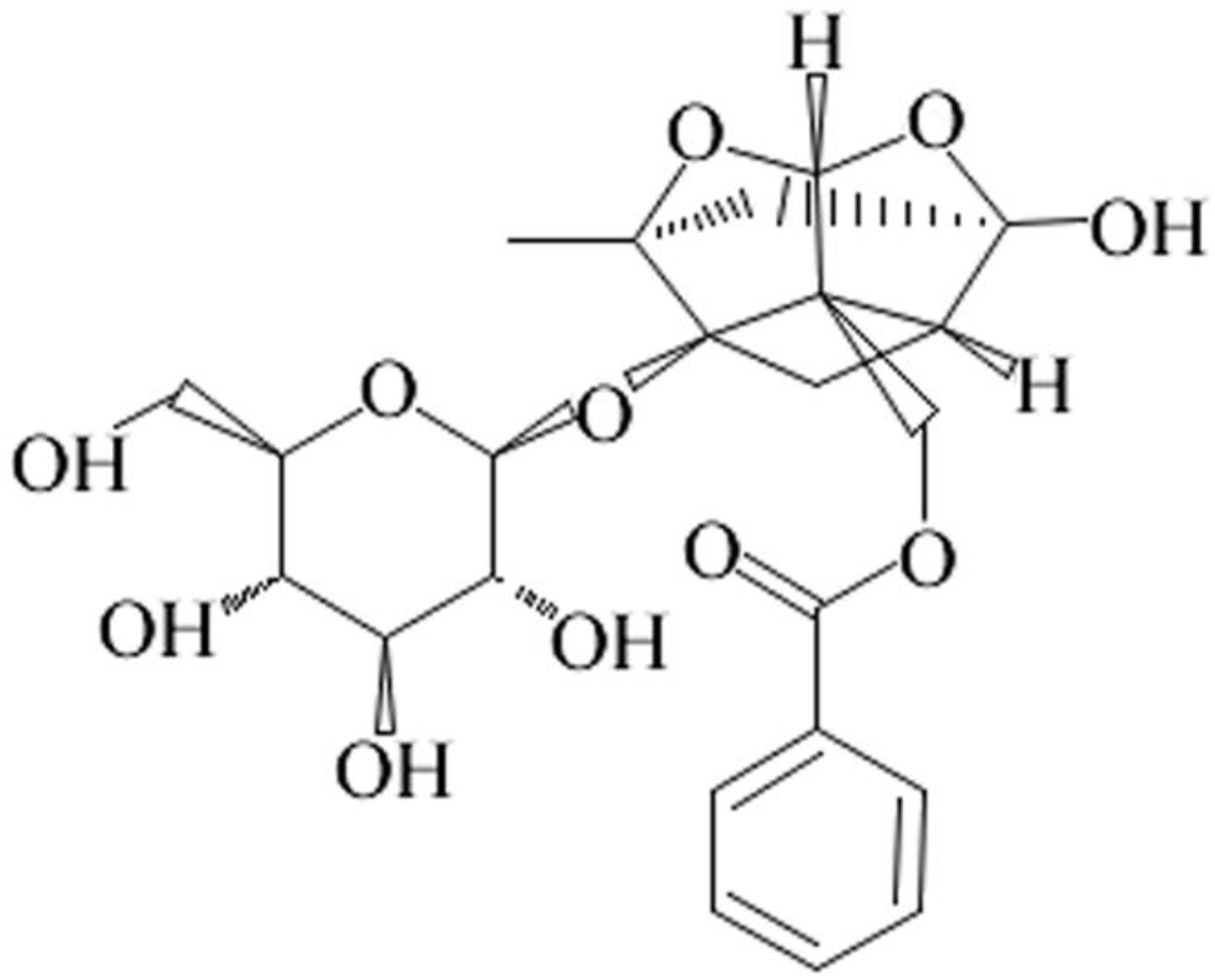
Paeoniflorin Ameliorates Fructose-Induced Insulin Resistance and Hepatic Steatosis by Activating LKB1/AMPK and AKT Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Oral Glucose Tolerance Test
2.4. Blood Biochemical Analysis
2.5. Hepatic Lipids Analyses
2.6. Histological Analysis
2.7. Quantitative Real-Time polymerase chain reaction (qRT-PCR)
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Paeoniflorin Reversed the Metabolic Abnormalities Induced by Fructose
3.2. Paeoniflorin Improved the Liver Function in Fructose Rats
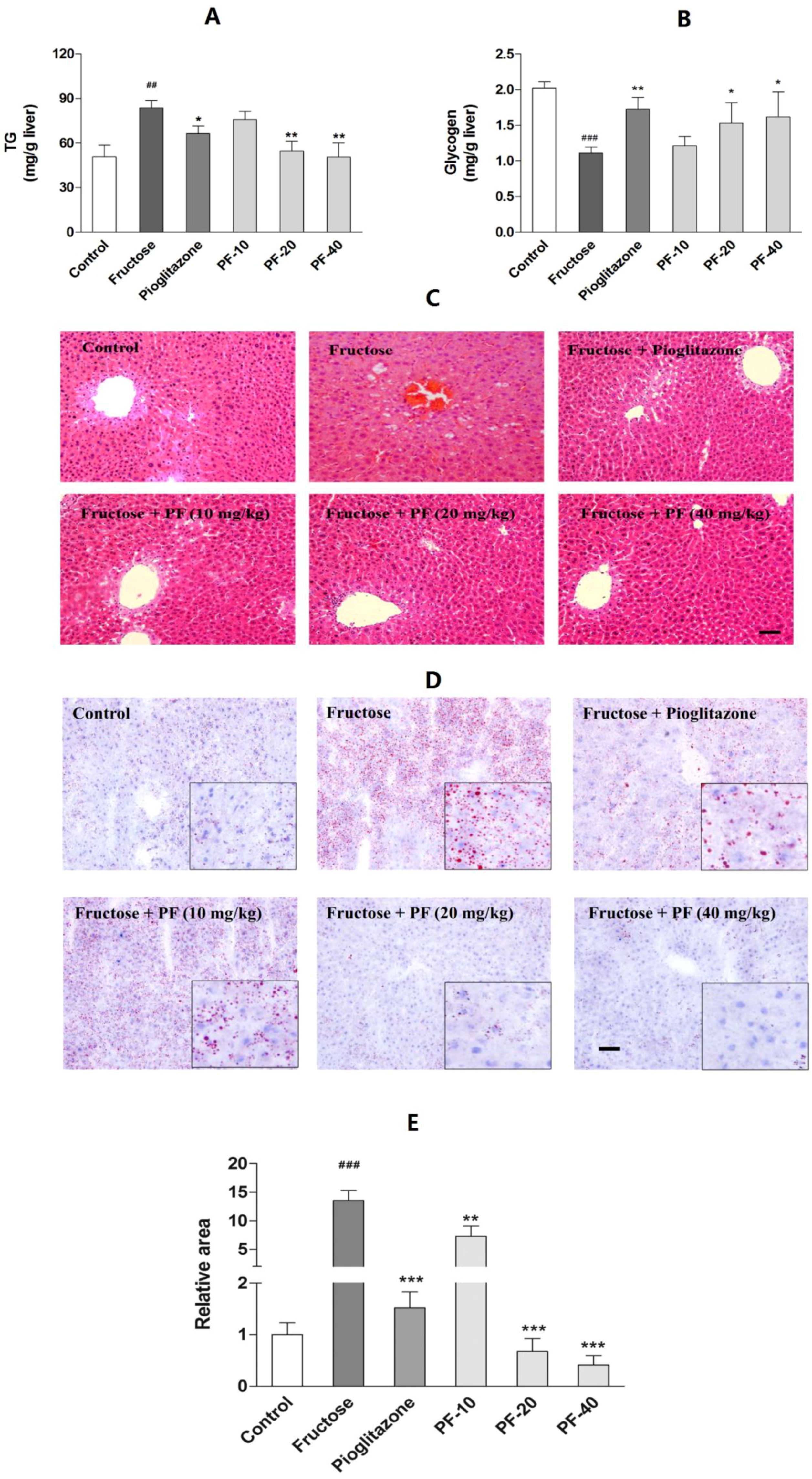
3.3. Paeoniflorin Reversed Fructose-Induced Hepatic Lipids Accumulation and the Reduction of Hepatic Glycogen
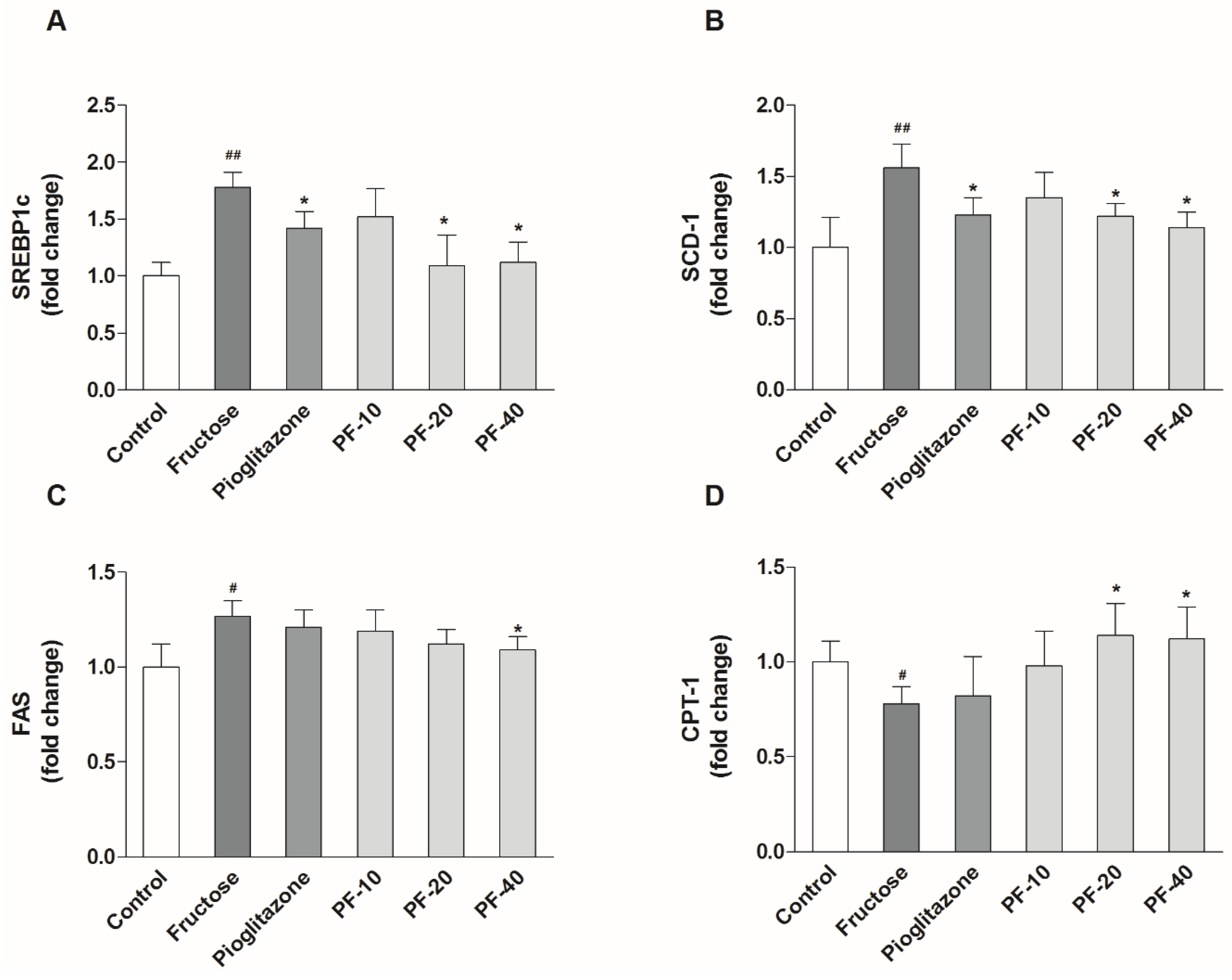
3.4. Paeoniflorin Inhibited the Hepatic Lipogenesis and Promoted Fatty Acid Oxidation
3.5. Paeoniflorin Enhanced the Activities of LKB1/AMPK and AKT Signaling in Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gisela, W. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Jornayvaz, F.R. Metabolic syndrome and nonalcoholic fatty liver disease: Is insulin resistance the link? Mol. Cell. Endocrinol. 2015, 418 Pt 1, 55–65. [Google Scholar] [CrossRef]
- Samuel, V.T. Fructose induced lipogenesis: From sugar to fat to insulin resistance. Trends Endocrinol. Metab. 2011, 22, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Mulligan, K.; Noworolski, S.M.; Tai, V.W.; Wen, M.J.; Erkin-Cakmak, A.; Gugliucci, A.; Schwarz, J.M. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity 2016, 24, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; Gunn, P.J.; Fielding, B.A. The role of dietary sugars and de novo lipogenesis in non-alcoholic fatty liver disease. Nutrients 2014, 6, 5679–5703. [Google Scholar] [CrossRef] [PubMed]
- Mamikutty, N.; Thent, Z.C.; Sapri, S.R.; Sahruddin, N.N.; Mohd Yusof, M.R.; Haji Suhaimi, F. The establishment of metabolic syndrome model by induction of fructose drinking water in male wistar rats. Biomed. Res. Int. 2014, 2014, 263897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, B.; Yu, B. Paeoniflorin protects against nonalcoholic fatty liver disease induced by a high-fat diet in mice. Biol. Pharm. Bull. 2015, 38, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, H.; Wang, W.; Guan, S.; Yi, J.; Chu, L. Paeoniflorin suppresses lipid accumulation and alleviates insulin resistance by regulating the Rho kinase/IRS-1 pathway in palmitate-induced HepG2 cells. Biomed. Pharmacother. 2017, 90, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chu, L.; Liu, H.; Li, J.; Zhang, Y.; Liu, W.; Dai, J.; Yi, J.; Gao, Y. Paeoniflorin alleviates non-alcoholic steatohepatitis in rats: Involvement with the ROCK/NF-κB pathway. Int. Immunopharmacol. 2016, 38, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Li, Y.C.; Kong, L.D.; Hu, Q.H. Curcumin inhibits hepatic protein-tyrosine phosphatase 1B and prevents hypertriglyceridemia and hepatic steatosis in fructose-fed rats. Hepatology 2010, 51, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Kalavalapalli, S.; Bril, F.; Koelmel, J.P.; Abdo, K.; Guingab, J.; Andrews, P.; Li, W.Y.; Jose, D.; Yost, R.A.; Frye, R.F.; et al. Pioglitazone improves hepatic mitochondrial function in a mouse model of nonalcoholic steatohepatitis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E163–E173. [Google Scholar] [CrossRef] [PubMed]
- Shahataa, M.G.; Mostafa-Hedeab, G.; Ali, E.F.; Mahdi, E.A.; Mahmoud, F.A. Effects of telmisartan and pioglitazone on high fructose induced metabolic syndrome in rats. Can. J. Physiol. Pharmacol. 2016, 94, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Abd El-Haleim, E.A.; Bahgat, A.K.; Saleh, S. Resveratrol and fenofibrate ameliorate fructose-induced nonalcoholic steatohepatitis by modulation of genes expression. World J. Gastroenterol. 2016, 22, 2931–2948. [Google Scholar] [CrossRef] [PubMed]
- Kraegen, E.W.; Clark, P.W.; Jenkins, A.B.; Daley, E.A.; Chisholm, D.J.; Storlien, L.H. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 1991, 40, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Hui, S.; Lu, W.; Cowan, A.J.; Morscher, R.J.; Lee, G.; Liu, W.; Tesz, G.J.; Birnbaum, M.J.; Rabinowitz, J.D. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 2018, 27, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.; Fielding, B.A.; Frayn, K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007, 85, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Bashmakov, Y.; Shimomura, I.; Shimano, H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl. Acad. Sci. USA 1998, 95, 5987–5992. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Dobrzyn, A.; Man, W.C.; Chu, K.; Sampath, H.; Kim, H.J.; Ntambi, J.M. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J. Biol. Chem. 2004, 279, 25164–25171. [Google Scholar] [CrossRef] [PubMed]
- Lirio, L.M.; Forechi, L.; Zanardo, T.C.; Batista, H.M.; Meira, E.F.; Nogueira, B.V.; Mill, J.G.; Baldo, M.P. Chronic fructose intake accelerates non-alcoholic fatty liver disease in the presence of essential hypertension. J. Diabetes Complicat. 2016, 30, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Mirrahimi, A.; Carleton, A.J.; Ha, V.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014, 68, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Lee, Y.S.; Cha, S.H.; Jeong, H.W.; Choe, S.S.; Lee, M.R.; Oh, G.T.; Park, H.S.; Lee, K.U.; Lane, M.D.; et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E812–E819. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Dai, W.; Scott, M.J.; Li, R.; Zhang, Y.Q.; Yang, Y.; Chen, L.Z.; Huang, X.S. Metformin improves nonalcoholic fatty liver disease in obese mice via down-regulation of apolipoprotein A5 as part of the AMPK/LXRα signaling pathway. Oncotarget 2017, 8, 108802–108809. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Bettermann, I.; Hechtl, C.; Gabele, E.; Hellerbrand, C.; Scholmerich, J.; Bollheimer, L.C. Dietary folic acid activates AMPK and improves insulin resistance and hepatic inflammation in dietary rodent models of the metabolic syndrome. Horm. Metab. Res. 2010, 42, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Hillgartner, F.B.; Salati, L.M.; Goodridge, A.G. Physiological and molecular mechanisms involved in nutritional regulation of fatty acid synthesis. Physiol. Rev. 1995, 75, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A. Fructose surges damage hepatic adenosyl-monophosphate-dependent kinase and lead to increased lipogenesis and hepatic insulin resistance. Med. Hypotheses 2016, 93, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003, 13, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.P.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Koike, T.; Qin, B.; Kubota, M.; Kawata, Y.; Jia, Y.J.; Oshida, Y. A high-fructose diet impairs Akt and PKCzeta phosphorylation and GLUT4 translocation in rat skeletal muscle. Horm. Metab. Res. 2008, 40, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Jin, R.; Yang, L.; Zhang, J.; Yang, Q.; Guo, Y.Y.; Li, X.B.; Liu, S.B.; Luo, X.X.; Zhao, M.G. Phosphatidylinositol 3 kinase/protein kinase b is responsible for the protection of paeoniflorin upon H2O2-induced neural progenitor cell injury. Neuroscience 2013, 240, 54–62. [Google Scholar] [CrossRef] [PubMed]


| Genes | Accession | Sense | Antisense |
|---|---|---|---|
| SREBP1c | NM_001276707 | 5′-CCATGGACGAGCTACCCTTC-3′ | 5′-GCCTGTGTCTCCTGTCTCAC-3′ |
| SCD-1 | AF509569.1 | 5′-CCTGGCTTACGACCGGAAA-3′ | 5′-CAGGAACTCAGAAGCCCAG-3′ |
| FAS | NM_012820.1 | 5′-TGTGGGGTGGAAATCATCGG-3′ | 5′-CATTGCTCCTTTGGGGTTGC-3′ |
| CPT-1 | NM_064320.3 | 5′-ACGAGCCGATTGGGCTAAA-3′ | 5′-ACCAACGATCGTGAGCCTTT-3′ |
| GAPDH | NM_017008.4 | 5′-AGTGCCAGCCTCGTCTCATA-3′ | 5′-GGTAACCAGGCGTCCGATA-3′ |
| Control | Fructose | Pioglitazone | PF-10 | PF-20 | PF-40 | |
|---|---|---|---|---|---|---|
| TG (mmol/L) | 0.87 ± 0.05 | 1.14 ± 0.10 # | 0.80 ± 0.06 ** | 0.96 ± 0.09 | 0.85 ± 0.08 ** | 0.71 ± 0.09 ** |
| TC (mmol/L) | 1.92 ± 0.10 | 2.37 ± 0.08 ## | 2.05 ± 0.05 * | 2.16 ± 0.12 | 2.05 ± 0.06 * | 1.97 ± 0.07 ** |
| HDL-C (mmol/L) | 0.63 ± 0.06 | 0.76 ± 0.05 # | 0.57 ± 0.03 * | 0.69 ± 0.07 | 0.60 ± 0.05 * | 0.62 ± 0.04 * |
| LDL-C (mmol/L) | 0.95 ± 0.07 | 1.59 ± 0.10 ### | 1.20 ± 0.05 ** | 1.37 ± 0.09 * | 1.23 ± 0.04 ** | 1.18 ± 0.08 ** |
| NEFA (mmol/L) | 0.92 ± 0.11 | 1.53 ± 0.07 ### | 0.88 ± 0.07 *** | 1.15 ± 0.08 ** | 1.07 ± 0.07 *** | 0.92 ± 0.05 *** |
| Insulin (mIU/L) | 11.7 ± 0.5 | 18.3 ± 0.5 ### | 12.6 ± 0.8 *** | 15.9 ± 0.3 * | 14.1 ± 0.9 ** | 14.5 ± 0.9 ** |
| Glucagon (pg/mL) | 194.0 ± 4.6 | 245.6 ± 8.6 ### | 227.7 ± 7.0 * | 235.4 ± 10.8 | 206.8 ± 9.4 ** | 188.7 ± 9.5 *** |
| Glucose (mmol/L) | 5.27 ± 0.09 | 5.43 ± 0.13 | 5.11 ± 0.16 | 5.19 ± 0.16 | 5.08 ± 0.13 | 4.98 ± 0.25 |
| ALB (g/L) | 37.9 ± 2.9 | 37.2 ± 1.4 | 37.4 ± 3.5 | 37.4 ± 4.3 | 38.9 ± 2.9 | 38.5 ± 1.9 |
| AST (IU/L) | 17.1 ± 0.7 | 18.8 ± 0.4 | 16.9 ± 0.6 | 15.3 ± 0.8 * | 12.8 ± 0.5 ** | 12.2 ± 0.9 ** |
| ALT (IU/L) | 7.70 ± 1.34 | 8.23 ± 0.75 | 9.42 ± 1.54 | 9.34 ± 0.82 | 8.23 ± 1.15 | 8.84 ± 0.95 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-C.; Qiao, J.-Y.; Wang, B.-Y.; Bai, M.; Shen, J.-D.; Cheng, Y.-X. Paeoniflorin Ameliorates Fructose-Induced Insulin Resistance and Hepatic Steatosis by Activating LKB1/AMPK and AKT Pathways. Nutrients 2018, 10, 1024. https://doi.org/10.3390/nu10081024
Li Y-C, Qiao J-Y, Wang B-Y, Bai M, Shen J-D, Cheng Y-X. Paeoniflorin Ameliorates Fructose-Induced Insulin Resistance and Hepatic Steatosis by Activating LKB1/AMPK and AKT Pathways. Nutrients. 2018; 10(8):1024. https://doi.org/10.3390/nu10081024
Chicago/Turabian StyleLi, Yu-Cheng, Jing-Yi Qiao, Bao-Ying Wang, Ming Bai, Ji-Duo Shen, and Yong-Xian Cheng. 2018. "Paeoniflorin Ameliorates Fructose-Induced Insulin Resistance and Hepatic Steatosis by Activating LKB1/AMPK and AKT Pathways" Nutrients 10, no. 8: 1024. https://doi.org/10.3390/nu10081024
APA StyleLi, Y.-C., Qiao, J.-Y., Wang, B.-Y., Bai, M., Shen, J.-D., & Cheng, Y.-X. (2018). Paeoniflorin Ameliorates Fructose-Induced Insulin Resistance and Hepatic Steatosis by Activating LKB1/AMPK and AKT Pathways. Nutrients, 10(8), 1024. https://doi.org/10.3390/nu10081024





