Effects of Multivitamin and Multimineral Supplementation on Blood Pressure: A Meta-Analysis of 12 Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Selection
2.2. Data Extraction and Quality Assessment
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Quality Assessment
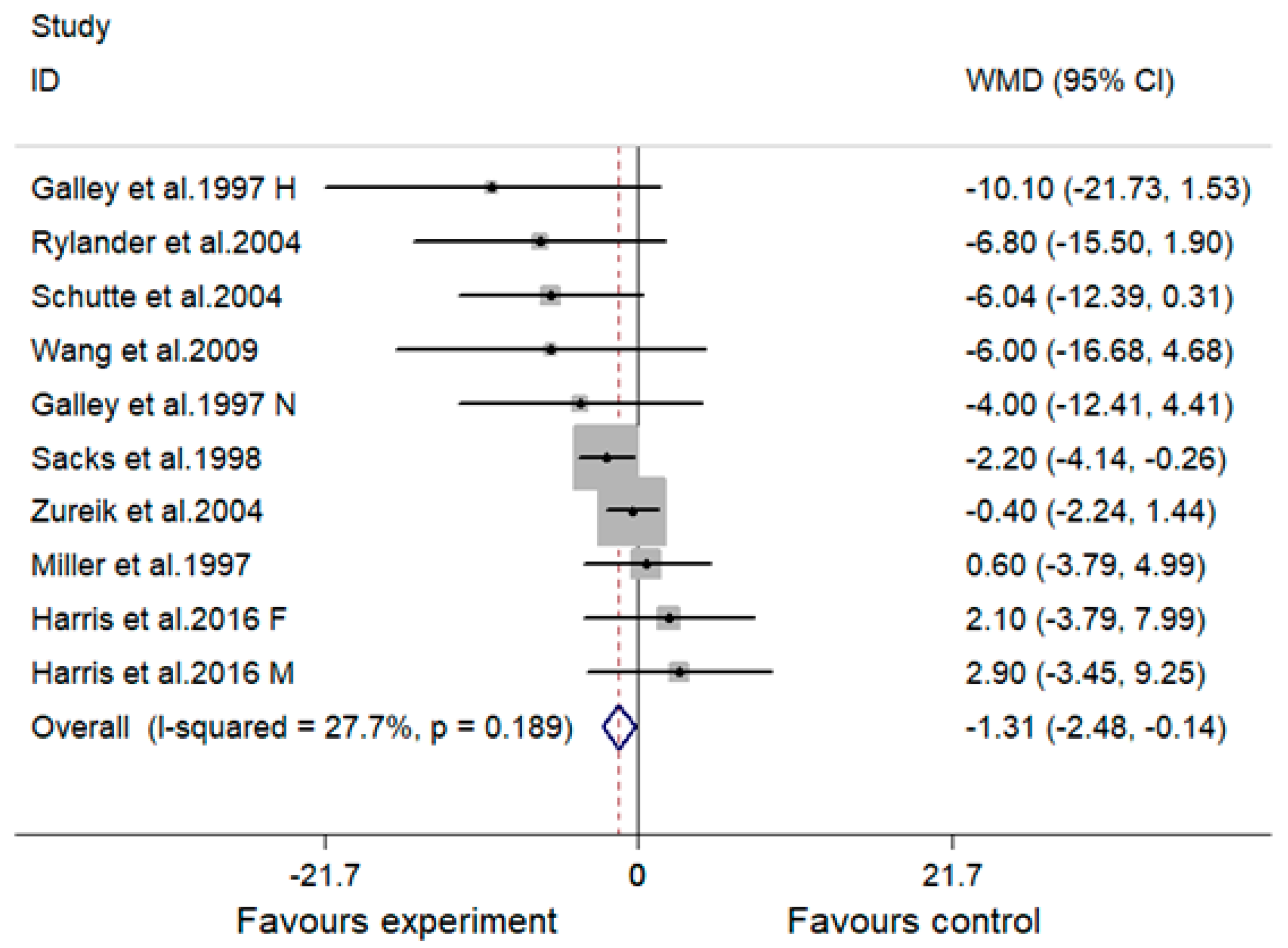
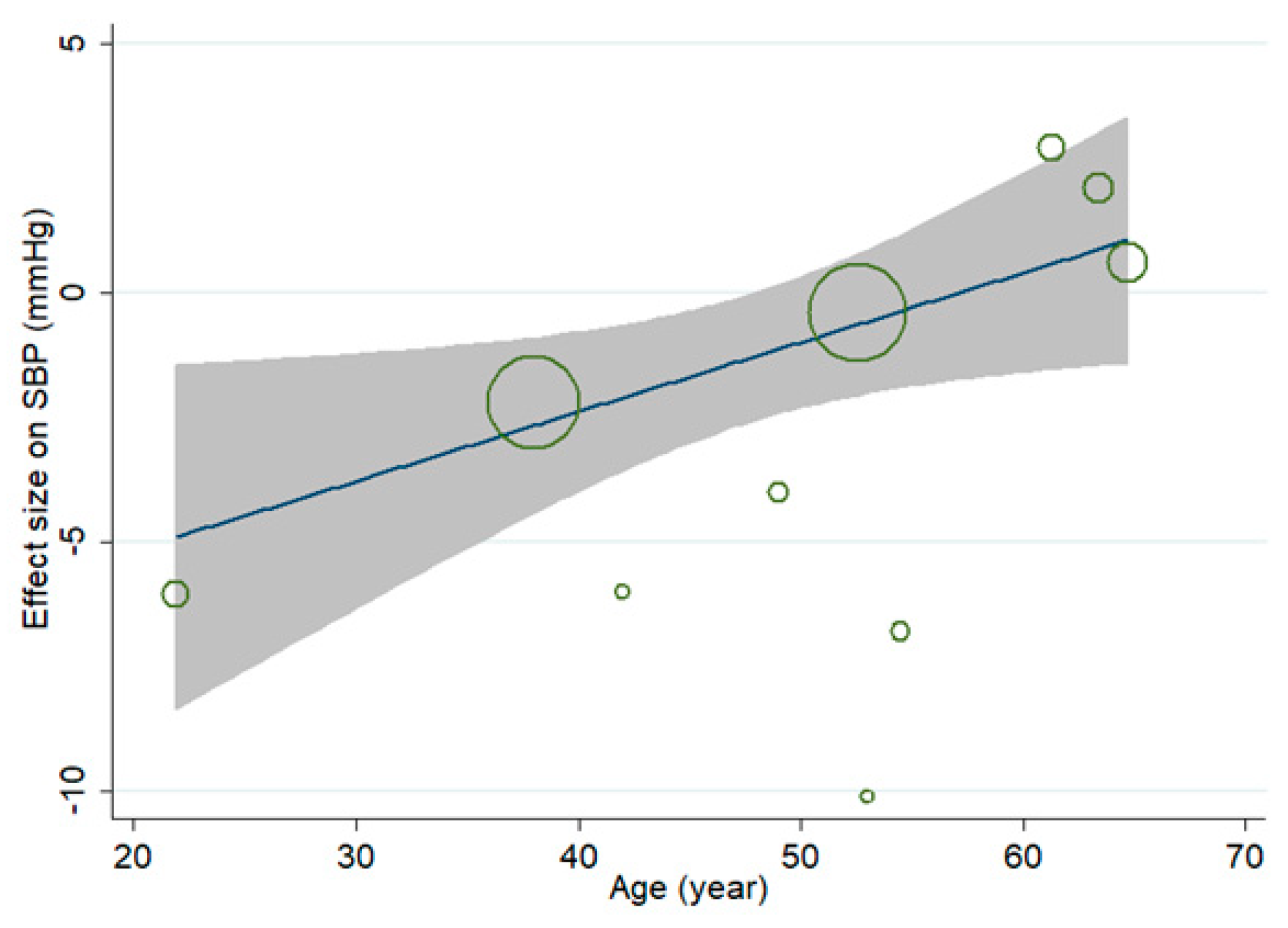
3.3. Effect of MVMS and SBP
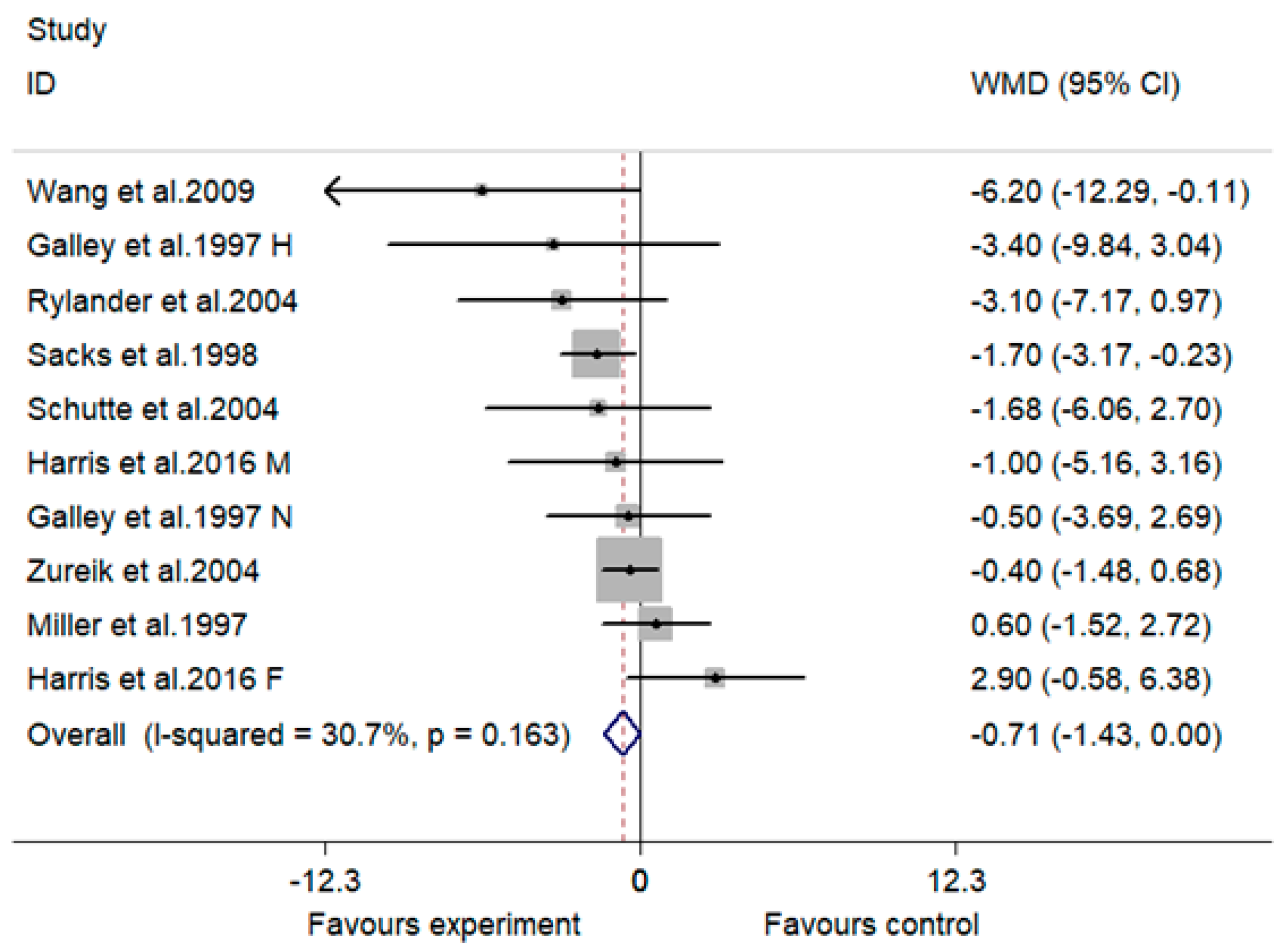
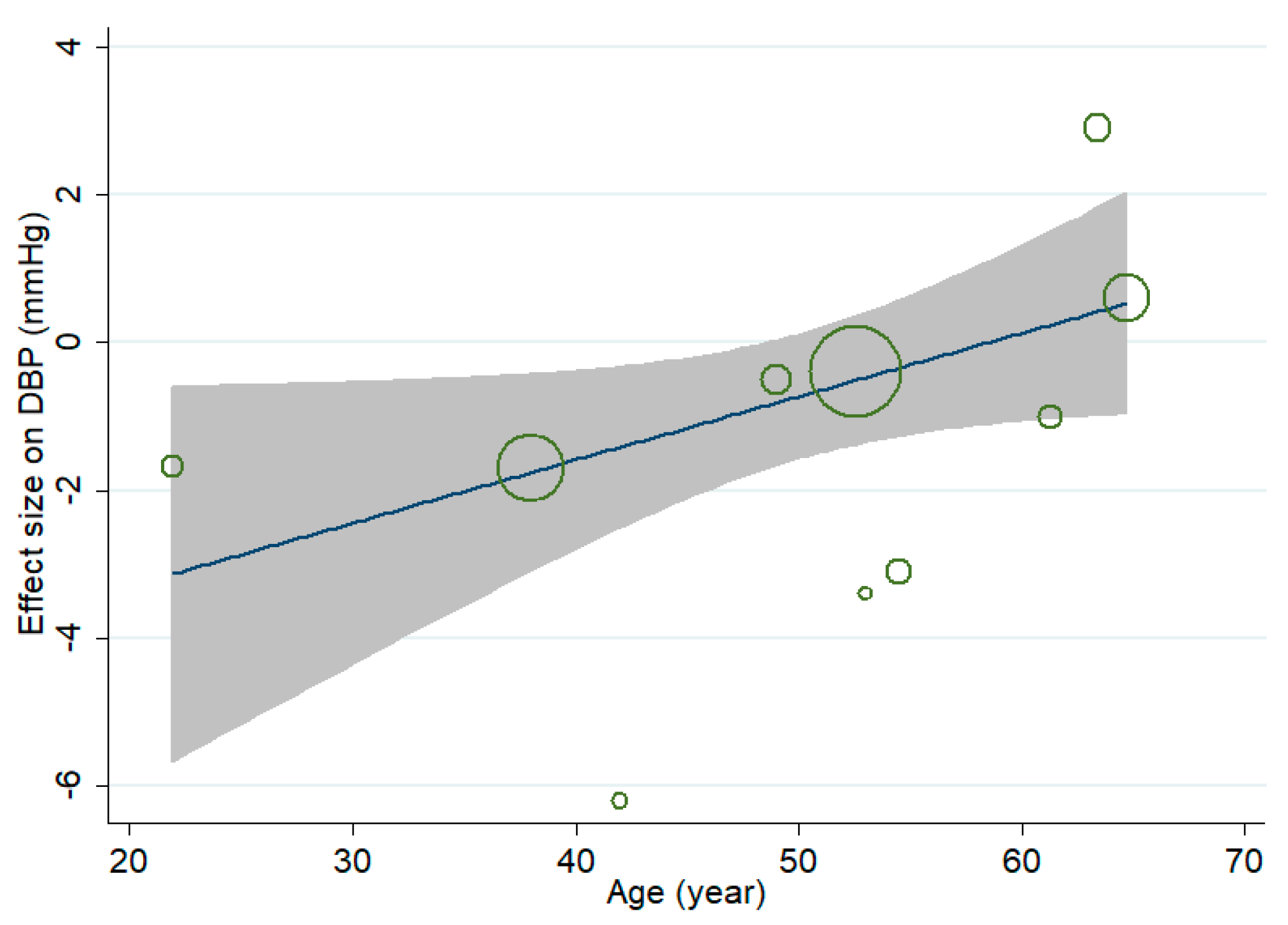
3.4. Effect of MVMS on DBP
3.5. Effect of MVMS on MAP
3.6. Effect of MVMS on Risk of Hypertension
3.7. Sensitivity Analysis
3.8. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Joffres, M.; Falaschetti, E.; Gillespie, C.; Robitaille, C.; Loustalot, F.; Poulter, N.; McAlister, F.A.; Johansen, H.; Baclic, O.; Campbell, N. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: A cross-sectional study. BMJ Open 2013, 3, e003423. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C. Recent studies on the effects of beta blockers on blood lipid levels. Am. Heart J. 1989, 117, 709–714. [Google Scholar] [CrossRef]
- Pollare, T.; Lithell, H.; Selinus, I.; Berne, C. Sensitivity to insulin during treatment with atenolol and metoprolol: A randomised, double blind study of effects on carbohydrate and lipoprotein metabolism in hypertensive patients. BMJ 1989, 298, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Dakshinamurti, K.; Paulose, C.S.; Viswanathan, M. Vitamin B6 and hypertension. Ann. N. Y. Acad. Sci. 1990, 585, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tare, M.; Emmett, S.J.; Coleman, H.A.; Skordilis, C.; Eyles, D.W.; Morley, R.; Parkington, H.C. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J. Physiol. 2011, 589, 4777–4786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altura, B.M.; Altura, B.T. Magnesium, electrolyte transport and coronary vascular tone. Drugs 1984, 28 (Suppl. 1), 120–142. [Google Scholar] [CrossRef] [PubMed]
- Zicha, J.; Behuliak, M.; Pinterova, M.; Bencze, M.; Kunes, J.; Vaneckova, I. The interaction of calcium entry and calcium sensitization in the control of vascular tone and blood pressure of normotensive and hypertensive rats. Physiol. Res. 2014, 63 (Suppl. 1), S19–S27. [Google Scholar] [PubMed]
- Mark, S.D.; Wang, W.; Fraumeni, J.F., Jr.; Li, J.Y.; Taylor, P.R.; Wang, G.Q.; Guo, W.; Dawsey, S.M.; Li, B.; Blot, W.J. Lowered risks of hypertension and cerebrovascular disease after vitamin/mineral supplementation: The Linxian nutrition intervention trial. Am. J. Epidemiol. 1996, 143, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Zureik, M.; Galan, P.; Bertrais, S.; Mennen, L.; Czernichow, S.; Blacher, J.; Ducimetière, P.; Hercberg, S. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Zhu, K.; Dong, Y.M.; Sun, C.H. Effects of supplementation with multivitamin and mineral on blood pressure and c-reactive protein in obese Chinese women with increased cardiovascular disease risk. Asia Pac. J. Clin. Nutr. 2009, 18, 121–130. [Google Scholar] [PubMed]
- Harris, E.; Rowsell, R.; Pipingas, A.; Macpherson, H. No effect of multivitamin supplementation on central blood pressure in healthy older people: A randomized controlled trial. Atherosclerosis 2016, 246, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Rylander, R.; Arnaud, M.J. Mineral water intake reduces blood pressure among subjects with low urinary magnesium and calcium levels. BMC Public Health 2004, 4, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Li, N.; Mei, Z.; Ye, R.; Li, Z.; Liu, J.; Serdula, M.K. Micronutrient supplementation during pregnancy and the risk of pregnancy-induced hypertension: A randomized clinical trial. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Thornton, J.; Howdle, P.D.; Walker, B.E.; Webster, N.R. Combination oral antioxidant supplementation reduces blood pressure. Clin. Sci. 1997, 92, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Willett, W.C.; Smith, A.; Brown, L.E.; Rosner, B.; Moore, T.J. Effect on blood pressure of potassium, calcium, and magnesium in women with low habitual intake. Hypertension 1998, 31, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Schutte, A.E.; Huisman, H.W.; Oosthuizen, W.; van Rooyen, J.M.; Jerling, J.C. Cardiovascular effects of oral supplementation of Vitamin C, E and folic acid in young healthy males. Int. J. Vitam. Nutr. Res. 2004, 74, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R., 3rd; Appel, L.J.; Levander, O.A.; Levine, D.M. The effect of antioxidant vitamin supplementation on traditional cardiovascular risk factors. J. Cardiovasc. Risk 1997, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Merchant, A.T.; Msamanga, G.; Villamor, E.; Saathoff, E.; O’Brien, M.; Hertzmark, E.; Hunter, D.J.; Fawzi, W.W. Multivitamin supplementation of HIV-positive women during pregnancy reduces hypertension. J. Nutr. 2005, 135, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Bertrais, S.; Blacher, J.; Galan, P.; Briançon, S.; Favier, A.; Safar, M.; Hercberg, S. Effect of supplementation with antioxidants upon long-term risk of hypertension in the SU.VI. Max study: Association with plasma antioxidant levels. J. Hypertens. 2005, 23, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Beyer, F.R.; Dickinson, H.O.; Nicolson, D.J.; Ford, G.A.; Mason, J. Combined calcium, magnesium and potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst. Rev. 2006, Cd004805. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Cena, H.; Barr, S.I.; Biesalski, H.K.; Dagach, R.U.; Delaney, B.; Frei, B.; Moreno Gonzalez, M.I.; Hwalla, N.; Lategan-Potgieter, R.; et al. The use of multivitamin/multimineral supplements: A modified delphi consensus panel report. Clin. Ther. 2018, 40, 640–657. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, H.; Pipingas, A.; Pase, M.P. Multivitamin-multimineral supplementation and mortality: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Robien, K.; Harnack, L.J.; Park, K.; Jacobs, D.R., Jr. Dietary supplements and mortality rate in older women: The Iowa women’s health study. Arch. Intern. Med. 2011, 171, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Sleeper, R.B.; Kennedy, S.M. Adverse reaction to a dietary supplement in an elderly patient. Ann. Pharmacother. 2003, 37, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Timbo, B.B.; Ross, M.P.; McCarthy, P.V.; Lin, C.T. Dietary supplements in a national survey: Prevalence of use and reports of adverse events. J. Am. Diet. Assoc. 2006, 106, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of marine-derived n-3 polyunsaturated fatty acids on c-reactive protein, interleukin 6 and tumor necrosis factor alpha: A meta-analysis. PLoS ONE 2014, 9, e88103. [Google Scholar]
- Cappuccio, F.P.; Kerry, S.M.; Forbes, L.; Donald, A. Blood pressure control by home monitoring: Meta-analysis of randomised trials. BMJ 2004, 329, 145. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Zheng, J.; Yang, B.; Huang, T.; Yu, Y.; Yang, J.; Li, D. Green tea and black tea consumption and prostate cancer risk: An exploratory meta-analysis of observational studies. Nutr. Cancer 2011, 63, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Juni, P.; Schulz, K.F.; Altman, D.G.; Bartlett, C.; Egger, M. Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’ research. Stat. Med. 2002, 21, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.C.; Suh, J.Y.; Kim, B.S.; Kang, J.H.; Kim, H.; Lee, M.H.; Park, J.R.; Kim, S.W. High sensitivity c-reactive protein as an independent risk factor for essential hypertension. Am. J. Hypertens. 2003, 16, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Buring, J.E.; Shih, J.; Matias, M.; Hennekens, C.H. Prospective study of c-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998, 98, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Earnest, C.P.; Wood, K.A.; Kampert, J.B. Reduction of c-reactive protein levels through use of a multivitamin. Am. J. Med. 2003, 115, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Resta, T.C.; Broughton, B.R.; Jernigan, N.L. Reactive oxygen species and rhoa signaling in vascular smooth muscle: Role in chronic hypoxia-induced pulmonary hypertension. Adv. Exp. Med. Biol. 2010, 661, 355–373. [Google Scholar] [PubMed]
- Touyz, R.M. Reactive oxygen species in vascular biology: Role in arterial hypertension. Expert Rev. Cardiovasc. Ther. 2003, 1, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.M. Factors in aging that effect the bioavailability of nutrients. J. Nutr. 2001, 131, 1359S–1361S. [Google Scholar] [CrossRef] [PubMed]
- Ireland, P.; Fordtran, J.S. Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J. Clin. Investig. 1973, 52, 2672–2681. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Quast, S.; Ullrich, M.; Schultze, H.; Bodis, M.; Geisel, J. Hyperhomocysteinemia in high-aged subjects: Relation of b-vitamins, folic acid, renal function and the methylenetetrahydrofolate reductase mutation. Atherosclerosis 1999, 144, 91–101. [Google Scholar] [CrossRef]
- Turnlund, J.R.; Durkin, N.; Costa, F.; Margen, S. Stable isotope studies of zinc absorption and retention in young and elderly men. J. Nutr. 1986, 116, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Belvedere, M.; Dominguez, L.J. Magnesium homeostasis and aging. Magnes. Res. 2009, 22, 235–246. [Google Scholar] [PubMed]
- Heidelbaugh, J.J. Proton pump inhibitors and risk of vitamin and mineral deficiency: Evidence and clinical implications. Ther. Adv. Drug Saf. 2013, 4, 125–133. [Google Scholar] [CrossRef] [PubMed]
- De Burgos, M.A.; Wartanowicz, M.; Ziemlanski, S. Blood vitamin and lipid levels in overweight and obese women. Eur. J. Clin. Nutr. 1992, 46, 803–808. [Google Scholar]
- Yanoff, L.B.; Menzie, C.M.; Denkinger, B.; Sebring, N.G.; McHugh, T.; Remaley, A.T.; Yanovski, J.A. Inflammation and iron deficiency in the hypoferremia of obesity. Int. J. Obes. 2007, 31, 1412–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trehan, N.; Afonso, L.; Levine, D.L.; Levy, P.D. Vitamin D deficiency, supplementation, and cardiovascular health. Crit. Pathw. Cardiol. 2017, 16, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Coodley, G.; Girard, D.E. Vitamins and minerals in HIV infection. J. Gen. Intern. Med. 1991, 6, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Wang, X.S.; Zhang, Z.R.; He, J.C.; Xie, C.L. A meta-analysis of folic acid in combination with anti-hypertension drugs in patients with hypertension and hyperhomocysteinemia. Front. Pharmacol. 2017, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wahlqvist, M.L.; Li, D. Nutrition, one-carbon metabolism and neural tube defects: A review. Nutrients 2016, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, H.L.T. Lowering blood homocysteine with folic acid based supplements: Meta-analysis of randomised trials. BMJ 1998, 316, 894–898. [Google Scholar] [CrossRef]
- Wald, D.S.; Law, M.; Morris, J.K. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Zhuang, L.; Wang, Y.; Ma, Y. Homocysteine levels and risk of essential hypertension: A meta-analysis of published epidemiological studies. Clin. Exp. Hypertens. 2017, 39, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Lichtenstein, A.H.; Howard, B.V.; Steinberg, D.; Witztum, J.L. Antioxidant vitamin supplements and cardiovascular disease. Circulation 2004, 110, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G.; Gongora, M.C. Oxidative stress and hypertension. Med. Clin. N. Am. 2009, 93, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Touyz, R.M. Oxidative stress and hypertension: Current concepts. Curr. Hypertens. Rep. 2010, 12, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.V.; Padmaja, G.; Kuppusamy, P.; Kutala, V.K. Oxidative stress in cardiovascular disease. Indian J. Biochem. Biophys. 2009, 46, 421–440. [Google Scholar] [PubMed]
- Houston, M.C.; Harper, K.J. Potassium, magnesium, and calcium: Their role in both the cause and treatment of hypertension. J. Clin. Hypertens. 2008, 10, 3–11. [Google Scholar] [CrossRef]

| Study | Design | Sample Size | Duration (Month) | Age (Year) | Sex | BMI | Race | Healthy Status | Baseline | |
|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | |||||||||
| Zureik et al., 2004 [10] | P | 1162 | 86.4 | 52.6 | M + F | 24.85 | Caucasian | Healthy | 125.5 | 81.3 |
| Wang et al., 2009 [11] | P | 59 | 6.5 | 42 | F | 30.9 | Chinese | Obesity | 128 | 85 |
| Harris et al., 2016 M [12] | P | 79 | 4 | 61.3 | M | 27.4 | Caucasian | Healthy | 137.5 | 84.1 |
| Harris et al., 2016 F [12] | P | 160 | 4 | 63.4 | F | 26.5 | Caucasian | Healthy | 134.9 | 82.7 |
| Rylander et al., 2004 [13] | P | 37 | 1 | 54.5 | NR | NR | Caucasian | Hypertension | 152.7 | 91.1 |
| Galley et al., 1997 H [15] | C | 21 | 2 | 53 | NR | NR | Caucasian | Hypertension | 165 | 88.9 |
| Galley et al., 1997 N [15] | C | 17 | 2 | 49 | NR | NR | Caucasian | Dyspepsia or other gastro-intestinal complaints | 132.8 | 79.6 |
| Sacks et al., 1998 [16] | P | 148 | 4 | 38 | NR | 23.2 | Caucasian | Healthy | 115.3 | 73 |
| Schutte et al., 2004 [17] | P | 31 | 3 | 21.9 | M | 25.23 | Caucasian | Healthy | 128.9 | 79.1 |
| Miller et al., 1997 [18] | P | 297 | 3 | 64.7 | M + F | NR | Caucasian | NR | NR | NR |
| Study | Design | Sample Size | Duration (Month) | Age (Year) | Sex | BMI | Race | Subjects | Baseline | |
|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | |||||||||
| Mark et al., 1996 M [9] | P | 1461 | 72 | 54.5 | M | 20.39 | Chinese | Esophageal dysplasia | 131.8 | 80 |
| Mark et al., 1996 F [9] | P | 1857 | 72 | 54.5 | F | 20.39 | Chinese | Esophageal dysplasia | 131.8 | 80 |
| Chen et al., 2018 [14] | P | 11,837 | 5 | <29 * | F | Normal # | Chinese | Pregnant women | Normal & | Normal & |
| Merchant et al., 2005 [19] | P | 955 | 5 | 24.7 | F | 23.3 | African | HIV-positive pregnant women | 106 | 66 |
| Czernichow et al., 2005 M [20] | P | 2153 | 78 | 52.3 | M | 25.1 | Caucasian | Healthy | Normal & | Normal & |
| Czernichow et al., 2005 F [20] | P | 2933 | 78 | 47.8 | F | 22.8 | Caucasian | Healthy | Normal & | Normal & |
| Subgroup Analysis | No. of Comparisons | SBP | DBP | ||||
|---|---|---|---|---|---|---|---|
| WMD (95% CI) | I2 (%) | p | WMD (95% CI) | I2 (%) | p | ||
| Overall | 10 | −1.31 (−2.48, −0.14) | 27.7 | 0.028 | −0.71 (−1.43, 0.00) | 30.7 | 0.051 |
| Design | |||||||
| Parallel | 8 | −1.17 (−2.35, 0.02) | 28.5 | 0.053 | −0.69 (−1.43, 0.05) | 43.1 | 0.068 |
| Crossover | 2 | −6.09 (−12.91, 0.72) | 0 | 0.08 | −1.07 (−3.93, 1.79) | 0 | 0.463 |
| Sex | |||||||
| Men | 2 | −1.57 (−6.06, 2.92) | 73.7 | 0.494 | −1.32 (−4.34, 1.70) | 0 | 0.39 |
| Women | 2 | 0.21 (−4.95, 5.37) | 41 | 0.936 | 0.65 (−2.37, 3.68) | 84.5 | 0.672 |
| Men and Women | 2 | −0.25 (−1.95, 1.44) | 0 | 0.772 | −0.19 (−1.16, 0.77) | 0 | 0.696 |
| Unclear | 4 | −2.68 (−4.50, −0.86) | 0 | 0.004 | −1.71 (−2.96, −0.47) | 0 | 0.007 |
| BMI | |||||||
| <30 kg/m2 | 5 | −1.13 (−2.38, 0.12) | 40.9 | 0.077 | −0.69 (−1.50, 0.13) | 37.4 | 0.099 |
| >30 kg/m2 | 1 | −6.00 (−16.68, 4.68) | NA | 0.271 | −6.20 (−12.29, −0.11) | NA | 0.046 |
| Unclear | 4 | −2.20 (−5.59, 1.20) | 34.6 | 0.205 | −0.45 (−2.02, 1.12) | 11.3 | 0.571 |
| Race | |||||||
| Caucasian | 9 | −1.25 (−2.43, −0.08) | 31.6 | 0.036 | −0.64 (−1.36, 0.09) | 18.6 | 0.084 |
| Chinese | 1 | −6.00 (−16.68, 4.68) | NA | 0.271 | −6.20 (−12.29, −0.11) | NA | 0.046 |
| Healthy status | |||||||
| Healthy | 5 | −1.13 (−2.38, 0.12) | 40.9 | 0.077 | −0.69 (−1.50, 0.13) | 37.4 | 0.099 |
| Chronic disease | 4 | −6.29 (−11.09, −1.50) | 0 | 0.01 | −2.32 (−4.50, −0.13) | 1.9 | 0.038 |
| Unclear | 1 | 0.60 (−3.79, 4.99) | NA | 0.789 | 0.60 (−1.52, 2.72) | NA | 0.579 |
| Baseline of blood pressure | |||||||
| Normotensive subjects | 7 | −1.25 (−2.48, −0.02) | 24.8 | 0.046 | −0.77 (−1.55, 0.02) | 36.9 | 0.055 |
| Hypertensive subjects | 2 | −7.98 (−14.95, −1.02) | 0 | 0.025 | −3.19 (−6.63, 0.25) | 0 | 0.07 |
| Unclear | 1 | 0.60 (−3.79, 4.99) | NA | 0.789 | 0.60 (−1.52, 2.72) | NA | 0.579 |
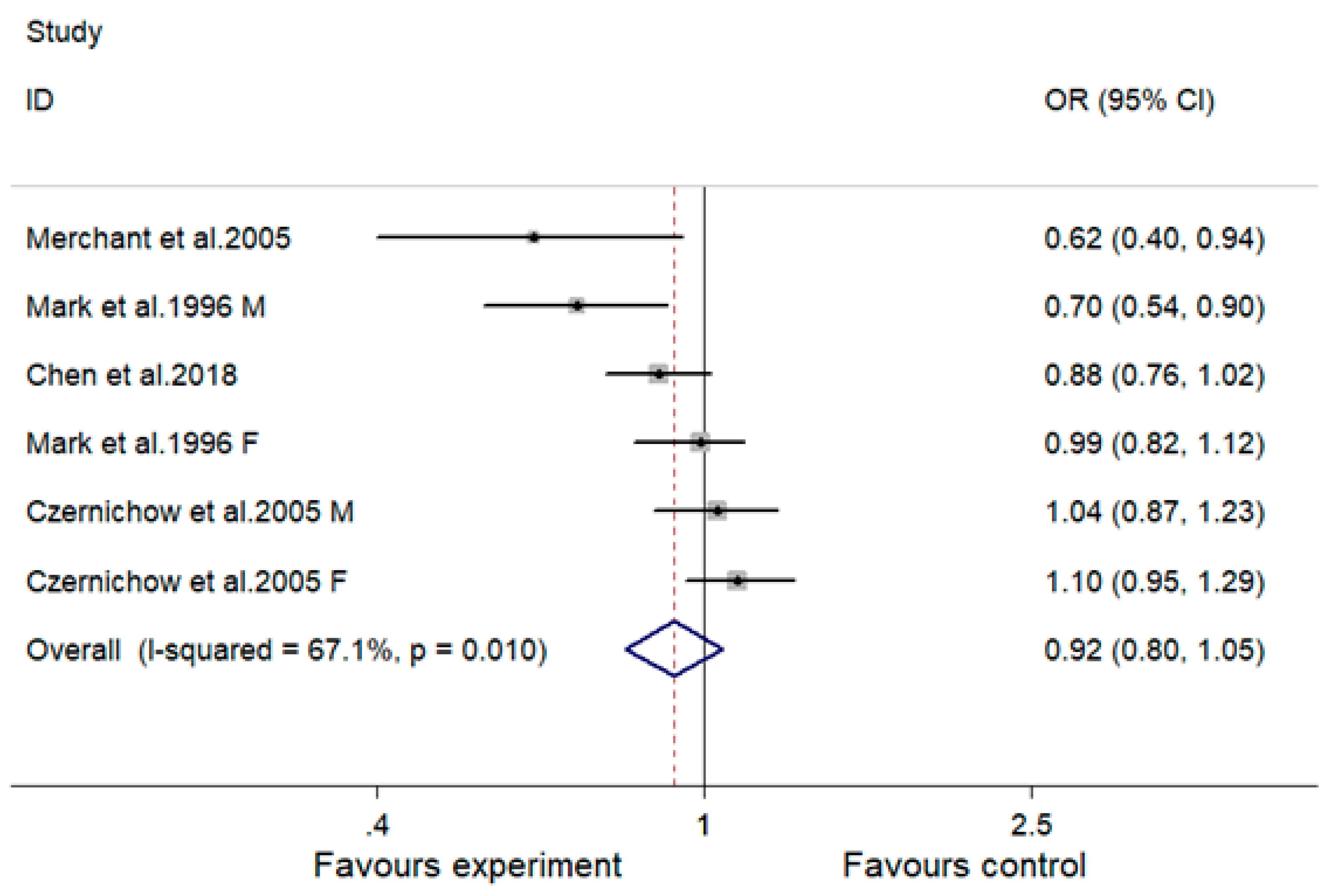
| Subgroup Analysis | No. of Comparisons | OR (95% CI) | I2 (%) | p |
|---|---|---|---|---|
| Overall | 6 | 0.92 (0.80, 1.05) | 67.1 | 0.216 |
| Duration | ||||
| <6 months | 2 | 0.78 (0.57, 1.08) | 67.4 | 0.14 |
| ≥6 years | 4 | 0.97 (0.83, 1.13) | 56.7 | 0.676 |
| Age | ||||
| Young (<30 years) | 2 | 0.78 (0.57, 1.08) | 56.7 | 0.14 |
| Middle aged (40–55 years) | 4 | 0.97 (0.83, 1.13) | 67.4 | 0.676 |
| Sex | ||||
| Men | 2 | 0.86 (0.59, 1.27) | 84.2 | 0.457 |
| Women | 4 | 0.94 (0.80, 1.10) | 64.9 | 0.437 |
| Race | ||||
| Caucasian | 2 | 1.07 (0.96, 1.20) | 0 | 0.227 |
| Chinese | 3 | 0.87 (0.74, 1.03) | 61.6 | 0.103 |
| African | 1 | 0.62 (0.40, 0.95) | NA | 0.028 |
| Healthy status | ||||
| Healthy subjects | 2 | 1.07 (0.96, 1.20) | 0 | 0.227 |
| Chronic disease | 2 | 0.85 (0.60, 1.19) | 80.6 | 0.33 |
| Infectious disease | 1 | 0.62 (0.40, 0.95) | NA | 0.028 |
| Unclear | 1 | 0.88 (0.76, 1.02) | NA | 0.089 |
| Pregnant or not | ||||
| Pregnant women | 2 | 0.78 (0.57, 1.08) | 56.7 | 0.14 |
| Normal adults | 4 | 0.97 (0.83, 1.13) | 67.4 | 0.676 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Liu, C.; Kuang, X.; Deng, Q.; Zhao, F.; Li, D. Effects of Multivitamin and Multimineral Supplementation on Blood Pressure: A Meta-Analysis of 12 Randomized Controlled Trials. Nutrients 2018, 10, 1018. https://doi.org/10.3390/nu10081018
Li K, Liu C, Kuang X, Deng Q, Zhao F, Li D. Effects of Multivitamin and Multimineral Supplementation on Blood Pressure: A Meta-Analysis of 12 Randomized Controlled Trials. Nutrients. 2018; 10(8):1018. https://doi.org/10.3390/nu10081018
Chicago/Turabian StyleLi, Kelei, Chunxiao Liu, Xiaotong Kuang, Qingxue Deng, Feng Zhao, and Duo Li. 2018. "Effects of Multivitamin and Multimineral Supplementation on Blood Pressure: A Meta-Analysis of 12 Randomized Controlled Trials" Nutrients 10, no. 8: 1018. https://doi.org/10.3390/nu10081018




