1. Introduction
IgE-mediated food allergy is an adverse food reaction triggered by the ingestion of allergenic proteins in sensitized individuals. This disorder affects more than 1%, but less than 10%, of the general population [
1]. Sensitization per se is not enough to trigger the symptoms associated with allergic reactions, but is essential for potential IgE-mediated allergic disease. The clinical manifestations of the condition vary from mild and transient symptoms to life-threatening anaphylaxis. Certainly, most cases of food allergy are associated with a limited number of allergens, such as peanut, tree nuts, hen’s egg, cow’s milk, fish and shellfish, although the number of food allergens is large [
2,
3]. This could be attributed to an increased ability of these foods to sensitize and trigger allergic reactions and suggests that “a spectrum of allergenic or sensitizing potentials exists amongst food proteins” [
4]. If such a spectrum does exist, the sensitizing potential of proteins, defined as the inherent capacity of proteins to trigger an IgE-mediated immune response, could be assessed not only in naturally occurring protein allergens, but also in food proteins derived from transgene products or proteins modified upon food processing.
One study suggested that only an in vivo model could be used to evaluate the allergenicity of proteins resulting from de novo sensitization [
5], but there are currently no validated animal models available to evaluate the sensitizing or allergenic potential of proteins [
6]. A model for the evaluation of such potentials should be able to identify and distinguish commonly allergenic from rarely allergenic proteins [
7] by utilizing a standardized adjuvant-free sensitization procedure. Although sensitization to proteins via intraperitoneal (ip) injection has some limitations, it seems to be the method of choice in the mouse model. This is mainly because ip sensitization avoids induction of oral tolerance to administered protein.
The BALB/c mouse strain is considered to favor type 2 immune responses with atopic-like phenotype [
8,
9], therefore protocols of ip sensitization of BALB/c mice have been proposed [
10,
11]. Notably, these protocols generate consistent and reproducible results from an intra-protocol point of view. However, the robustness of the IgE antibody response triggered after the ip administration of the reference allergen ovalbumin (OVA; the major allergenic component of egg protein) significantly differ between the proposed BALB/c protocols [
11]. In order to validate animal models for the evaluation of the sensitizing potential of proteins, the sensitization protocols should be evaluated using common, weak, and rare allergens. In particular, to evaluate whether the protocol distinguishes commonly allergenic from rarely allergenic proteins. Thus, the aim of this study was to evaluate the use of the BALB/c mouse model to measure sensitizing potential of the common allergens OVA and cow milk protein (CMP), and the rarely allergenic potato protein (PAP) using three different IgE protocols.
2. Materials and Methods
2.1. Animals
Six- to Eight-week-old female BALB/c mice were purchased from Bioterium Claude Bernard (Benemérita Universidad Autónoma de Puebla, Puebla, México). The mice were maintained on a cow’s milk-egg-potato protein-free standard diet for at least three generations (Mazuri Rat and Mouse Diet #5663) and housed in an animal room at 23 ± 3 °C and 50 ± 10% relative humidity with a 12:12-h light-dark cycle. Water and diet were available ad libitum. The ethics review board of the Autonomous University of Sinaloa (Universidad Autónoma de Sinaloa) approved the study design (Ethical approval number: CE-UACNyG-2014-JUL-001).
2.2. Test Materials
The reference allergen OVA (grade V ≥98% pure) and the hypoallergenic potato acid phosphatase (PAP, 0.5–3.0 unit/mg solid) [
10,
12] were obtained from Sigma Chemical. Cow’s milk protein rich in casein (milk protein) was obtained from MP Biomedicals (Solon, OH, USA) (protein content ≥ 99%, Cat. 0219509605-5). For ip injection, the proteins were solubilized in sterile PBS 7.4 (SIGMA) as follows: (a) 0.02% OVA solution, (b) 10% PAP solution, and (c) 1, 0.5 and 0.1 mg/mL milk protein solutions. Milk protein was macerated until a fine powder was obtained. Samples (5 mg/mL) of milk protein powder in PBS were heated at 50 °C for 1 h with continuous shaking (400 RPM) in a ThermoMixer
® C (Eppendorf, Hamburg, Germany). After this, the casein samples were centrifuged at 13,400×
g for 5 min and the supernatants collected and stored at −80 °C until their use. The protein content of the milk protein preparations was determined using the bicinchoninic acid method according to the manufacturer’s instructions (BCA assay, PierceTM Thermo Scientific, Rockford, IL, USA).
2.3. Milk Protein Gel Electrophoresis
The purity analysis of cow milk proteins (MP Biomedicals) was carried out by gel electrophoresis in reducing conditions (SDS-PAGE) according to the method of Laemmli [
13]. Commercially available 4–15% polyacrylamide electrophoresis gels were utilized (Mini-PROTEAN
®TGX Stain-Free, BIO-RAD, Hercules, California, USA). Standard markers (BIO-RAD, Cat. 161-0363) containing 10 proteins ranging in size from 10 to 250 KDa were included on the gels. The cow milk proteins were dissolved in 1X Laemmli buffer (BIO-RAD, Cat. 161-0747) to a final concentration of 1 mg/mL. Samples (20 μL) at different concentrations (2, 4, 6, 8, 10, 12, 14, 16 μg of cow milk proteins) were subjected to SDS-PAGE. Protein bands were visualized with the ChemiDoc Imaging System (BIO-RAD) and analyzed with the Image Lab™ Software version 5.2.1 (BIO-RAD).
2.4. Sensitization Procedure
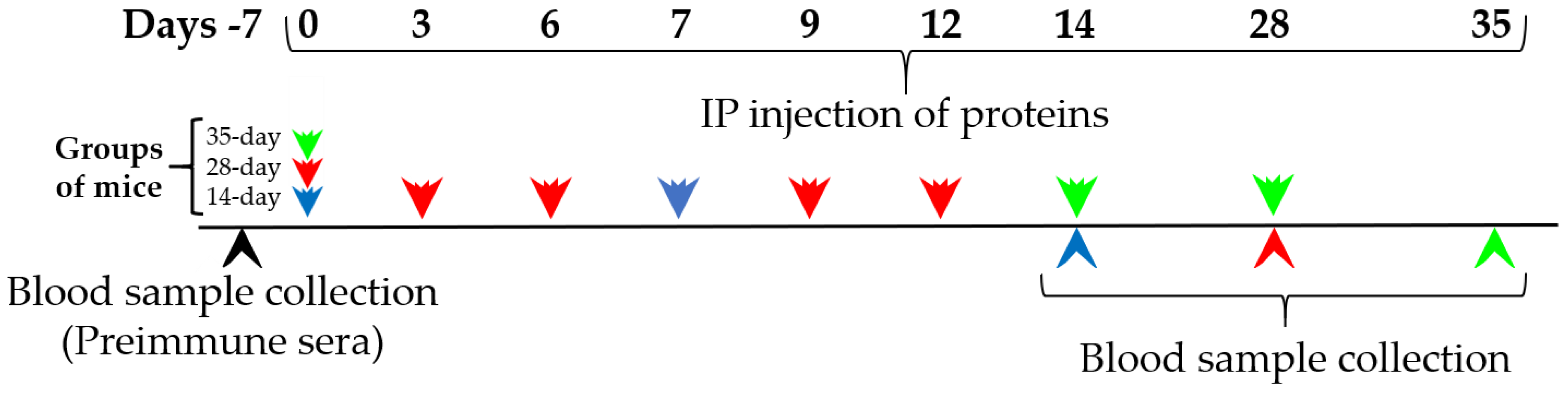
Groups of mice (n = 4–6) were injected with 250 μL of 0.02% OVA (0.05 mg per mouse), 10% PAP (250.0 mg per mouse) or a range of concentrations of CMP (1, 0.2 and 0.1 mg/mL) in PBS. The control groups (n = 6) were ip injected with 250 μL of PBS only (Sigma-Aldrich cat P5368, Saint Louis, Missouri). The immunization treatments with OVA, PAP and CMP were repeated at different time points: days 0 and 7 for 14-day protocol, days 0, 3, 6, 9 and 12 for 28-day protocol, and days 0, 14 and 28 for 35-day protocol. The mice were exsanguinated (from the tail vein) after 14, 28 or 35 days following exposure to the proteins. The three protocols are described in short in
Figure 1. Individual serum samples were stored at −80 °C until analysis.
2.5. IgG Depletion of Serum Samples
Serum samples were diluted 1:10 with ELISA diluent (BioLegend. Cat. 408804, San Diego, CA, USA). IgG antibodies were removed from the diluted serum samples. For this purpose, we took advantage of the SureBeads™ Magnetic Beads system (BIO-RAD. Cat. 161-4823). The system contains magnetic beads designed to capture IgG or IgA antibodies. The beats can attach IgG or IgA antibodies from the FC region. The procedure was carried as follows: 100 μL of magnetic beads (SureBeads protein G) were transferred to 1.5 mL tubes. The beads were washed three times with 1 mL of PBS + 0.01% Tween 20. After magnetization of the beads, the wash solution was discarded and 350 μL of diluted serum samples were added into the tube. Samples were left rotating for 10 min at RT and magnetized again. The supernatants (serum samples depleted in IgG) were collected and stored at −80 °C until analysis.
2.6. Specific Antibody Analyses
OVA-, PAP- or cow milk protein-specific IgE and IgG were detected using enzyme-linked immunosorbent assay (ELISA). Ninety six-well flat-bottomed polystyrene plates (NUNC Maxisorb #442404) were coated with 20 μg of individual proteins in 100 μL of coating buffer pH 9.5 (BioLegend. Cat. 421701). The plates were incubated overnight at 4 °C, then washed twice with PBS + 0.05% Tween 20 and incubated for 2 h at RT with 200 μL of blocking solution (10% FCS in PBS). The blocking solution was discarded and 100 μL of serum samples diluted 1:10 in ELISA diluent (BioLegend, cat. 421203) were added to individual wells. After overnight incubation at 4 °C, wells were washed three times with PBS + 0.05% Tween 20. 100 μL of the biotinylated detection antibody at 2 μg/mL (Biotin rat IgG1 anti-mouse IgE, BioLegend. Cat. 408804) was added to each well and incubated for 1 h at RT. Wells were washed as before, then 100 μL of streptavidin-horseradish peroxidase (Diluted 1:1000 in ELISA diluent, BioLegend. Cat. 405210) was added to each well and incubated for 30 min at RT. Finally, the assays were developed with 100 μL of tetramethylbenzidine (TMB, Thermo Scientific, Cat. 34028, Rockford, IL, USA) substrate for 30 min at RT. H2SO4 2M (50 μL) was used as stop solution. An automated ELISA reader (Multiskan™ FC Microplate Photometer, Thermo Scientific, Cat. 34028, Rockford, IL, USA) was used to measure absorbance at 450 nm. The specific IgG antibodies were determined as stated above, but serum samples were diluted 1:1000 and the biotinylated detection antibody was an anti-mouse IgG at 2 μg/mL (Diluted 1-250 in ELISA diluent, BioLegend. Cat. 405303). The assays were developed for 5 min at RT.
2.7. Statistical Analysis
Data were analyzed using GraphPad Prism Version 5.0 (GraphPad Software, San Diego, CA, USA). Normality testing of the data was carried out using Kolmogorov–Smirnov test. Unpaired t-tests were used to compare the difference between two different groups and paired t-tests were used to compare the levels of antibodies in the same group of animals. One-way ANOVA followed by Tukey’s multiple comparison test for comparison of more than two groups. When normality test failed, Kruskall–Wallis tests followed by Dunn’s multiple comparison tests were used for comparison of more than two groups. Significance was taken to be p < 0.05. IgG or IgE antibody responses to antigen were assessed following subtraction of background responses (pre-immune serum samples).
4. Discussion
Mouse models of allergy to a variety of food allergens have been developed [
14], but there are no mouse models available to evaluate the inherent sensitizing or allergenic potential of proteins. A model for the evaluation of the previously mentioned potentials requires specific characteristics, such as adjuvant-free conditions, capability to discriminate between commonly allergenic and rarely allergenic proteins, capability to detect allergens that hardly triggers IgE responses under adjuvant-free experimental conditions, and capability to discriminate among common, weak, and rare allergens. Particularly, CMP hardly sensitize mice under adjuvant-free experimental conditions although it is a well-known allergen [
15,
16]. Contrarily, adjuvant-free protocols to sensitize mice to OVA have been well-documented [
11,
14,
17,
18]. In this study, BALB/c mice were used to assess the sensitizing potentials of two commonly allergenic proteins (OVA and CMP) and one rarely allergenic protein (PAP). Two previously published protocols of ip sensitization were followed in detail [
8,
9,
11]. An additional 35-day protocol of ip sensitization was evaluated for comparative purposes, but no anti-CMP IgE immune responses were triggered in the mice that underwent this protocol. These results highlight that the source of allergen is of particular relevance for the evaluation of an animal model for assessing the inherent sensitizing or allergenic potential of proteins.
Previous studies have shown that a 14-day protocol with two ip injections can distinguish commonly allergenic from rarely allergenic proteins [
17,
18]. However, in these studies the IgE immune response was evaluated by passive cutaneous anaphylaxis assays, an assay that in our hands is technically demanding and difficult to implement. Alternatively, a 28-day protocol to evaluate the allergenic potential of proteins has been proposed [
11]. The authors claim that the 28-day protocol triggers more robust IgE immune responses than the 14-day using the reference standard OVA [
11] and, notably, the anti-OVA IgE antibodies produced could be readily detected using ELISA. Our results highlight that BALB/c mice that underwent the 28-day protocol with five ip injections and OVA doses of 0.05 mg triggered significantly higher anti-OVA IgE responses than the 14-day protocol with two ip injections. Furthermore, the 28-day protocol not only triggers robust IgE immune responses, but also can discriminate between commonly allergenic and rarely allergenic proteins, such as OVA and PAP respectively. Therefore, our data support the notion that the 28-day protocol is more suitable than 14-day to develop a BALB/c mouse model to evaluate the inherent sensitizing or allergenic potential of proteins.
To corroborate our data with previous findings, the three protocols were carried out utilizing CMP. This commonly allergenic protein was chosen because there is scarce information describing adjuvant-free protocols to sensitize mice to CMP [
14]. Previous studies have shown that a six-week-transdermal sensitization protocol is effective to sensitize BALB/c mice to CMP under adjuvant-free conditions [
15]. Others have shown that BALB/c mice can be sensitized to CMP without the use of adjuvants after ip injections, but the mice have to be born and grew up in germ-free conditions [
16]. The 28-day protocol has the advantages of being shorter than the six-week-transdermal sensitization procedure, and that BALB/c mice can be sensitized after ip injections without the need of germ-free animal facilities. Furthermore, our results show that the 28-day protocol can detect allergens that hardly trigger IgE immune responses under adjuvant-free experimental conditions. Most notably, the IgE responses are robust in a dose dependent manner and are readily detected using ELISA. Overall, the findings highlight a critical role for the dose of allergen, as well as the number and frequency of immunizations, to successfully sensitize BALB/c mice to food proteins, as stated by others [
16,
19].
Additionally, to show that the undetectable serum levels of anti-CMP IgE antibodies from the 14-day protocol mice were not due to IgG masking of epitopes [
20,
21], the serum levels of anti-CMP IgE antibodies were evaluated after IgG depletion. After the depletion treatment, anti-CMP IgE antibodies remained undetectable in most serum samples. Contrary, serum samples with detectable levels of anti-CMP IgE antibodies significantly increased the level of detection after IgG depletion showing that IgG masking of epitopes negatively impact on the detection of allergen-specific IgE antibodies.
Animal models have been widely used to evaluate the immune response to antigens in health and disease. The mouse seems to be the animal model of choice to evaluate the inherent sensitizing and allergenic potential of proteins, largely due to the availability of many mouse-specific immunological reagents, their short generation time, the possibility of large experimental groups of animals, and the low cost of purchase and maintenance [
22]. Furthermore, it has been reported that the overall structure of the mouse immune system is quite similar to humans [
23]. Certainly, the genetic background of each mouse strain has a close association with the type and intensity of the immune response they trigger after antigen exposure, and this characteristic should be taken into account in the search of a mouse model to evaluate the inherent sensitizing or allergenic potential of food proteins [
24]. Notably, the BALB/c mouse strain is considered to favor type 2 immune responses with atopic-like phenotype) [
8,
9,
25,
26]. Thus, this mouse strain has been widely used in the search for a validated murine model to evaluate the sensitizing or allergenic potential of proteins [
4,
5,
8,
9,
11].
We should acknowledge that our study has some limitations. Firstly, the anti-IgG immune responses using the 35-day protocol were evaluated in four mice only, and groups of a minimum of five mice have been suggested to evaluate the allergenic potential of food proteins [
27]. Furthermore, due to the inter-individual variability in the immune responses, more than six mice should be tested. This consideration should be taken into account in future studies. Secondly, weak allergens were not tested in this study although the results justify their evaluation. Further studies to demonstrate the capability of the 28-day protocol to discriminate between strong and weak allergens are warranted. Thirdly, our data do not support an explanation about the reasons why the 14-day protocol efficiently sensitizes to OVA, but not to CMP. Despite the previous, the results highlight that the 28-day protocol with five ip immunizations triggers a robust IgE immune response that is readily detected using ELISA, can differentiate between commonly allergenic and rarely allergenic proteins, and can detect allergens that hardly trigger IgE immune responses under adjuvant-free experimental conditions. Therefore, the 28-day protocol could be used as an animal model to evaluate the inherent sensitizing or allergenic potential of naturally occurring proteins, biotechnology-derived proteins, or the impact of food processing on the allergenic potential of proteins.