Kahweol Ameliorates the Liver Inflammation through the Inhibition of NF-κB and STAT3 Activation in Primary Kupffer Cells and Primary Hepatocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemical
2.2. Isolation of Primary Kupffer Cells (KC) and Primary Hepatocytes (HC)
2.3. Plating of Primary KC and Primary HC Co-Cultures
2.4. Quantitative Real-Time (qRT)-RCR
2.5. Determination of Inflammatory Cytokine Levels
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
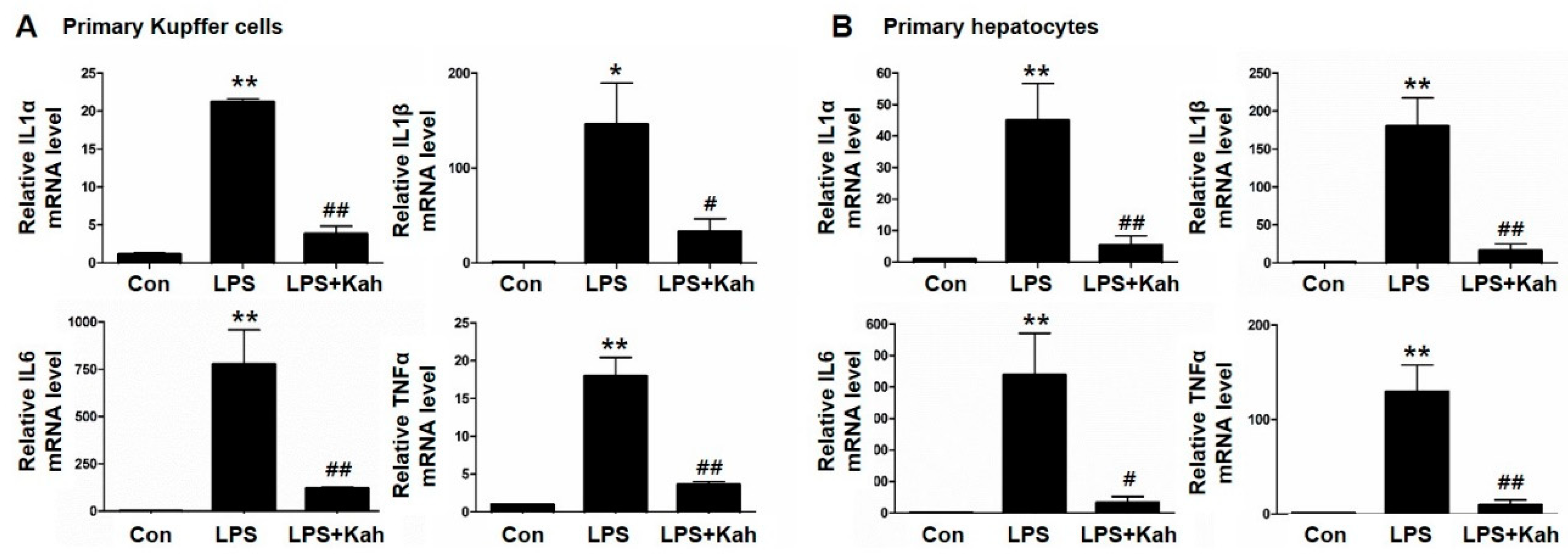
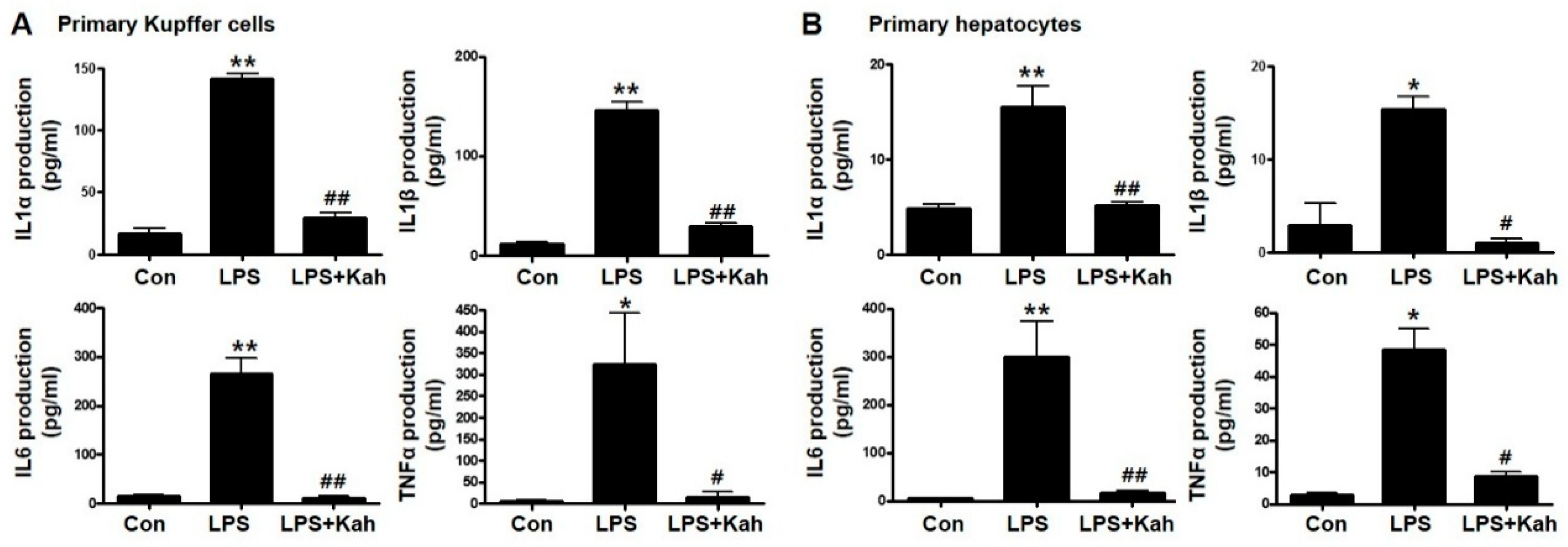
3.1. Kahweol Inhibited LPS-Stimulated Inflammatory Cytokine Levels in Primary KC and Primary HC
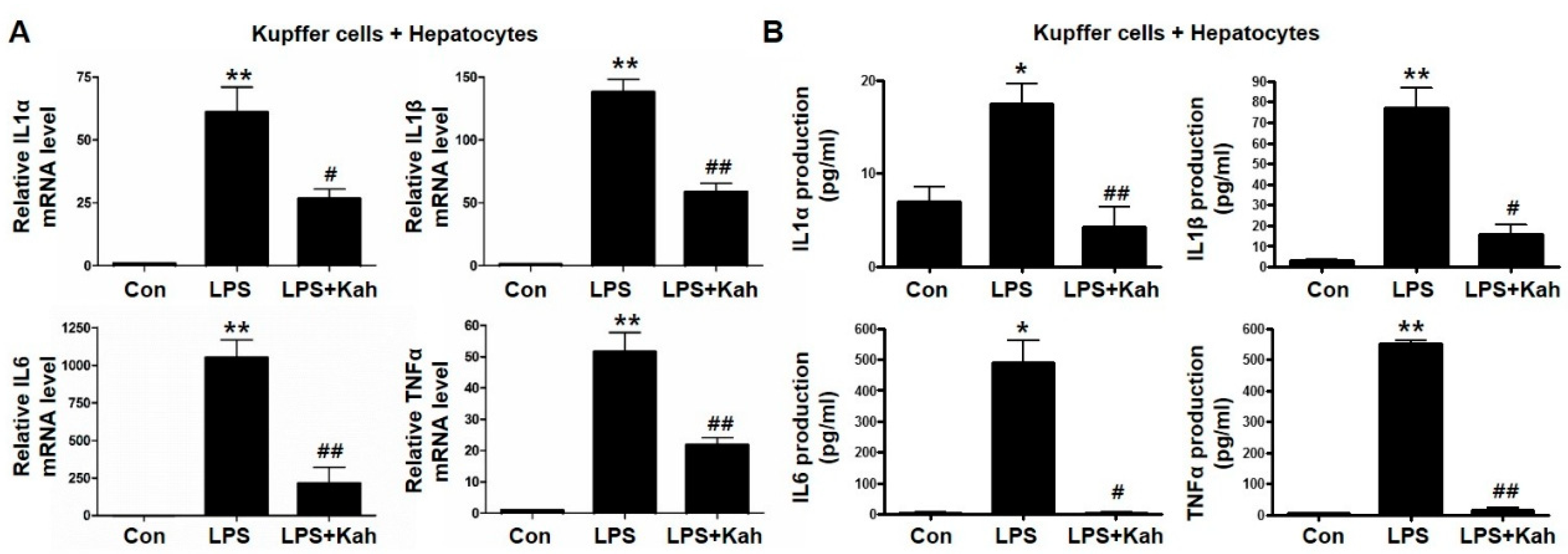
3.2. Kahweol Inhibited Inflammatory Cytokine Levels in Primary KC and Primary HC Co-Cultures
3.3. Kahweol Inhibited the NF-κB Activation
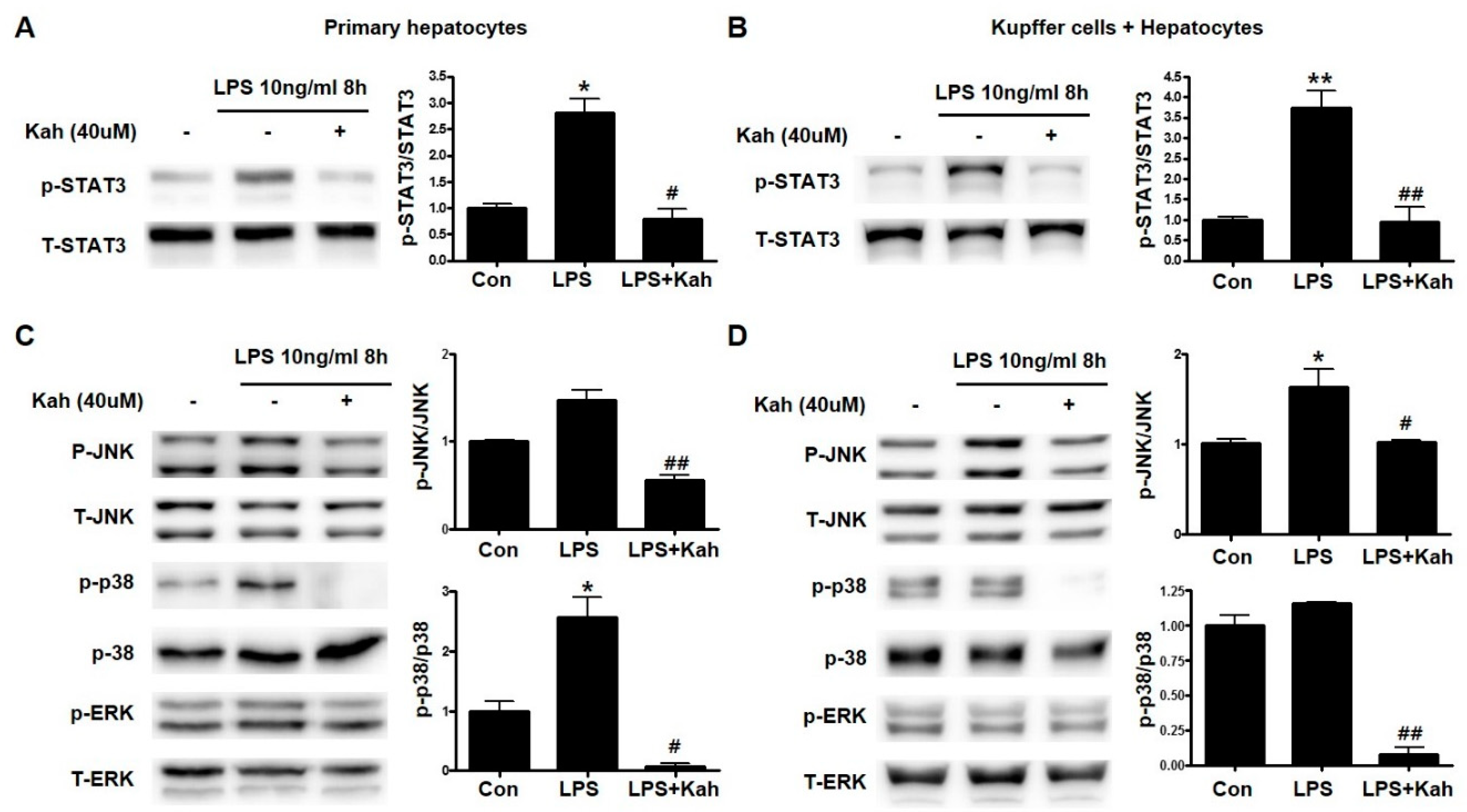
3.4. Involvement of STAT3 and Mitogen-Activated Protein Kinase (MAPK) Activation in the Inhibitory Effect of Kahweol on Inflammation
3.5. Kahweol Inhibited Primary HC Inflammation Induced by the Conditioned Media Obtained from LPS Treated Primary KC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Larrosa, M.; Azorin-Ortuno, M.; Yanez-Gascon, M.J.; Garcia-Conesa, M.T.; Tomas-Barberan, F.; Espin, J.C. Lack of Effect of Oral Administration of Resveratrol in Lps-Induced Systemic Inflammation. Eur. J. Nutr. 2011, 50, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Sebai, H.; Ben-Attia, M.; Sani, M.; Aouani, E.; Ghanem-Boughanmi, N. Protective Effect of Resveratrol in Endotoxemia-Induced Acute Phase Response in Rats. Arch. Toxicol. 2009, 83, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Bala, S.; Petrasek, J.; Gattu, A. Gut-Liver Axis and Sensing Microbes. Dig. Dis. 2011, 28, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I.; Ward, K.; Peters, T. The Leaky Gut of Alcoholism: Possible Route of Entry for Toxic Compounds. Lancet 1984, 323, 179–182. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Farhadi, A.; Forsyth, C.B.; Rangan, J.; Jakate, S.; Shaikh, M.; Banan, A.; Fields, J.Z. Evidence That Chronic Alcohol Exposure Promotes Intestinal Oxidative Stress, Intestinal Hyperpermeability and Endotoxemia Prior to Development of Alcoholic Steatohepatitis in Rats. J. Hepatol. 2009, 50, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J. Hepatic Inflammation and Progressive Liver Fibrosis in Chronic Liver Disease. World J. Gastroenterol. 2014, 20, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Mehal, W.Z. Sterile Inflammation in the Liver. Gastroenterology 2012, 143, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Ai, Q.; Lin, L.; Dai, J.; Jia, M.; Zhou, D.; Che, Q.; Wan, J.; Jiang, R.; Zhang, L. Protective Effects of Garcinol in Mice with Lipopolysaccharide/D-Galactosamine-Induced Apoptotic Liver Injury. Int. Immunopharmacol. 2014, 19, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, D.X.; Lv, J.-W.; Ning, H.; Wei, W. Melatonin Attenuates Lipopolysaccharide (Lps)-Induced Apoptotic Liver Damage in D-Galactosamine-Sensitized Mice. Toxicology 2007, 237, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor Nf-Kappab Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Huang, S.S.; Deng, J.S. Anti-Inflammatory Activities of Inotilone from Phellinus Linteus through the Inhibition of Mmp-9, Nf-Kappab, and Mapk Activation in Vitro and in Vivo. PLoS ONE 2012, 7, e35922. [Google Scholar] [CrossRef]
- He, G.; Karin, M. Nf-Kappab and Stat3—Key Players in Liver Inflammation and Cancer. Cell Res. 2011, 21, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Li Volti, G.; Galvano, F.; Grosso, G. Coffee Components and Cardiovascular Risk: Beneficial and Detrimental Effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Sciacca, S.; Pajak, A.; Martinez-Gonzalez, M.A.; Giovannucci, E.L.; Galvano, F. Coffee Consumption and Risk of All-Cause, Cardiovascular, and Cancer Mortality in Smokers and Non-Smokers: A Dose-Response Meta-Analysis. Eur. J. Epidemiol. 2016, 31, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Salomone, F.; Godos, J.; Pluchinotta, F.; Del Rio, D.; Mistretta, A.; Grosso, G. Coffee and Tea Consumption in Relation with Non-Alcoholic Fatty Liver and Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. 2016, 35, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Bes-Rastrollo, M.; Galvano, F.; Martinez-Gonzalez, M.A. Long-Term Coffee Consumption is Associated with Decreased Incidence of New-Onset Hypertension: A Dose-Response Meta-Analysis. Nutrients 2017, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, M.; Larsson, S.C. Coffee Consumption and Reduced Risk of Developing Type 2 Diabetes: A Systematic Review with Meta-Analysis. Nutr. Rev. 2018, 76, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, S.; Zhu, C.; Huang, H.; Wu, L.; Wan, X.; Yang, X.; Zhang, H.; Miao, R.; He, L.; et al. Coffee and Cancer Risk: A Meta-Analysis of Prospective Observational Studies. Sci. Rep. 2016, 6, 33711. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Jaccaud, E.; Huggett, A.C. Analysis of the Content of the Diterpenes Cafestol and Kahweol in Coffee Brews. Food Chem. Toxicol. 1997, 35, 547–554. [Google Scholar] [CrossRef]
- Cardenas, C.; Quesada, A.R.; Medina, M.A. Anti-Angiogenic and Anti-Inflammatory Properties of Kahweol, a Coffee Diterpene. PLoS ONE 2011, 6, e23407. [Google Scholar] [CrossRef]
- Tao, K.S.; Wang, W.; Wang, L.; Cao, D.Y.; Li, Y.Q.; Wu, S.X.; Dou, K.F. The Multifaceted Mechanisms for Coffee’s Anti-Tumorigenic Effect on Liver. Med. Hypotheses 2008, 71, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Micek, A.; Marranzano, M.; Salomone, F.; Rio, D.D.; Ray, S. Coffee Consumption and Risk of Biliary Tract Cancers and Liver Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutrients 2017, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.Y.; Jung, Y.A.; Lee, S.H.; Hwang, J.S.; Park, K.G.; Kim, M.K.; Jang, B.K. Kahweol Decreases Hepatic Fibrosis by Inhibiting the Expression of Connective Tissue Growth Factor Via the Transforming Growth Factor-Beta Signaling Pathway. Oncotarget 2017, 8, 87086–87094. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Ben-Ari, Z.; Masoud, R.; Pappo, O.; Harats, D.; Kamari, Y.; Safran, M. Interleukin-1α and Interleukin-1β Play a Central Role in the Pathogenesis of Fulminant Hepatic Failure in Mice. PLoS ONE 2017, 12, e0184084. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. Il-6 Pathway in the Liver: From Physiopathology to Therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer Cells in the Liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, K.A.; Holman, N.S.; Green, A.M.; Andersen, M.E.; LeCluyse, E.L. Co-Culture of Hepatocytes and Kupffer Cells as an In vitro Model of Inflammation and Drug-Induced Hepatotoxicity. J. Pharm. Sci. 2016, 105, 950–964. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-κB in the Liver—Linking Injury, Fibrosis and Hepatocellular Carcinoma. Nature reviews. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of Kupffer Cells in the Pathogenesis of Liver Disease. World J. Gastroenterol. 2006, 12, 7413–7420. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, T.; Froh, M.; Arteel, G.E.; Bradford, B.U.; Thurman, R.G. Toll-Like Receptor 4 Is Involved in the Mechanism of Early Alcohol-Induced Liver Injury in Mice. Hepatology 2001, 34, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Parlesak, A.; Schafer, C.; Schutz, T.; Bode, J.C.; Bode, C. Increased Intestinal Permeability to Macromolecules and Endotoxemia in Patients with Chronic Alcohol Abuse in Different Stages of Alcohol-Induced Liver Disease. J. Hepatol. 2000, 32, 742–747. [Google Scholar] [CrossRef]
- Rao, R. Endotoxemia and Gut Barrier Dysfunction in Alcoholic Liver Disease. Hepatology 2009, 50, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-Like Receptor-4 Signaling and Kupffer Cells Play Pivotal Roles in the Pathogenesis of Non-Alcoholic Steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut Microbiome and Liver Diseases. Gut 2016, 65, 2035–3044. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G. Gut-Liver Axis in Alcoholic Liver Disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, K.S.; Jeong, H.G. Suppressive Effects of the Kahweol and Cafestol on Cyclooxygenase-2 Expression in Macrophages. FEBS Lett. 2004, 569, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, K.S.; Lee, K.J.; Na, H.K.; Chun, H.K.; Kho, Y.H.; Jeong, H.G. The Coffee Diterpene Kahweol Suppress the Inducible Nitric Oxide Synthase Expression in Macrophages. Cancer Lett. 2004, 213, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Park, Y.C.; Kim, S.H.; Lee, J.; Cho, J.Y. Nuclear Factor-Kappab/Signal Transducers and Activators of Transcription-1-Mediated Inflammatory Responses in Lipopolysaccharide-Activated Macrophages Are a Major Inhibitory Target of Kahweol, a Coffee Diterpene. Biol. Pharm. Bull. 2010, 33, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Hwang, Y.P.; Jeong, H.G. Kahweol Blocks Stat3 Phosphorylation and Induces Apoptosis in Human Lung Adenocarcinoma A549 Cells. Toxicol. Lett. 2009, 187, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Ukairo, O.; Khetani, S.R.; McVay, M.; Kanchagar, C.; Seghezzi, W.; Ayanoglu, G.; Irrechukwu, O.; Evers, R. Establishment of a Hepatocyte-Kupffer Cell Coculture Model for Assessment of Proinflammatory Cytokine Effects on Metabolizing Enzymes and Drug Transporters. Drug Metab. Dispos. 2015, 43, 774–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoebe, K.H.N.; Witkamp, R.F.; Fink-Gremmels, J.; Van Miert, A.S.; Monshouwer, M. Direct Cell-to-Cell Contact between Kupffer Cells and Hepatocytes Augments Endotoxin-Induced Hepatic Injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G720–G728. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.A.; Ganey, P.E.; Ju, C.; Kamendulis, L.M.; Rusyn, I.; Klaunig, J.E. Role of the Kupffer Cell in Mediating Hepatic Toxicity and Carcinogenesis. Toxicol. Sci. 2007, 96, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Khor, T.O.; Saw, C.L.; Lin, W.; Wu, T.; Huang, Y.; Kong, A.N. Role of Nrf2 in Suppressing Lps-Induced Inflammation in Mouse Peritoneal Macrophages by Polyunsaturated Fatty Acids Docosahexaenoic Acid and Eicosapentaenoic Acid. Mol. Pharm. 2010, 7, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Scollick, C.; Traore, K.; Yates, M.; Trush, M.A.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 Dependent Protection from Lps Induced Inflammatory Response and Mortality by Cddo-Imidazolide. Biochem. Biophys. Res. Commun. 2006, 351, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jiang, Y.F.; Ponnusamy, M.; Diallo, M. Role of Nrf2 in Chronic Liver Disease. World J. Gastroenterol. 2014, 20, 13079–13087. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Jeong, H.G. The Coffee Diterpene Kahweol Induces Heme Oxygenase-1 Via the Pi3k and P38/Nrf2 Pathway to Protect Human Dopaminergic Neurons from 6-Hydroxydopamine-Derived Oxidative Stress. FEBS Lett. 2008, 582, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear Heme Oxygenase-1 (Ho-1) Modulates Subcellular Distribution and Activation of Nrf2, Impacting Metabolic and Anti-Oxidant Defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.-Y.; Kim, M.-K.; Lee, S.-H.; Hwang, J.S.; Park, K.-G.; Jang, B.K. Kahweol Ameliorates the Liver Inflammation through the Inhibition of NF-κB and STAT3 Activation in Primary Kupffer Cells and Primary Hepatocytes. Nutrients 2018, 10, 863. https://doi.org/10.3390/nu10070863
Seo H-Y, Kim M-K, Lee S-H, Hwang JS, Park K-G, Jang BK. Kahweol Ameliorates the Liver Inflammation through the Inhibition of NF-κB and STAT3 Activation in Primary Kupffer Cells and Primary Hepatocytes. Nutrients. 2018; 10(7):863. https://doi.org/10.3390/nu10070863
Chicago/Turabian StyleSeo, Hye-Young, Mi-Kyung Kim, So-Hee Lee, Jae Seok Hwang, Keun-Gyu Park, and Byoung Kuk Jang. 2018. "Kahweol Ameliorates the Liver Inflammation through the Inhibition of NF-κB and STAT3 Activation in Primary Kupffer Cells and Primary Hepatocytes" Nutrients 10, no. 7: 863. https://doi.org/10.3390/nu10070863





