Intake of Baked Cod Fillet Resulted in Lower Serum Cholesterol and Higher Long Chain n-3 PUFA Concentrations in Serum and Tissues in Hypercholesterolemic Obese Zucker fa/fa Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Ethics
2.3. Analyses of Diets
2.4. Analyses in Serum
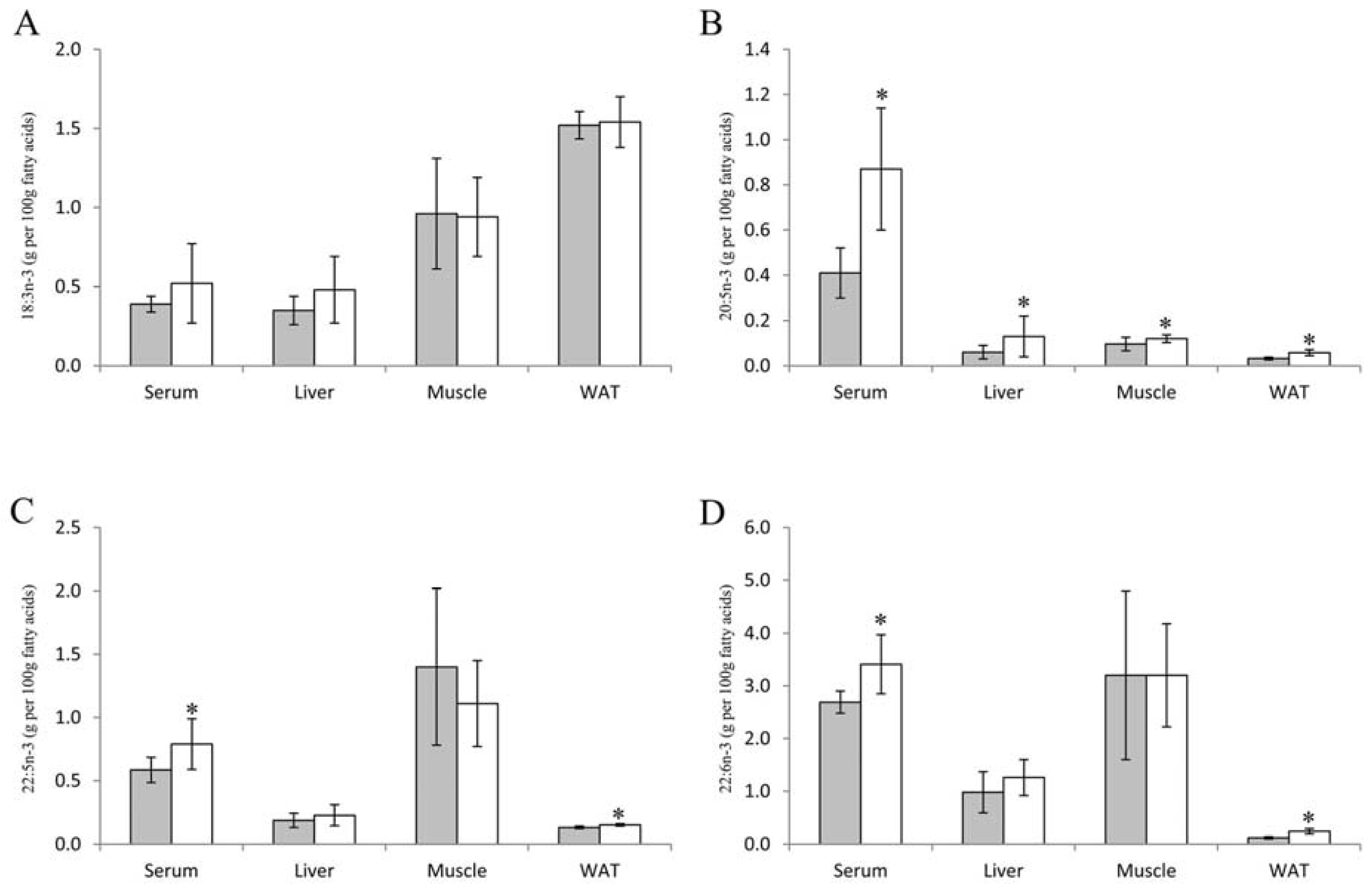
2.5. Fatty Acids in Serum, Liver, Muscle and Adipose Tissue
2.6. Analyses of Cholesterol in Liver, Muscle and Faeces
2.7. Bile Acids in Liver and Faeces
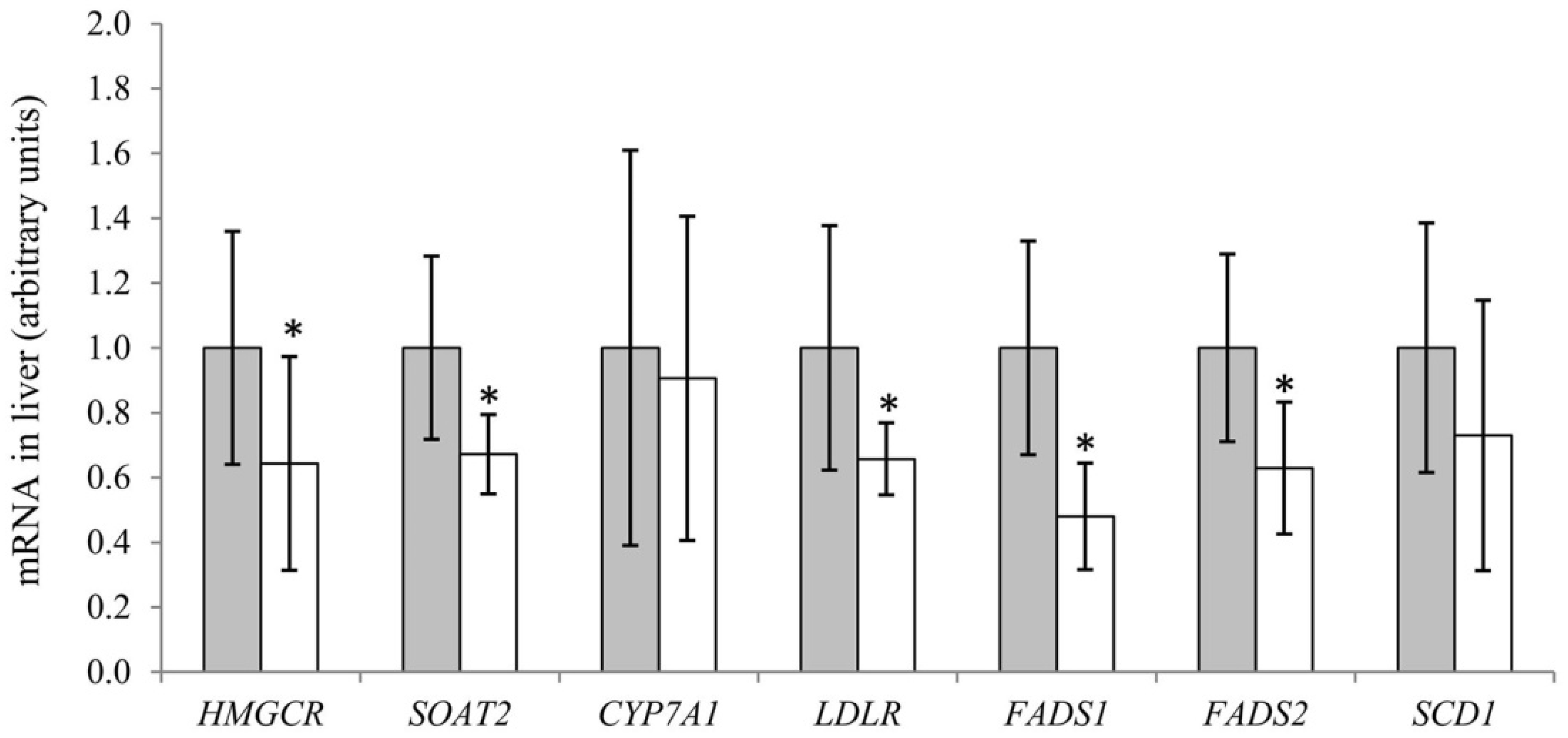
2.8. Analyses of mRNA Expression of Genes in Liver
2.9. Outcome Measurements
2.10. Sample Size
2.11. Statistical Analysis
3. Results
3.1. Diets and Anthropometry
3.2. Cholesterol and Bile Acids
3.3. Fatty Acids in Serum, Liver, Skeletal Muscle and White Adipose Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. Obesity and Overweight; Fact Sheet; World Health Organisation: Geneva, Switzerland, 2016; Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 11 September 2018).
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Noncommunicable Diseases; Fact Sheet; World Health Organisation: Geneva, Switzerland, 2017; Available online: http://www.who.int/mediacentre/factsheets/fs355/en/ (accessed on 19 February 2018).
- Prospective Studies Collaboration; Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [PubMed]
- Law, M.R.; Wald, N.J.; Thompson, S.G. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ 1994, 308, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. Fish oils and plasma lipid and lipoprotein metabolism in humans: A critical review. J. Lipid Res. 1989, 30, 785–807. [Google Scholar] [PubMed]
- Harris, W.S. N-3 fatty acids and serum lipoproteins: Human studies. Am. J. Clin. Nutr. 1997, 65, 1645S–1654S. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Barraj, L.M.; Kantor, M.; Sahyoun, N.R. Relationship between fish intake, n-3 fatty acids, mercury and risk markers of CHD (National Health and Nutrition Examination Survey 1999–2002). Public Health Nutr. 2009, 12, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; De Goede, J. Update on cardiometabolic health effects of omega-3 fatty acids. Curr. Opin. Lipidol. 2014, 25, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Eslick, G.D.; Howe, P.R.; Smith, C.; Priest, R.; Bensoussan, A. Benefits of fish oil supplementation in hyperlipidemia: A systematic review and meta-analysis. Int. J. Cardiol. 2009, 136, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Telle-Hansen, V.H.; Larsen, L.N.; Hostmark, A.T.; Molin, M.; Dahl, L.; Almendingen, K.; Ulven, S.M. Daily intake of cod or salmon for 2 weeks decreases the 18:1n-9/18:0 ratio and serum triacylglycerols in healthy subjects. Lipids 2012, 47, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, E.B.; Skou, H.A.; Christensen, J.H.; Dyerberg, J. N-3 fatty acids from fish and coronary artery disease: Implications for public health. Public Health Nutr. 2000, 3, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Bao, D.Q.; Burke, V.; Puddey, I.B.; Watts, G.F.; Beilin, L.J. Dietary fish as a major component of a weight-loss diet: Effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am. J. Clin. Nutr. 1999, 70, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsdottir, I.; Tomasson, H.; Kiely, M.; Martinez, J.A.; Bandarra, N.M.; Morais, M.G.; Thorsdottir, I. Inclusion of fish or fish oil in weight-loss diets for young adults: Effects on blood lipids. Int. J. Obes. 2008, 32, 1105–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindqvist, H.M.; Langkilde, A.M.; Undeland, I.; Sandberg, A.S. Herring (Clupea harengus) intake influences lipoproteins but not inflammatory and oxidation markers in overweight men. Br. J. Nutr. 2009, 101, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Tinker, L.F.; Parks, E.J.; Behr, S.R.; Schneeman, B.O.; Davis, P.A. (N-3) fatty acid supplementation in moderately hypertriglyceridemic adults changes postprandial lipid and apolipoprotein B responses to a standardized test meal. J. Nutr. 1999, 129, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Katz, R. N-3 long-chain polyunsaturated fatty acids in type 2 diabetes: A review. J. Am. Diet. Assoc. 2005, 105, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.J.; Economou, M.; Wallace, A.M.; Rumley, A.; Lowe, G.; Slater, C.; Caslake, M.; Sattar, N.; Lean, M.E. Benefits of salmon eating on traditional and novel vascular risk factors in young, non-obese healthy subjects. Atherosclerosis 2007, 193, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Mozaffarian, D.; Chiuve, S.E.; Rimm, E.B. Fish consumption and risk of major chronic disease in men. Am. J. Clin. Nutr. 2008, 88, 1618–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Huang, T.; Yu, Y.; Hu, X.; Yang, B.; Li, D. Fish consumption and CHD mortality: An updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012, 15, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Akinkuolie, A.O.; Wu, J.H.; Ding, E.L.; Gaziano, J.M. Fish consumption, omega-3 fatty acids and risk of heart failure: A meta-analysis. Clin. Nutr. 2012, 31, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung Yinko, S.S.; Stark, K.D.; Thanassoulis, G.; Pilote, L. Fish consumption and acute coronary syndrome: A meta-analysis. Am. J. Med. 2014, 127, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Aadland, E.K.; Lavigne, C.; Graff, I.E.; Eng, O.; Paquette, M.; Holthe, A.; Mellgren, G.; Jacques, H.; Liaset, B. Lean-seafood intake reduces cardiovascular lipid risk factors in healthy subjects: Results from a randomized controlled trial with a crossover design. Am. J. Clin. Nutr. 2015, 102, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Torris, C.; Molin, M.; Smastuen, M.C. Lean Fish Consumption Is Associated with Beneficial Changes in the Metabolic Syndrome Components: A 13-Year Follow-Up Study from the Norwegian Tromso Study. Nutrients 2017, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Vikoren, L.A.; Nygard, O.K.; Lied, E.; Rostrup, E.; Gudbrandsen, O.A. A randomised study on the effects of fish protein supplement on glucose tolerance, lipids and body composition in overweight adults. Br. J. Nutr. 2013, 109, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Demonty, I.; Deshaies, Y.; Jacques, H. Dietary proteins modulate the effects of fish oil on triglyceridemia in the rat. Lipids 1998, 33, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Beynen, A.C. Influence of dietary fish proteins on plasma and liver cholesterol concentrations in rats. Br. J. Nutr. 1993, 69, 767–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, C.; Galibois, I.; Jacques, H. Fasting and postprandial lipid and glucose metabolism are modulated by dietary proteins and carbohydrates: Role of plasma insulin concentrations. J. Nutr. Biochem. 1995, 6, 540–546. [Google Scholar] [CrossRef]
- De Artinano, A.A.; Castro, M.M. Experimental rat models to study the metabolic syndrome. Br. J. Nutr. 2009, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Kamanna, V.S.; Kirschenbaum, M.A. Association between very-low-density lipoprotein and glomerular injury in obese Zucker rats. Am. J. Nephrol. 1993, 13, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Poston, W.C.; Haddock, C.K. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis 2007, 193, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Weisnagel, S.J.; Marois, J.; Bergeron, J.; Julien, P.; Gougeon, R.; Tchernof, A.; Holub, B.J.; Jacques, H. Dietary cod protein reduces plasma C-reactive protein in insulin-resistant men and women. J. Nutr. 2008, 138, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Drotningsvik, A.; Mjos, S.A.; Pampanin, D.M.; Slizyte, R.; Carvajal, A.; Remman, T.; Hogoy, I.; Gudbrandsen, O.A. Dietary fish protein hydrolysates containing bioactive motifs affect serum and adipose tissue fatty acid compositions, serum lipids, postprandial glucose regulation and growth in obese Zucker fa/fa rats. Br. J. Nutr. 2016, 116, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Vikoren, L.A.; Drotningsvik, A.; Bergseth, M.T.; Mjos, S.A.; Mola, N.; Leh, S.; Mellgren, G.; Gudbrandsen, O.A. Effects of baked and raw salmon fillet on lipids and n-3 PUFAs in serum and tissues in Zucker fa/fa rats. Food Nutr. Res. 2017, 61, 1333395. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Mjos, S.A.; Joensen, H.; Grahl-Nielsen, O. Validation of a one-step extraction/methylation method for determination of fatty acids and cholesterol in marine tissues. J. Chromatogr. A 2006, 1104, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Sciotto, C.; Mjos, S.A. Trans isomers of EPA and DHA in omega-3 products on the European market. Lipids 2012, 47, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Wasta, Z.; Mjos, S.A. A database of chromatographic properties and mass spectra of fatty acid methyl esters from omega-3 products. J. Chromatogr. A 2013, 1299, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Drotningsvik, A.; Mjos, S.A.; Hogoy, I.; Remman, T.; Gudbrandsen, O.A. A low dietary intake of cod protein is sufficient to increase growth, improve serum and tissue fatty acid compositions, and lower serum postprandial glucose and fasting non-esterified fatty acid concentrations in obese Zucker fa/fa rats. Eur. J. Nutr. 2015, 54, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Suckling, K.E.; Benson, G.M.; Bond, B.; Gee, A.; Glen, A.; Haynes, C.; Jackson, B. Cholesterol lowering and bile acid excretion in the hamster with cholestyramine treatment. Atherosclerosis 1991, 89, 183–190. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hagen, I.V.; Helland, A.; Bratlie, M.; Brokstad, K.A.; Rosenlund, G.; Sveier, H.; Mellgren, G.; Gudbrandsen, O.A. High intake of fatty fish, but not of lean fish, affects serum concentrations of TAG and HDL-cholesterol in healthy, normal-weight adults: A randomised trial. Br. J. Nutr. 2016, 116, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Helland, A.; Bratlie, M.; Hagen, I.V.; Mjos, S.A.; Sornes, S.; Ingvar Halstensen, A.; Brokstad, K.A.; Sveier, H.; Rosenlund, G.; Mellgren, G.; et al. High intake of fatty fish, but not of lean fish, improved postprandial glucose regulation and increased the n-3 PUFA content in the leucocyte membrane in healthy overweight adults: A randomised trial. Br. J. Nutr. 2017, 117, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Farris, E.J.; Griffith, J.Q. The Rat in Laboratory Investigation; J. B. Lippincott Company: Philadelphia, PA, USA, 1949. [Google Scholar]
- Ramel, A.; Martinez, J.A.; Kiely, M.; Bandarra, N.M.; Thorsdottir, I. Effects of weight loss and seafood consumption on inflammation parameters in young, overweight and obese European men and women during 8 weeks of energy restriction. Eur. J. Clin. Nutr. 2010, 64, 987–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iritani, N.; Narita, R.; Fujita, T.; Tanaka, T. Effects of dietary fish protein, soybean protein and casein on cholesterol turnover in rats. J. Nutr. Sci. Vitaminol. 1985, 31, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ogawa, H.; Kishida, T.; Ebihara, K. The mechanism of the cholesterol-lowering effect of water-insoluble fish protein in ovariectomised rats. Br. J. Nutr. 2009, 102, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Ogawa, H.; Kishida, T.; Ebihara, K. Hypocholesterolaemic effect of water-insoluble fish protein from Alaska pollock in ovariectomised rats is not abolished by methionine addition. Br. J. Nutr. 2011, 106, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, A.; Bettzieche, A.; Hirche, F.; Brandsch, C.; Stangl, G.I.; Eder, K. Dietary fish protein alters blood lipid concentrations and hepatic genes involved in cholesterol homeostasis in the rat model. Br. J. Nutr. 2006, 96, 674–682. [Google Scholar] [PubMed]
- Hosomi, R.; Fukunaga, K.; Arai, H.; Kanda, S.; Nishiyama, T.; Yoshida, M. Fish protein decreases serum cholesterol in rats by inhibition of cholesterol and bile acid absorption. J. Food Sci. 2011, 76, H116–H121. [Google Scholar] [CrossRef] [PubMed]
- Wergedahl, H.; Liaset, B.; Gudbrandsen, O.A.; Lied, E.; Espe, M.; Muna, Z.; Mork, S.; Berge, R.K. Fish Protein Hydrolysate Reduces Plasma Total Cholesterol, Increases the Proportion of HDL Cholesterol, and Lowers Acyl-CoA: Cholesterol Acyltransferase Activity in Liver of Zucker Rats. J. Nutr. 2004, 134, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Wergedahl, H.; Gudbrandsen, O.A.; Rost, T.H.; Berge, R.K. Combination of fish oil and fish protein hydrolysate reduces the plasma cholesterol level with a concurrent increase in hepatic cholesterol level in high-fat-fed Wistar rats. Nutrition 2009, 25, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D.; Tepper, S.A.; Czarnecki, S.K.; Klurfeld, D.M. Atherogenicity of animal and vegetable protein. Influence of the lysine to arginine ratio. Atherosclerosis 1982, 41, 429–431. [Google Scholar] [CrossRef]
- Morita, T.; Oh-hashi, A.; Takei, K.; Ikai, M.; Kasaoka, S.; Kiriyama, S. Cholesterol-lowering effects of soybean, potato and rice proteins depend on their low methionine contents in rats fed a cholesterol-free purified diet. J. Nutr. 1997, 127, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, R.; Fukunaga, K.; Arai, H.; Kanda, S.; Nishiyama, T.; Yoshida, M. Effect of combination of dietary fish protein and fish oil on lipid metabolism in rats. J. Food Sci. Technol. 2013, 50, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Nara, Y.; Yamori, Y. Taurine accelerates the regression of hypercholesterolemia in stroke-prone spontaneously hypertensive rats. Life Sci. 1996, 58, 1643–1651. [Google Scholar] [CrossRef]
- Chen, W.; Guo, J.X.; Chang, P. The effect of taurine on cholesterol metabolism. Mol. Nutr. Food Res. 2012, 56, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J. The conjugation of glycine with cholic acid and benzoic acid in rat liver homogenate. Acta Chem. Scand. 1955, 9, 268–271. [Google Scholar] [CrossRef]
- Hosomi, R.; Fukunaga, K.; Arai, H.; Nishiyama, T.; Yoshida, M. Effects of dietary fish protein on serum and liver lipid concentrations in rats and the expression of hepatic genes involved in lipid metabolism. J. Agric. Food Chem. 2009, 57, 9256–9262. [Google Scholar] [CrossRef] [PubMed]
- Gudbrandsen, O.A.; Wergedahl, H.; Liaset, B.; Espe, M.; Berge, R.K. Dietary proteins with high isoflavone content or low methionine-glycine and lysine-arginine ratios are hypocholesterolaemic and lower the plasma homocysteine level in male Zucker fa/fa rats. Br. J. Nutr. 2005, 94, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.M.; Blond, J.P.; Juaneda, P.; Durand, G.; Bezard, J. Effect of low levels of dietary fish oil on fatty acid desaturation and tissue fatty acids in obese and lean rats. Lipids 1995, 30, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.R. Hormonal modulation of delta6 and delta5 desaturases: Case of diabetes. Prostaglandins Leukot. Essent. Fatty Acids 2003, 68, 151–162. [Google Scholar] [CrossRef]
- Bergen, W.G.; Mersmann, H.J. Comparative aspects of lipid metabolism: Impact on contemporary research and use of animal models. J. Nutr. 2005, 135, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- De Grooth, G.J.; Klerkx, A.H.; Stroes, E.S.; Stalenhoef, A.F.; Kastelein, J.J.; Kuivenhoven, J.A. A review of CETP and its relation to atherosclerosis. J. Lipid Res. 2004, 45, 1967–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulgoni, V.L., 3rd. Current protein intake in America: Analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008, 87, 1554S–1557S. [Google Scholar] [CrossRef] [PubMed]
- The Norwegian Directorate of Health. Norkost 3, National Dietary Survey Conducted among Adults in Norway Aged 18–70 Years (En Landsomfattende Kostholdsundersøkelse Blant Menn og Kvinner i Norge i Alderen 18–70 år, 2010–2011). 2012. Available online: http://helsedirektoratet.no/publikasjoner/norkost-3-en-landsomfattende-kostholdsundersokelse-blant-menn-og-kvinner-i-norge-i-alderen-18-70-ar/Sider/default.aspx (accessed on 19 October 2017).
- The Norwegian Directorate of Health. Recommendations Regarding Diet, Nutrition and Physical Activity (Anbefalinger om Kosthold, Ernæring og Fysisk Aktivitet). 2014. Available online: http://helsedirektoratet.no/publikasjoner/anbefalinger-om-kosthold-ernering-og-fysisk-aktivitet/Sider/default.aspx (accessed on 19 October 2017).
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
| g/kg Diet | Control Diet | Baked Cod Diet |
|---|---|---|
| Casein protein 1 | 216.22 | 162.16 |
| Freeze dried baked cod 2 | - | 62.50 |
| Corn starch | 511.67 | 503.22 |
| Sucrose | 90.00 | 90.00 |
| Cellulose | 50.00 | 50.00 |
| Soybean Oil | 70.00 | 70.00 |
| t-Butylhydroquinone | 0.014 | 0.014 |
| Mineral Mix (AIN-93-MX) | 35.00 | 35.00 |
| Vitamin Mix (AIN-93-VX) | 10.00 | 10.00 |
| L-Methionine | 1.60 | 1.60 |
| L-Cystine | 3.00 | 3.00 |
| Choline Bitartrate 3 | 2.50 | 2.50 |
| Growth and Maintenance Supplement 4 | 10.00 | 10.00 |
| Per kg Diet | Control Diet | Baked Cod Diet |
|---|---|---|
| Amino Acids, g | ||
| Arginine | 6.4 | 7.1 |
| Glycine | 3.6 | 4.2 |
| Histidine | 5.1 | 4.8 |
| Isoleucine | 9.2 | 8.9 |
| Leucine | 16.0 | 16.0 |
| Lysine | 14.0 | 14.0 |
| Methionine | 6.4 | 6.8 |
| Phenylalanine | 9.1 | 8.8 |
| Threonine | 7.2 | 7.3 |
| Valine | 12.0 | 12.0 |
| Taurine | ND | 0.3 |
| Lysine/ arginine ratio | 2.2 | 2.0 |
| Methionine/ glycine ratio | 1.8 | 1.6 |
| Fatty acids, g | ||
| 18:2n-6 | 32.7 | 30.4 |
| 18:3n-3 | 3.9 | 3.6 |
| 20:5n-3 | ND | 0.33 |
| 22:5n-3 | ND | 0.03 |
| 22:6n-3 | ND | 0.81 |
| Cholesterol, mg | 340 | 97 |
| Control Group | Baked Cod Group | p | |
|---|---|---|---|
| Bodyweight at endpoint, g | 548 ± 32 | 566 ± 20 | 0.17 |
| Body weight-to-square body length without tail ratio, kg/m2 | 9.8 ± 0.6 | 10.3 ± 0.4 | 0.062 |
| Relative liver weight, g/100g body weight | 4.5 ± 0.9 | 4.0 ± 0.6 | 0.11 |
| Relative thigh muscle weight, g/100 g body weight | 0.30 ± 0.04 | 0.29 ± 0.03 | 0.58 |
| Relative amount of WAT 1, g/100 g body weight | 7.4 ± 0.6 | 7.4 ± 0.5 | 0.97 |
| Faecal output, g dry weight/24 h | 2.8 ± 0.9 | 2.9 ± 0.9 | 0.76 |
| Energy intake, kcal/kg bodyweight/24 h | 266 ± 33 | 269 ± 51 | 0.91 |
| Control Group | Baked Cod Group | p | |
|---|---|---|---|
| Total cholesterol, mmol/L | 6.6 ± 1.1 | 5.6 ± 0.9 | 0.032 |
| HDL cholesterol, mmol/L | 5.3 ± 0.6 | 3.8 ± 1.6 | 0.018 |
| LDL cholesterol, mmol/L | 1.7 ± 0.6 | 1.1 ± 0.5 | 0.0025 |
| Total bile acids, umol/L | 43 ± 24 | 29 ± 18 | 0.17 |
| Control Group | Baked Cod Group | p | |
|---|---|---|---|
| Liver cholesterol, umol/g liver | 9.3 ± 2.4 | 8.2 ± 1.9 | 0.27 |
| Liver total bile acids, umol/g protein | 15.6 ± 2.4 | 16.6 ± 2.4 | 0.38 |
| Skeletal muscle cholesterol, umol/g | 2.5 ± 1.1 | 2.3 ± 1.2 | 0.71 |
| Faeces cholesterol, umol/24 h | 21.2 ± 12.9 | 18.2 ± 5.3 | 0.52 |
| Faeces total bile acids, umol/24 h | 8.8 ± 2.7 | 10.4 ± 4.1 | 0.36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikøren, L.A.; Drotningsvik, A.; Bergseth, M.T.; Mjøs, S.A.; Austgulen, M.H.; Mellgren, G.; Gudbrandsen, O.A. Intake of Baked Cod Fillet Resulted in Lower Serum Cholesterol and Higher Long Chain n-3 PUFA Concentrations in Serum and Tissues in Hypercholesterolemic Obese Zucker fa/fa Rats. Nutrients 2018, 10, 840. https://doi.org/10.3390/nu10070840
Vikøren LA, Drotningsvik A, Bergseth MT, Mjøs SA, Austgulen MH, Mellgren G, Gudbrandsen OA. Intake of Baked Cod Fillet Resulted in Lower Serum Cholesterol and Higher Long Chain n-3 PUFA Concentrations in Serum and Tissues in Hypercholesterolemic Obese Zucker fa/fa Rats. Nutrients. 2018; 10(7):840. https://doi.org/10.3390/nu10070840
Chicago/Turabian StyleVikøren, Linn A., Aslaug Drotningsvik, Marthe T. Bergseth, Svein A. Mjøs, Maren H. Austgulen, Gunnar Mellgren, and Oddrun A. Gudbrandsen. 2018. "Intake of Baked Cod Fillet Resulted in Lower Serum Cholesterol and Higher Long Chain n-3 PUFA Concentrations in Serum and Tissues in Hypercholesterolemic Obese Zucker fa/fa Rats" Nutrients 10, no. 7: 840. https://doi.org/10.3390/nu10070840





