A Double-Blind, Randomized Controlled, Acute Feeding Equivalence Trial of Small, Catalytic Doses of Fructose and Allulose on Postprandial Blood Glucose Metabolism in Healthy Participants: The Fructose and Allulose Catalytic Effects (FACE) Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Trial Design
2.3. Treatments
2.4. Protocol for Blood Sample Collection
2.5. Outcome Measures
2.6. Plasma Glucose and Insulin Analyses
2.7. Calculations
2.8. Statistical Analyses
3. Results
3.1. Flow of Participants
3.2. Participant Characteristics
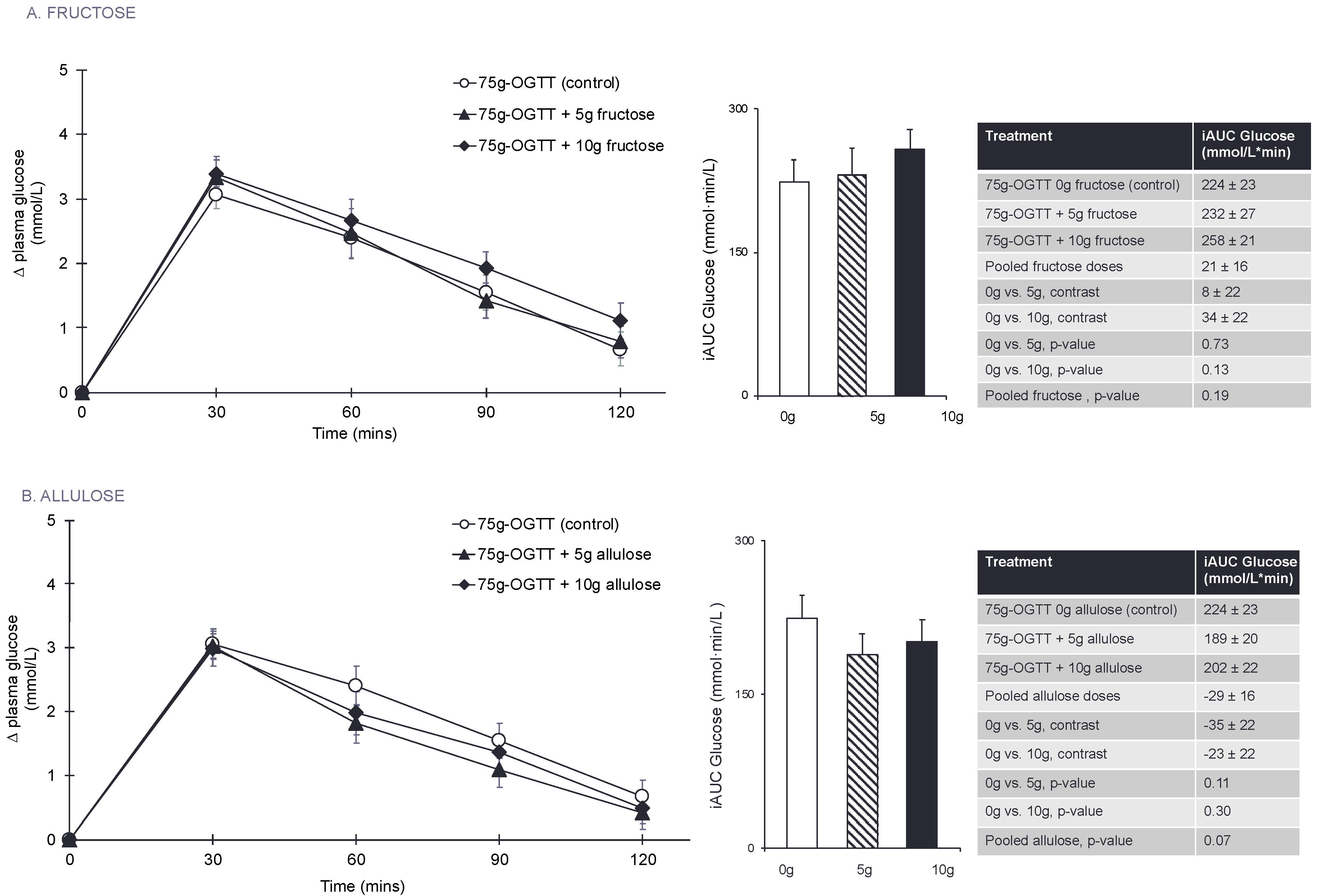
3.3. Primary Outcome: Glucose iAUC
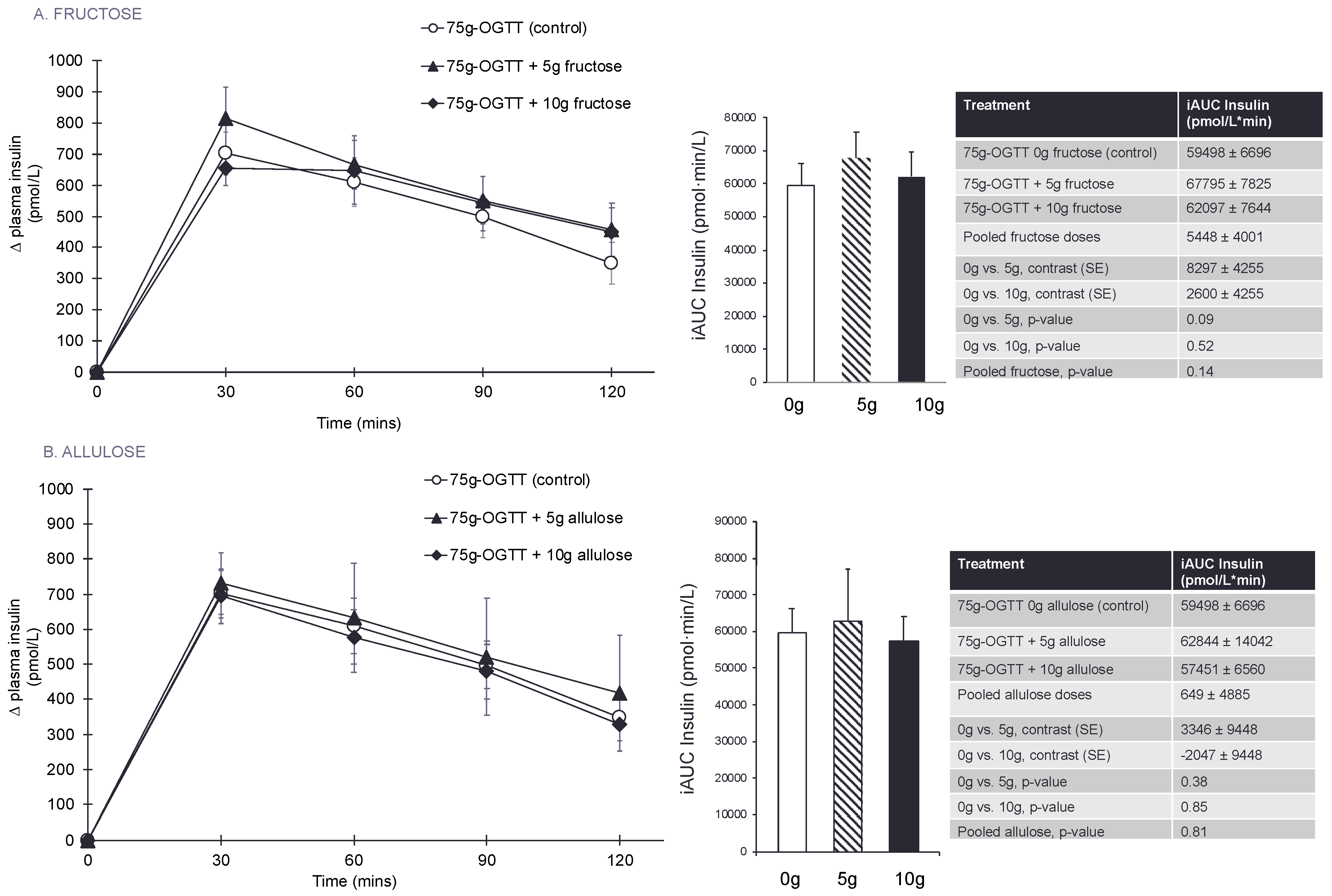
3.4. Secondary Outcomes and Exploratory Outcome Measures
3.5. Subgroup Analyses
3.6. Sensitivity Analyses
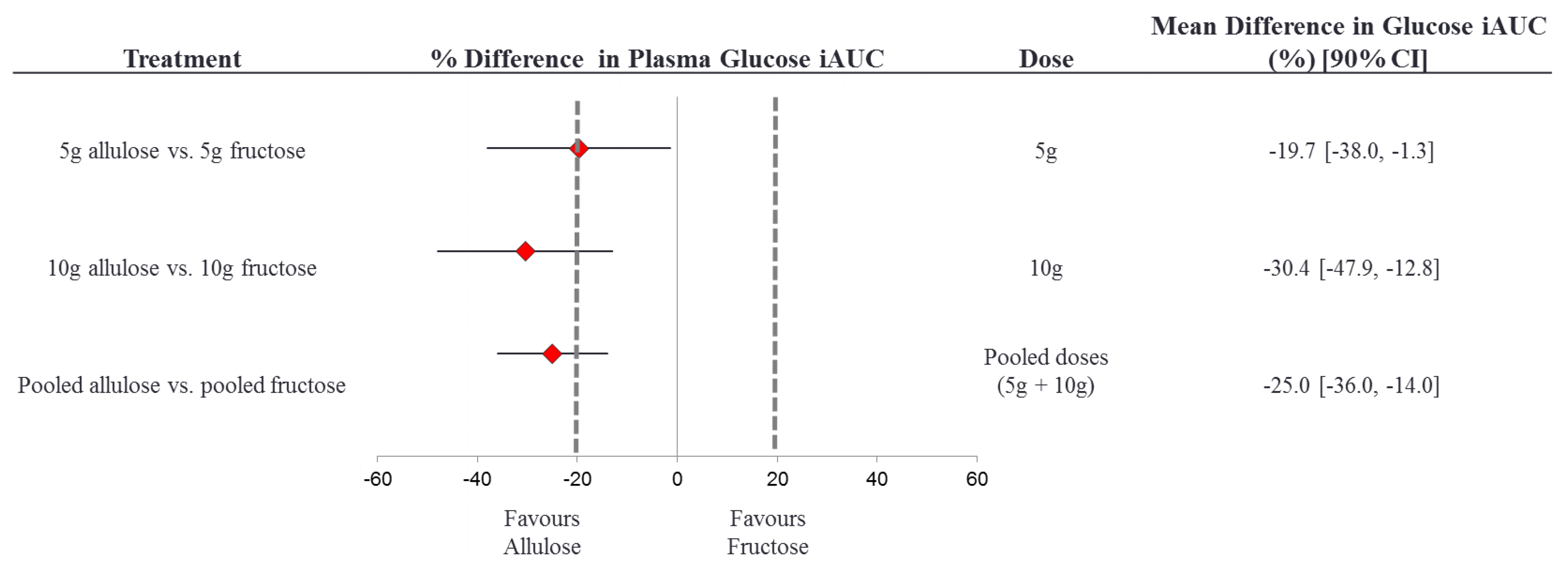
3.7. Equivalence Testing
3.8. Side Effects
4. Discussion
4.1. Results in the Context of Previous Studies
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Sugar Intake for Adults and Children; WHO: Geneva, Switzerland, 2015; p. 49. [Google Scholar]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans; U.S. Government Printing Office: Washington, DC, USA, 2015.
- Public Health England. Sugar Reduction: Achieving the 20% A Technical Report Outlining Progress to Date, Guidelines for Industry, 2015 Baseline Levels in Key Foods and Next Steps; Wellington House: London, UK, 2017.
- Sievenpiper, J.L. Fructose: Back to the future? Am. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Lucan, S.C. Added fructose: A principal driver of type 2 diabetes mellitus and its consequences. Mayo Clin. Proc. 2015, 90, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H. Sickeningly Sweet: Does Sugar Cause Type 2 Diabetes? Yes. Can. J. Diabetes 2016, 40, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Taubes, G. The Case Against Sugar; Alfred A Knopf Canada, Random House of Canada, a Division of Penguin Random House of Canada Limited: Toronto, ON, Canada, 2016. [Google Scholar]
- Sievenpiper, J.L.; de Souza, R.J.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Beyene, J.; Chiavaroli, L.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on body weight in controlled feeding trials: A systematic review and meta-analysis. Ann. Intern Med. 2012, 156, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; de Souza, R.J.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Wang, D.D.; Yu, M.; Carleton, A.J.; Di Buono, M.; Jenkins, A.L.; et al. Effect of Fructose on Established Lipid Targets: A Systematic Review and Meta-Analysis of Controlled Feeding Trials. J. Am. Heart Assoc. 2015, 4, e001700. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; de Souza, R.J.; Chiavaroli, L.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; DiBuono, M.; Jenkins, A.L.; et al. Effect of Fructose on Uric Acid: A Meta-Analysis of Controlled Feeding Trials. J. Nutr. 2012, 142, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; de Souza, R.J.; Cozma, A.I.; Chiavaroli, L.; Ha, V.; Mirrahimi, A.; Carleton, A.J.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on postprandial triglycerides: A systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014, 232, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Mirrahimi, A.; Carleton, A.J.; Ha, V.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2014, 68, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Tappy, L.; Brouns, F. Fructose as a Driver of Diabetes: An Incomplete View of the Evidence. Mayo Clin. Proc. 2015, 90, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L. Sickeningly Sweet: Does Sugar Cause Chronic Disease? No. Can. J. Diabetes 2016, 40, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: Meta-analyses and meta-regression models of intervention studies. Am. J. Clin. Nutr. 2008, 88, 1419–1437. [Google Scholar] [PubMed]
- Cozma, A.I.; Sievenpiper, J.L.; de Souza, R.J.; Chiavaroli, L.; Ha, V.; Wang, D.D.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Di Buono, M.; et al. Effect of fructose on glycemic control in diabetes: A systematic review and meta-analysis of controlled feeding trials. Diabetes Care 2012, 35, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Chiavaroli, L.; de Souza, R.J.; Mirrahimi, A.; Cozma, A.I.; Ha, V.; Wang, D.D.; Yu, M.E.; Carleton, A.J.; Beyene, J.; et al. ‘Catalytic’ doses of fructose may benefit glycaemic control without harming cardiometabolic risk factors: A small meta-analysis of randomised controlled feeding trials. Br. J. Nutr. 2012, 108, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Chiavaroli, L.; Ha, V.; Mirrahimi, A. Fructose vs. glucose and metabolism: Do the metabolic differences matter? Curr. Opin. Lipidol. 2014, 25, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.I.T.; Yamada, T.; Okuma, K.; Takehara, I.; Yamamoto, T.; Yamada, K.; Tokuda, M. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects. Biosci. Biotechnol. Biochem. 2010, 74, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Kishimoto, Y.; Yoshikawa, Y.; Hayashi, N.; Okuma, K.; Tohi, M.; Yagi, K.; Matsuo, T.; Izumori, K. Acute D-Psicose Administration Decreases the Glycemic Responses to an Oral Maltodextrin Tolerance Test in Normal Adults. J. Nutr. Sci. Vitaminol. 2008, 54, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.C.; Mann, S.L.; Davis, S.N. Acute fructose administration decreases the glycemic response to an oral glucose tolerance test in normal adults. J. Clin. Endocrinol. Metab. 2000, 85, 4515–4519. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.C.; Mann, S.L.; Cherrington, A.D. Acute fructose administration improves oral glucose tolerance in adults with type 2 diabetes. Diabetes Care 2001, 24, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Heacock, P.M.; Wolf, B.W. Fructose prefeeding reduces the glycemic response to a high-glycemic index, starchy food in humans. J. Nutr. 2002, 132, 2601–2604. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Hayashi, N.; Yamada, T.; Yoshikawa, Y.; Miyazato, S.; Kishimoto, Y.; Okuma, K.; Tokuda, M.; Izumori, K. Failure of D-psicose absorbed in the small intestine to metabolize into energy and its low large intestinal fermentability in humans. Metab. Clin. Exp. 2010, 59, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.; Punthakee, Z. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Definition, classification, and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 2013, 37, S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0). Available online: http://www.randomizer.org/ (accessed on 12 February 2018).
- Sievenpiper, J.L.; Jenkins, D.J.; Josse, R.G.; Vuksan, V. Dilution of the 75-g oral glucose tolerance test improves overall tolerability but not reproducibility in subjects with different body compositions. Diabetes Res. Clin. Pract. 2001, 51, 87–95. [Google Scholar] [CrossRef]
- Group, W.S. Diabetes Mellitus: Report of a WHO Study Group; WHO: Geneva, Switzerland, 1985; p. 99. [Google Scholar]
- Schmidt, F.H. Enzymatic determination of glucose and fructose simultaneously. Klinische Wochensch. 1961, 39, 1244–1247. [Google Scholar] [CrossRef]
- Peterson, J.I.; Young, D.S. Evaluation of the hexokinase-glucose-6-phosphate dehydrogenase method of determination of glucose in urine. Anal. Biochem. 1968, 23, 301–316. [Google Scholar] [CrossRef]
- Liverey, J.H. Effect of time, temperature and freezing on the stability of immunoreactive LH, FSH, TSH, growth hormone, prolactin and insulin in plasma. Clin. Biochem. 1980, 13, 151–155. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M. Insulin sensitivity indicies obtained from oral glucose tolerance testing: Comparison witht he euglycemic clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I.; Hales, C.N.; Osmond, C. Understanding oral glucose tolerance: Comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet. Metab. 1995, 12, 931. [Google Scholar] [CrossRef]
- Health Canada. Draft Guidance Document on Food Health Claims Related to the Reduction in Post-Prandial Glycaemic Response Bureau of Nutritional Sciences; Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch, 2013; p. 12.
- Williams, J.D. A Multiple Regression Approach to Multiple Comparisons for Comparing Several Treatments with a Control. J. Exp. Educ. 1971, 39, 93–96. [Google Scholar] [CrossRef]
- Detry, M.A.; Ma, Y. Analyzing Repeated Measurements Using Mixed Models. JAMA 2016, 315, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; Group, C. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S. The Glycemic Index: A Physiological Classification of Dietary Carbohydrate; Biddles Ltd.: King’s Lynn, UK, 2006. [Google Scholar]
- Wolever, T.M.; Chiasson, J.L.; Csima, A.; Hunt, J.A.; Palmason, C.; Ross, S.A.; Ryan, E.A. Variation of postprandial plasma glucose, palatability, and symptoms associated with a standardized mixed test meal versus 75 g oral glucose. Diabetes Care 1998, 21, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Chiasson, J.L.; Hunt, J.A.; Palmason, C.; Ross, S.A.; Ryan, E.A. Similarity of relative glycaemic but not relative insulinaemic responses in normal, IGT and diabetic subjects. Nutr. Res. 1998, 18, 1667–1676. [Google Scholar] [CrossRef]
- Guyton, A.C. Textbook of Medical Physiology, 11th ed.; Elsevier Inc.: Philadelphia, PA, USA, 2006. [Google Scholar]
- Triplitt, C.L. Examining the mechanisms of glucose regulation. Am. J. Manag. Care 2012, 18, S4–S10. [Google Scholar] [PubMed]
- Colagiuri, S.; Sandbaek, A.; Carstensen, B.; Christensen, J.; Glumer, C.; Lauritzen, T.; Borch-Johnsen, K. Comparability of venous and capillary glucose measurements in blood. Diabetes Med. 2003, 20, 953–956. [Google Scholar] [CrossRef]
- Haeckel, R.; Brinck, U.; Colic, D.; Janka, H.U.; Puntmann, I.; Schneider, J.; Viebrock, C. Comparability of blood glucose concentrations measured in different sample systems for detecting glucose intolerance. Clin. Chem. 2002, 48, 936–939. [Google Scholar] [PubMed]
- Venn, B.S.; Williams, S.M.; Mann, J.I. Comparison of postprandial glycaemia in Asians and Caucasians. Diabetes Med. 2010, 27, 1205–1208. [Google Scholar] [CrossRef]
- Kataoka, M.; Venn, B.J.; Williams, S.M.; Te Morenga, L.A.; Heemels, I.M.; Mann, J.I. Glycaemic responses to glucose and rice in people of Chinese and European ethnicity. Diabetes Med. 2012, 30. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Vorster, H.H.; Bjorck, I.; Brand-Miller, J.; Brighenti, F.; Mann, J.I.; Ramdath, D.D.; Granfeldt, Y.; Holt, S.; Perry, T.L.; et al. Determination of the glycaemic index of foods: Interlaboratory study. Eur. J. Clin. Nutr. 2003, 57, 475–482. [Google Scholar] [CrossRef] [PubMed]
- International Standards Organisation. Food Products—Determination of the Glycaemic Index (GI) and Recommendation for Food Classification; ISO 26642–2010; International Standards Organisation: Geneva, Switzerland, 2010. [Google Scholar]
| Characteristics | Total, n = 25 | Males, n = 13 | Females, n = 12 |
|---|---|---|---|
| Age (years) | 37 ± 16 | 40 ± 15 | 35 ± 17 |
| Weight (kg) | 69.3 ± 13.9 | 78.3 ± 11.7 | 59.5 ± 8.4 |
| BMI (kg/m2) | 24.7 ± 3.4 | 25.7 ± 3.9 | 23.6 ± 2.6 |
| SBP (mmHg) | 116 ± 8 | 120 ± 9 | 112 ± 6 |
| DBP (mmHg) | 70 ± 8 | 68 ± 9 | 71 ± 8 |
| WC (cm) | 81.2 ± 11.4 | 87.8 ± 11.0 | 74.3 ± 7.1 |
| Outcome Measures * | 75 g-OGTT 0 g Fructose Control | 75 g-OGTT+ 5 g Fructose | 75 g-OGTT+ 10 g Fructose | 5 g vs. Control, Contrast | 10 g vs. Control, Contrast | Pooled Doses vs. Control | 5 g vs. Control, p-Value † | 10 g, vs. Control, p-Value † | Pooled Doses vs. Control, p-Value † |
|---|---|---|---|---|---|---|---|---|---|
| Secondary | |||||||||
| Abs Cmax PG (mmol/L) | 8.6 ± 0.3 | 8.8 ± 0.3 | 8.8 ± 0.2 | 0.2 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.2 | 0.67 | 0.91 | 0.25 |
| TmaxPG (min) ** | 41.4 ± 2.5 | 40.8 ± 4.5 | 39.6 ± 3.3 | −0.6 ± 4.3 | −1.8 ± 4.3 | −1.2 ± 2.8 | 0.89 | 0.68 | 0.67 |
| Inc PG (mmol/L) | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.8 ± 0.2 | 0.07 ± 0.2 | 0.29 ± 0.2 | 0.2 ± 0.1 | 0.68 | 0.07 | 0.14 |
| iAUC PI (pmol·min/L) | 59,498 ± 6696 | 67,795 ± 7825 | 62,097 ± 7644 | 8297 ± 4255 | 2600 ± 4255 | 5448 ± 4001 | 0.09 | 0.52 | 0.14 |
| Abs Cmax PI (pmol/L) | 894 ± 79 | 976 ± 105 | 887 ± 107 | 82 ± 68 | −7 ± 68 | 37 ± 56 | 0.45 | 0.46 | 0.82 |
| Tmax PI (min) ** | 49.8 ± 4.2 | 43.2 ± 3.9 | 54.0 ± 5.5 | −6.6 ± 5.9 | 4.2 ± 5.9 | −1.2 ± 4.1 | 0.26 | 0.47 | 0.77 |
| Inc PI (pmol/L) | 431 ± 51 | 498 ± 60 | 458 ± 58 | 66 ± 32 | 27 ± 32 | 47 ± 31 | 0.04 | 0.40 | 0.10 |
| ∆PI30-0/∆PG30-0 | 316 ± 66 | 357 ± 76 | 214 ± 23 | 42 ± 73 | −101 ± 73 | −30 ± 71 | 0.70 | 0.12 | 0.81 |
| Matsuda ISIOGTT | 3.9 ± 0.5 | 3.9 ± 0.6 | 3.6 ± 0.4 | 0.05 ± 0.3 | −0.28 ± 0.3 | −0.1 ± 0.2 | 0.45 | 0.34 | 0.45 |
| Exploratory | |||||||||
| Total AUC PG (mmol·min/L) | 854 ± 27 | 864 ± 31 | 880 ± 24 | 10 ± 22 | 26 ± 22 | 18 ± 16 | 0.65 | 0.24 | 0.26 |
| Inc Cmax PG (mmol/L) | 3.3 ± 0.2 | 3.5 ± 0.3 | 3.7 ± 0.2 | 0.2 ± 0.3 | 0.3 ± 0.3 | 0.2 ± 0.2 | 0.54 | 0.22 | 0.16 |
| Abs mean PG (mmol/L) | 6.8 ± 0.2 | 6.9 ± 0.2 | 7.0 ± 0.2 | 0.1 ± 0.2 | 0.2 ± 0.2 | 0.1 ± 0.1 | 0.61 | 0.21 | 0.25 |
| Total AUC Insulin (pmol·min/L) | 68,192 ± 7201 | 75,494 ± 8358 | 70,241 ± 7909 | 7302 ± 4436 | 2049 ± 4436 | 4675 ± 4313 | 0.18 | 0.57 | 0.26 |
| Inc Cmax PI (pmol/L) | 821 ± 75 | 911 ± 101 | 818 ± 105 | 90 ± 67 | −3 ± 67 | 44 ± 53 | 0.34 | 0.48 | 0.70 |
| Abs mean PI (pmol/L) | 504 ± 55 | 562 ± 65 | 527 ± 60 | 58 ± 34 | 23 ± 34 | 40 ± 33 | 0.17 | 0.41 | 0.21 |
| ISSI-2 | 289 ± 27 | 305 ± 25 | 275 ± 16 | 16 ± 18 | −14 ± 18 | 0.8 ± 14 | 0.23 | 0.64 | 0.47 |
| Outcome Measures * | 75 g-OGTT 0 g Allulose (Control) | 75 g-OGTT+ 5 g Allulose | 75 g-OGTT+ 10 g Allulose | 5 g vs. Control, Contrast | 10 g vs. Control, Contrast | Pooled Doses vs. Control, Contrast | 5 g vs. Control, p-Value † | 10 g, vs. Control, p-Value † | Pooled Doses vs. Control, p-Value † |
|---|---|---|---|---|---|---|---|---|---|
| Secondary | |||||||||
| Abs Cmax PG (mmol/L) | 8.6 ± 0.3 | 8.4 ± 0.3 | 8.5 ± 0.3 | −0.2 ± 0.3 | −0.2 ± 0.3 | −0.2 ± 0.2 | 0.44 | 0.53 | 0.30 |
| TmaxPG (min) ** | 41.4 ± 2.5 | 33.6 ± 2.6 | 37.2 ± 3.1 | −7.8 ± 3.5 | −4.2 ± 3.5 | −6.0 ± 2.7 | 0.03 | 0.23 | 0.03 |
| Inc PG (mmol/L) | 1.5 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.2 | −0.3 ± 0.2 | −0.2 ± 0.2 | −0.2 ± 0.1 | 0.11 | 0.30 | 0.05 |
| iAUC PI (pmol·min/L) | 59,498 ± 6696 | 62,844 ± 14042 | 57,451 ± 6560 | 3346 ± 9448 | −2047 ± 9448 | 649 ± 4885 | 0.38 | 0.85 | 0.81 |
| Abs Cmax PI (pmol/L) | 894 ± 79 | 957 ± 163 | 898 ± 82 | 63 ± 116 | 4 ± 116 | 34 ± 63 | 0.64 | 0.94 | 0.84 |
| Tmax PI (min) ** | 49.8 ± 4.2 | 40.8 ± 3.4 | 48.0 ± 3.9 | −9.0 ± 3.6 | −1.8 ± 3.6 | −5.4 ± 3.3 | 0.01φ | 0.62 | 0.13 |
| Inc PI (pmol/L) | 431 ± 51 | 461 ± 110 | 416 ± 50 | 29 ± 75 | −16 ± 75 | 7 ± 37 | 0.36 | 0.83 | 0.80 |
| ∆PI30-0/∆PG30-0 θ | 316 ± 66 | 374 ± 129 | 217 ± 87 | 58 ± 127 | −99 ± 127 | −21 ± 94 | 0.93 | 0.34 | 0.77 |
| Matsuda ISIOGTT | 3.9 ± 0.5 | 4.4 ± 0.7 | 3.7 ± 0.4 | 0.5 (0.3) | −0.2 (0.3) | 0.1 ± 0.2 | 0.92 | 0.73 | 0.77 |
| Exploratory | |||||||||
| tAUC PG (mmol·min/L) | 854 ± 27 | 821 ± 24 | 827 ± 24 | −33 ± 25 | −26 ± 25 | −30 ± 17 | 0.18 | 0.29 | 0.07 |
| Inc Cmax PG (mmol/L) | 3.3 ± 0.2 | 3.1 ± 0.2 | 3.2 ± 0.2 | −0.2 (0.2) | −0.1 (0.2) | −0.2 ± 0.17 | 0.35 | 0.56 | 0.26 |
| Abs mean PG (mmol/L) | 6.8 ± 0.2 | 6.6 ± 0.2 | 6.6 ± 0.2 | −0.2 (0.2) | −0.2 (0.2) | −0.2 ± 0.1 | 0.19 | 0.28 | 0.07 |
| tAUC Insulin (pmol·min/L) | 68,192 ± 7201 | 72,734 ± 14510 | 66,174 ± 6805 | 4541 ± 9617 | −2018 ± 9617 | 1262 ± 4786 | 0.45 | 0.99 | 0.96 |
| Inc Cmax PI (pmol/L) | 821 ± 75 | 874 ± 158 | 825 ± 82 | 53 ± 115 | 4 ± 115 | 28 ± 63 | 0.63 | 0.97 | 0.90 |
| Abs mean PI (pmol/L) | 504 ± 55 | 543 ± 113 | 489 ± 52 | 39 ± 76 | −15 ± 76 | 12 ± 37 | 0.45 | 0.99 | 0.99 |
| ISSI-2 | 289 ± 27 | 300 ± 28 | 306 ± 26 | 11 ± 18 | 16 ± 18 | 14 ± 12 | 0.62 | 0.42 | 0.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braunstein, C.R.; Noronha, J.C.; Glenn, A.J.; Viguiliouk, E.; Noseworthy, R.; Khan, T.A.; Au-Yeung, F.; Blanco Mejia, S.; Wolever, T.M.S.; Josse, R.G.; et al. A Double-Blind, Randomized Controlled, Acute Feeding Equivalence Trial of Small, Catalytic Doses of Fructose and Allulose on Postprandial Blood Glucose Metabolism in Healthy Participants: The Fructose and Allulose Catalytic Effects (FACE) Trial. Nutrients 2018, 10, 750. https://doi.org/10.3390/nu10060750
Braunstein CR, Noronha JC, Glenn AJ, Viguiliouk E, Noseworthy R, Khan TA, Au-Yeung F, Blanco Mejia S, Wolever TMS, Josse RG, et al. A Double-Blind, Randomized Controlled, Acute Feeding Equivalence Trial of Small, Catalytic Doses of Fructose and Allulose on Postprandial Blood Glucose Metabolism in Healthy Participants: The Fructose and Allulose Catalytic Effects (FACE) Trial. Nutrients. 2018; 10(6):750. https://doi.org/10.3390/nu10060750
Chicago/Turabian StyleBraunstein, Catherine R., Jarvis C. Noronha, Andrea J. Glenn, Effie Viguiliouk, Rebecca Noseworthy, Tauseef A. Khan, Fei Au-Yeung, Sonia Blanco Mejia, Thomas M.S. Wolever, Robert G. Josse, and et al. 2018. "A Double-Blind, Randomized Controlled, Acute Feeding Equivalence Trial of Small, Catalytic Doses of Fructose and Allulose on Postprandial Blood Glucose Metabolism in Healthy Participants: The Fructose and Allulose Catalytic Effects (FACE) Trial" Nutrients 10, no. 6: 750. https://doi.org/10.3390/nu10060750
APA StyleBraunstein, C. R., Noronha, J. C., Glenn, A. J., Viguiliouk, E., Noseworthy, R., Khan, T. A., Au-Yeung, F., Blanco Mejia, S., Wolever, T. M. S., Josse, R. G., Kendall, C. W. C., & Sievenpiper, J. L. (2018). A Double-Blind, Randomized Controlled, Acute Feeding Equivalence Trial of Small, Catalytic Doses of Fructose and Allulose on Postprandial Blood Glucose Metabolism in Healthy Participants: The Fructose and Allulose Catalytic Effects (FACE) Trial. Nutrients, 10(6), 750. https://doi.org/10.3390/nu10060750





