A Functional Food Mixture “Protector” Reinforces the Protective Immune Parameters against Viral Flu Infection in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Extracts and “Protector” Mixture
2.2. Animals
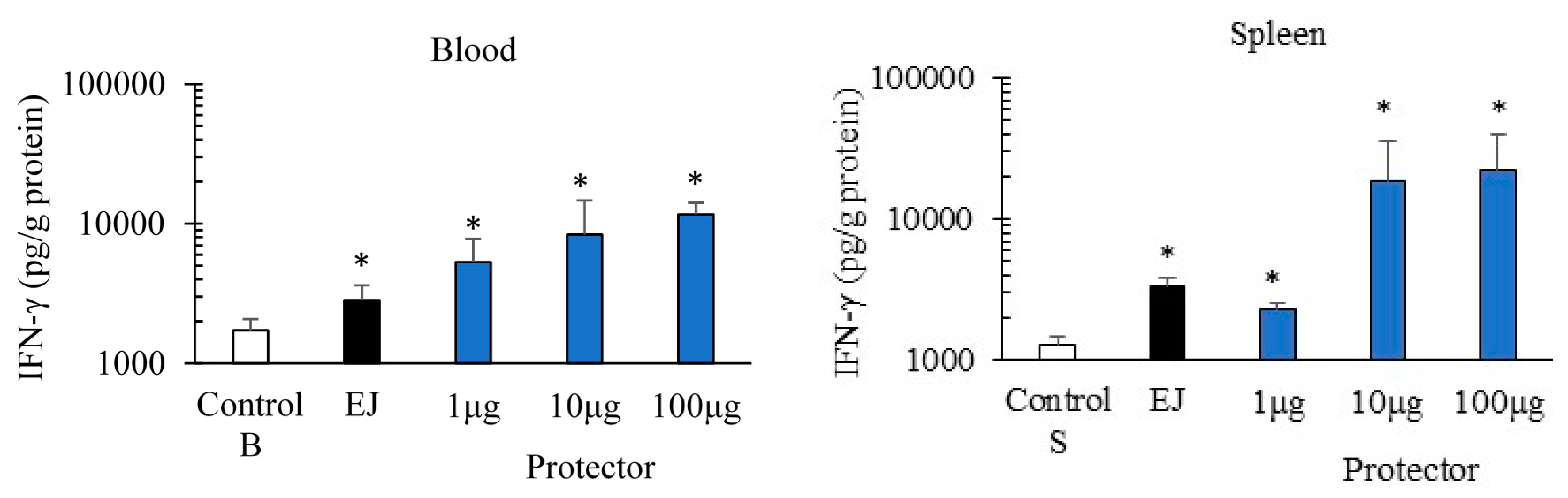
2.3. Systemic Administration of “Protector” and Cytokines Levels
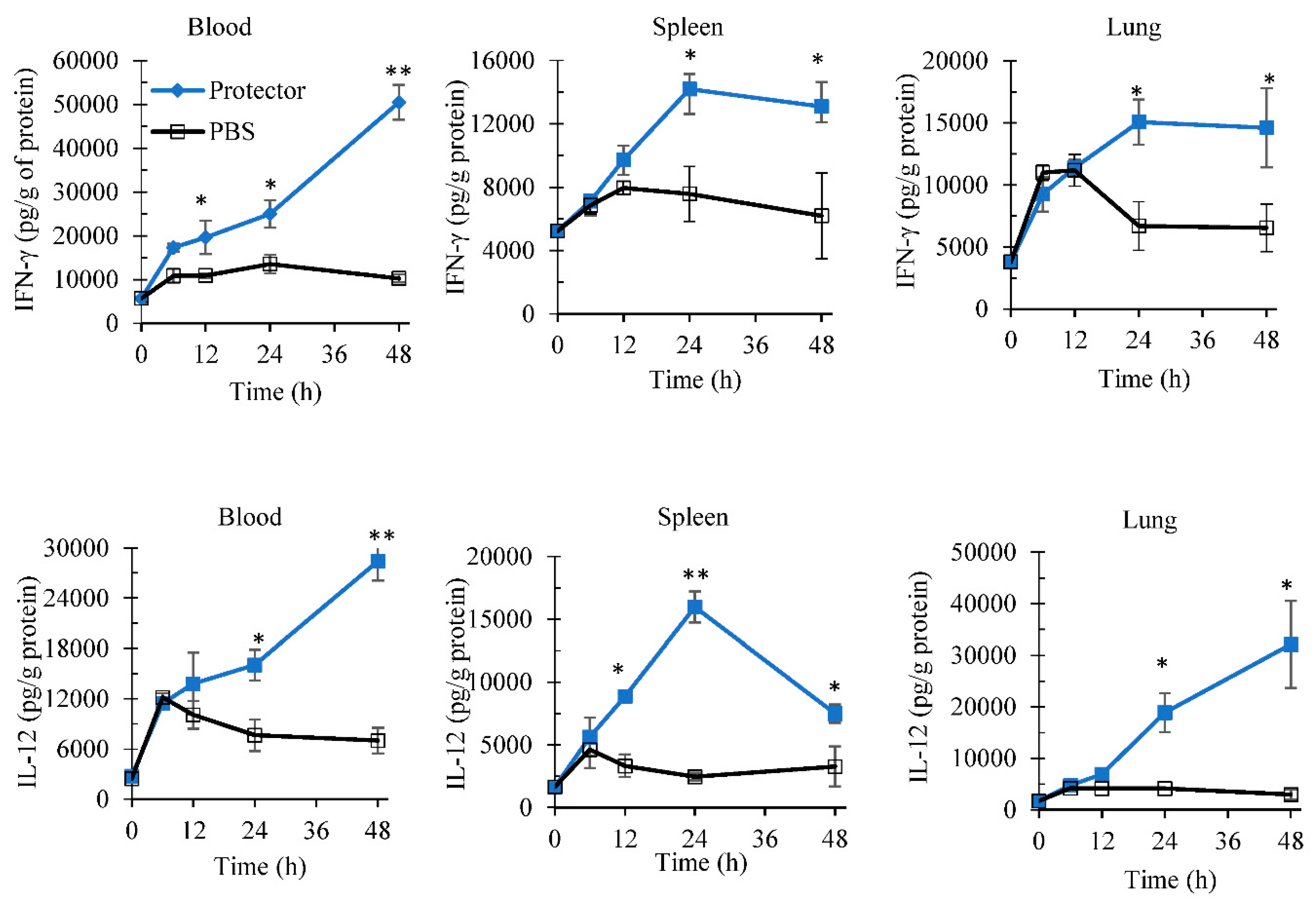
2.4. Time Profile of Cytokines Following Systemic Administration of “Protector”
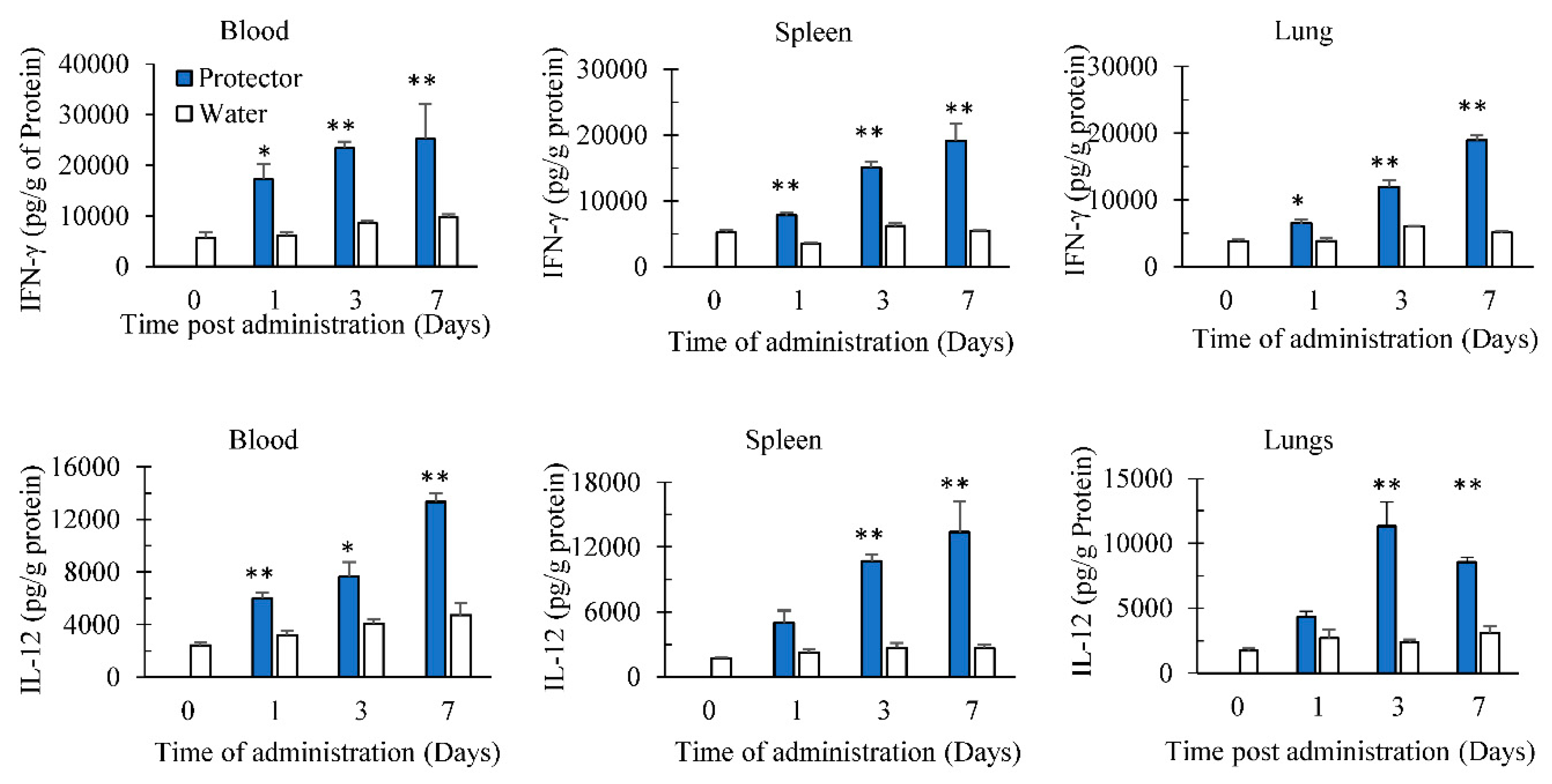
2.5. Oral Dosing of “Protector” and Cytokines Production
2.6. Influenza Virus Vaccine
2.7. Immunization Protocol
2.8. Hemagglutination Inhibition (HAI) Antibodies Titer Determination
2.9. Cytokine Determinations
2.10. Statistical Analysis
3. Results
3.1. Systemic Administration of “Protector” Enhanced IFN-γ Production Levels in Blood and Spleen
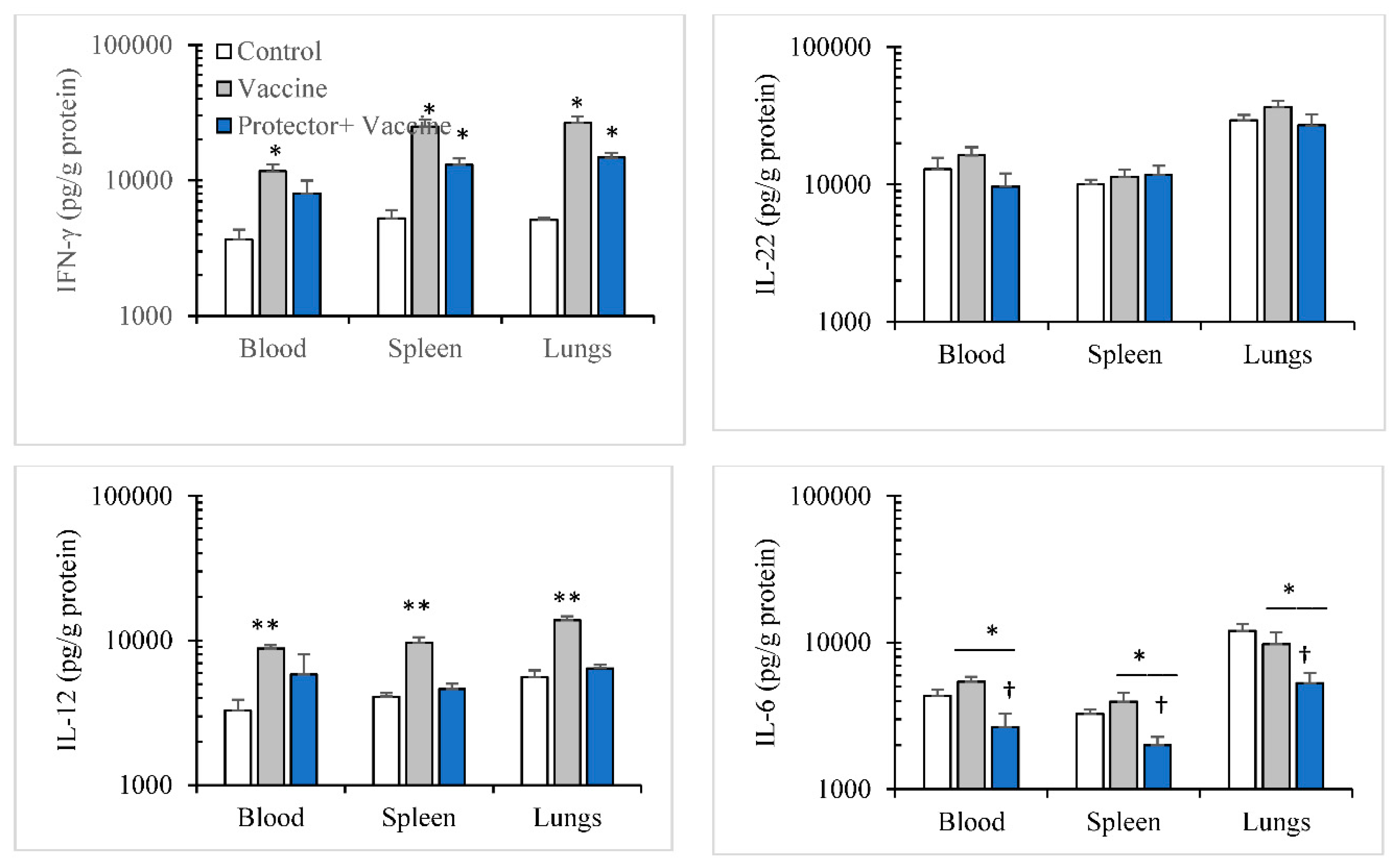
3.2. Oral Administration of “Protector” Enhanced IFN-γ and IL-12 Production in Tissues
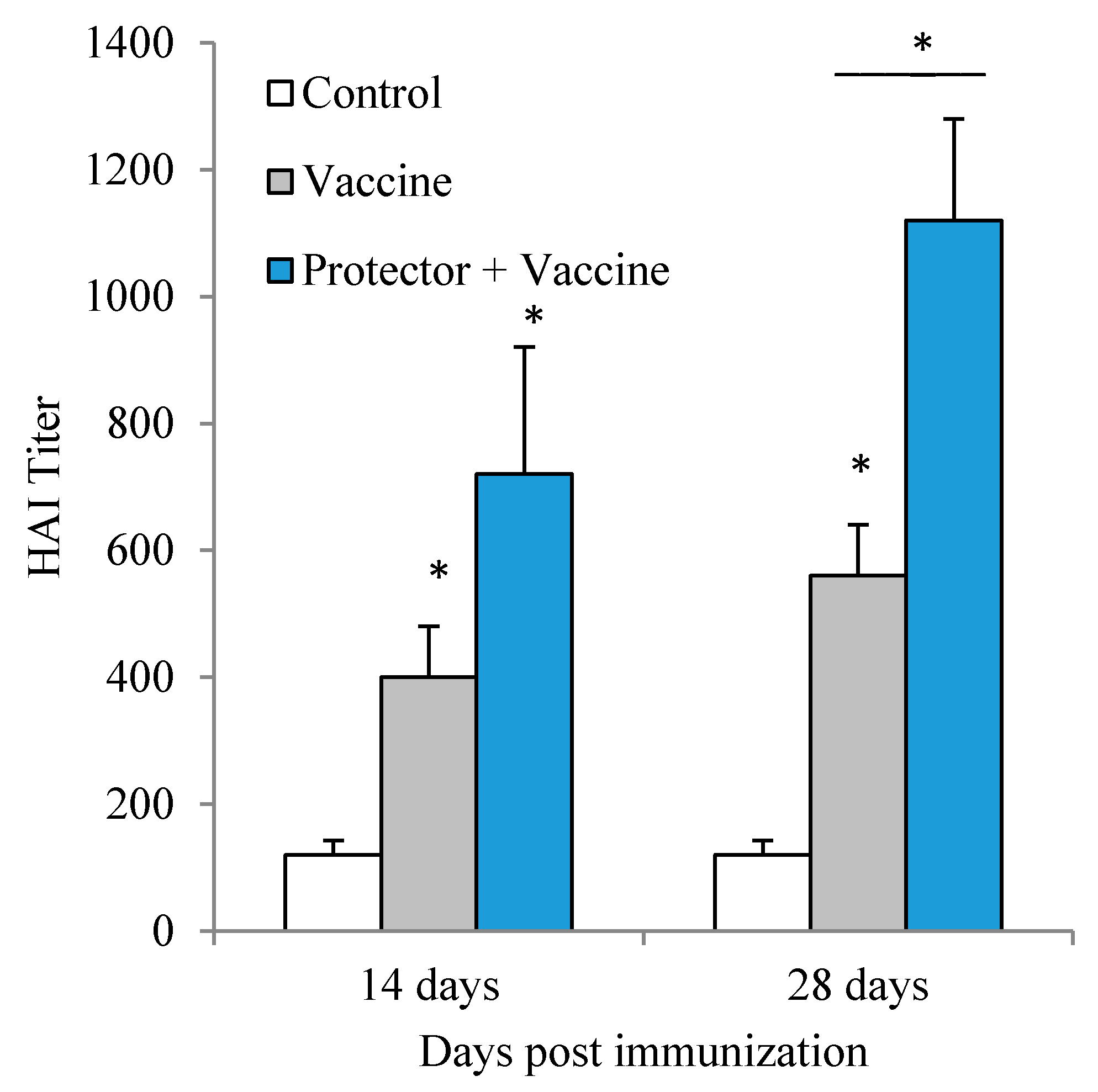
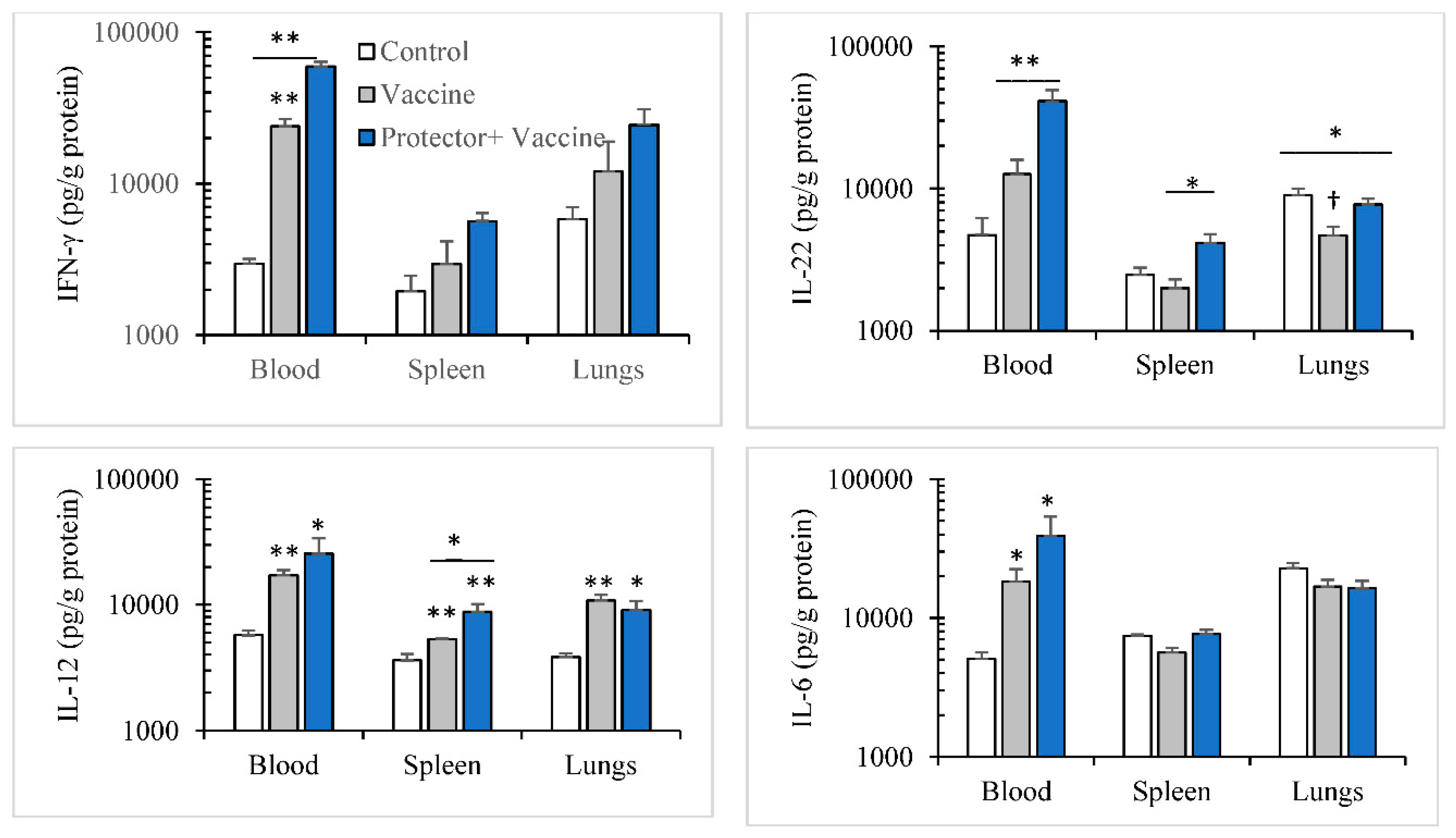
3.3. Oral Administration of “Protector” Enhanced HAI Antibodies against Influenza Virus
4. Discussion
Author Contributions
Conflicts of Interest
References
- Tripathi, S.; White, M.R.; Hartshorn, K.L. The amazing innate immune response to influenza A virus infection. Innate Immun. 2015, 21, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Openshaw, P.J. Antiviral B cell and T cell immunity in the lungs. Nat. Immunol. 2015, 16, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Thomas, P.G. Balancing Immune Protection and Immune Pathology by CD8+ T-Cell Responses to Influenza Infection. Front. Immunol. 2016, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Alshaker, H.A.; Matalka, K.Z. IFN-γ, IL-17 and TGF-β involvement in shaping the tumor microenvironment: The significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell Int. 2011, 11, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced recruitment of NK cells to lymph nodes provides IFN gamma for T(H)1 priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Digby, M.R.; Lowenthal, J.W. Cloning and expression of the chicken interferon gamma gene. J. Interferon Cytokine Res. 1995, 15, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, J.W.; York, J.J.; O’Neil, T.E.; Rhodes, S.; Prowse, S.J.; Strom, D.G.; Digby, M.R. In vivo effects of chicken interferon-gamma during infection with Eimeria. J. Interferon Cytokine Res. 1997, 17, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Gorres, J.P.; Lager, K.M.; Kong, W.P.; Royals, M.; Todd, J.P.; Vincent, A.L.; Wei, C.J.; Loving, C.L.; Zanella, E.L.; Janke, B.; et al. DNA vaccination elicits protective immune responses against pandemic and classic swine influenza viruses in pigs. Clin. Vaccine Immunol. 2011, 18, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, H.; Kapczynski, D.R. Chicken interferon alpha pretreatment reduces virus replication of pandemic H1N1 and H5N9 avian influenza viruses in lung cell cultures from different avian species. Virol. J. 2011, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Yuk, S.S.; Lee, D.H.; Park, J.K.; Tseren-Ochir, E.O.; Kwon, J.H.; Noh, J.Y.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Pre-immune state induced by chicken interferon gamma inhibits the replication of H1N1 human and H9N2 avian influenza viruses in chicken embryo fibroblasts. Virol. J. 2016, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Lee, H.J.; Lee, D.H.; Lee, Y.J.; Mo, I.P.; Nahm, S.S.; Kim, M.J.; Lee, J.B.; Park, S.Y.; Choi, I.S.; et al. Immune responses and pathogenesis in immunocompromised chickens in response to infection with the H9N2 low pathogenic avian influenza virus. Virus Res. 2008, 133, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Bot, A.; Bot, S.; Bona, C.A. Protective role of gamma interferon during the recall response to influenza virus. J. Virol. 1998, 72, 6637–6645. [Google Scholar] [PubMed]
- Karupiah, G.; Chen, J.H.; Mahalingam, S.; Nathan, C.F.; MacMicking, J.D. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 1998, 188, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.D.; Wald, O.; Wald, H.; Beider, K.; Abraham, M.; Galun, E.; Nagler, A.; Peled, A. IFN-gamma treatment at early stages of influenza virus infection protects mice from death in a NK cell-dependent manner. J. Interferon Cytokine Res. 2010, 30, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Wong, C.K.; Chan, P.K.; Chan, M.C.; Wong, R.Y.; Lun, S.W.; Ngai, K.L.; Lui, G.C.; Wong, B.C.; Lee, S.K.; et al. Cytokine response patterns in severe pandemic 2009 H1N1 and seasonal influenza among hospitalized adults. PLoS ONE 2011, 6, e26050. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Thakar, M.S.; Ouyang, W.; Malarkannan, S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013, 6, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.H.; Yang, Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.M.; Kim, J.; Tenson, T.; Min, J.Y.; Kainov, D.E. Influenza virus infection, interferon response, viral counter-response, and apoptosis. Viruses 2017, 9, E223. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.I.; Sullivan, S.G.; Subbarao, K.; Fauci, A.S. Chasing seasonal influenza—The need for a universal influenza vaccine. N. Engl. J. Med. 2018, 378, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Kitagawa, T.; Koyanagi, N.; Chujo, H.; Maeda, H.; Kohno-Murase, J.; Imamura, J.; Tachibana, H.; Yamada, K. Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition 2006, 22, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.; Narciso, C.D.; Bisinotto, R.S.; Perdomo, M.C.; Ballou, M.A.; Dreher, M.; Santos, J.E. Effects of feeding polyphenols from pomegranate extract on health, growth, nutrient digestion, and immunocompetence of calves. J. Dairy Sci. 2010, 93, 4280–4291. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.M.; Aravind, S.R.; Varghese, S.; Mini, S.; Sreelekha, T.T. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2012, 5, 489–496. [Google Scholar] [PubMed]
- Rowe, C.A.; Nantz, M.P.; Nieves, C., Jr.; West, R.L.; Percival, S.S. Regular consumption of concord grape juice benefits human immunity. J. Med. Food 2011, 14, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, F.A.; Porcino, C.; Cerezuela, R.; Cuesta, A.; Faggio, C.; Esteban, M.A. Impact of date palm fruits extracts and probiotic enriched diet on antioxidant status, innate immune response and immune-related gene expression of European seabass (Dicentrarchus labrax). Fish Shellfish Immunol. 2016, 52, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Cerezuela, R.; Guardiola, F.A.; Cuesta, A.; Esteban, M.Á. Enrichment of gilthead seabream (Sparus aurata L.) diet with palm fruit extracts and probiotics: Effects on skin mucosal immunity. Fish Shellfish Immunol. 2016, 49, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Khalili, M.; Rufchaei, R.; Raeisi, M.; Attar, M.; Cordero, H.; Esteban, M.Á. Effects of date palm fruit extracts on skin mucosal immunity, immune related genes expression and growth performance of common carp (Cyprinus carpio) fry. Fish Shellfish Immunol. 2015, 47, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, Y.; Yang, X.; Rui, K.; Tang, X.; Ma, J.; Chen, J.; Xu, H.; Lu, L.; Wang, S. Ficus carica polysaccharides promote the maturation and function of dendritic cells. Int. J. Mol. Sci. 2014, 15, 12469–12479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.M.; Yu, W.; Ou, Z.P.; Ma, H.L.; Liu, W.M.; Ji, X.L. Antioxidant and immunity activity of water extract and crude polysaccharide from Ficus carica L. fruit. Plant Foods Hum. Nutr. 2009, 64, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Schoenknecht, C.; Andersen, G.; Schmidts, I.; Schieberle, P. Quantitation of gingerols in human plasma by newly developed stable isotope dilution assays and assessment of their immunomodulatory potential. J. Agric. Food Chem. 2016, 64, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kim, B.S.; Kim, K.S.; Lee, S.; Shin, K.S.; Lim, J.S. Immune-suppressive activity of punicalagin via inhibition of NFAT activation. Biochem. Biophys. Res. Commun. 2008, 371, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Bachoual, R.; Talmoudi, W.; Boussetta, T.; Braut, F.; El-Benna, J. An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem. Toxicol. 2011, 49, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar]
- Chacón, M.R.; Ceperuelo-Mallafré, V.; Maymó-Masip, E.; Mateo-Sanz, J.M.; Arola, L.; Guitiérrez, C.; Fernandez-Real, J.M.; Ardèvol, A.; Simón, I.; Vendrell, J. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine 2009, 47, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Mossalayi, M.D.; Rambert, J.; Renouf, E.; Micouleau, M.; Mérillon, J.M. Grape polyphenols and propolis mixture inhibits inflammatory mediator release from human leukocytes and reduces clinical scores in experimental arthritis. Phytomedicine 2014, 21, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, S.; Villareal, M.O.; Fujitsuka, T.; Aida, K.; Isoda, H. Anti-inflammatory and anti-arthritic effects of pentacyclic triterpenoids maslinic acid through NF-κB inactivation. Mol. Nutr. Food Res. 2016, 60, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Sánchez-Fidalgo, S.; Alarcón-de-la-Lastra, C. Extra virgin olive oil polyphenolic extracts downregulate inflammatory responses in LPS-activated murine peritoneal macrophages suppressing NFκB and MAPK signalling pathways. Food Funct. 2014, 5, 1270–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luettig, J.; Rosenthal, R.; Lee, I.M.; Krug, S.M.; Schulzke, J.D. The ginger component 6-shogaol prevents TNF-α-induced barrier loss via inhibition of PI3K/Akt and NF-κB signaling. Mol. Nutr. Food Res. 2016, 60, 2576–2586. [Google Scholar] [CrossRef] [PubMed]
- Matalka, K.Z.; Ali, D.; Khawad, A.E.; Qadan, F. The differential effect of Eriobotrya japonica hydrophilic leaf extract on cytokines production and modulation. Cytokine 2007, 40, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Alshaker, H.A.; Qinna, N.A.; Qadan, F.; Bustami, M.; Matalka, K.Z. Eriobotrya japonica hydrophilic extract modulates cytokines in normal tissues and within the tumor of Meth-A-fibrosarcoma bearing mice with enhancing their survival time. BMC Complement. Altern. Med. 2011, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matalka, K.Z.; Abdulridha, N.A.; Badr, M.M.; Mansoor, K.; Qinna, N.A.; Qadan, F. Eriobotrya japonica water extract characterization: An inducer of interferon-gamma production mainly by the JAK-STAT pathway. Molecules 2016, 21, 722. [Google Scholar] [CrossRef] [PubMed]
- Matalka, K.Z.; Tutunji, M.F.; Abu-Baker, M.; Abu Baker, Y. Measurement of protein cytokines in tissue extracts by enzyme-linked immunosorbent assays: Application to lipopolysaccharide-induced differential milieu of cytokines. Neuroendocrinol. Lett. 2005, 26, 231–236. [Google Scholar] [PubMed]
- Halperin, S.A.; Smith, B.; Mabrouk, T.; Germain, M.; Trepanier, P.; Hassell, T.; Treanor, J.; Gauthier, R.; Mills, E.L. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in healthy adults, seniors, and children. Vaccine 2002, 20, 1240–1247. [Google Scholar] [CrossRef]
- Guebre-Xabier, M.; Hammond, S.A.; Ellingsworth, L.R.; Glenn, G.M. Immunostimulant patch enhances immune responses to influenza virus vaccine in aged mice. J. Virol. 2004, 78, 7610–7618. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansoor, K.A.; Qadan, F.; Schmidt, M.; Qinna, N.A.; Badr, M.; Matalka, K.Z. A Functional Food Mixture “Protector” Reinforces the Protective Immune Parameters against Viral Flu Infection in Mice. Nutrients 2018, 10, 743. https://doi.org/10.3390/nu10060743
Mansoor KA, Qadan F, Schmidt M, Qinna NA, Badr M, Matalka KZ. A Functional Food Mixture “Protector” Reinforces the Protective Immune Parameters against Viral Flu Infection in Mice. Nutrients. 2018; 10(6):743. https://doi.org/10.3390/nu10060743
Chicago/Turabian StyleMansoor, Kenza A., Fadi Qadan, Mathias Schmidt, Nidal A. Qinna, Mujtaba Badr, and Khalid Z. Matalka. 2018. "A Functional Food Mixture “Protector” Reinforces the Protective Immune Parameters against Viral Flu Infection in Mice" Nutrients 10, no. 6: 743. https://doi.org/10.3390/nu10060743



