The Role of Magnesium in Neurological Disorders
Abstract
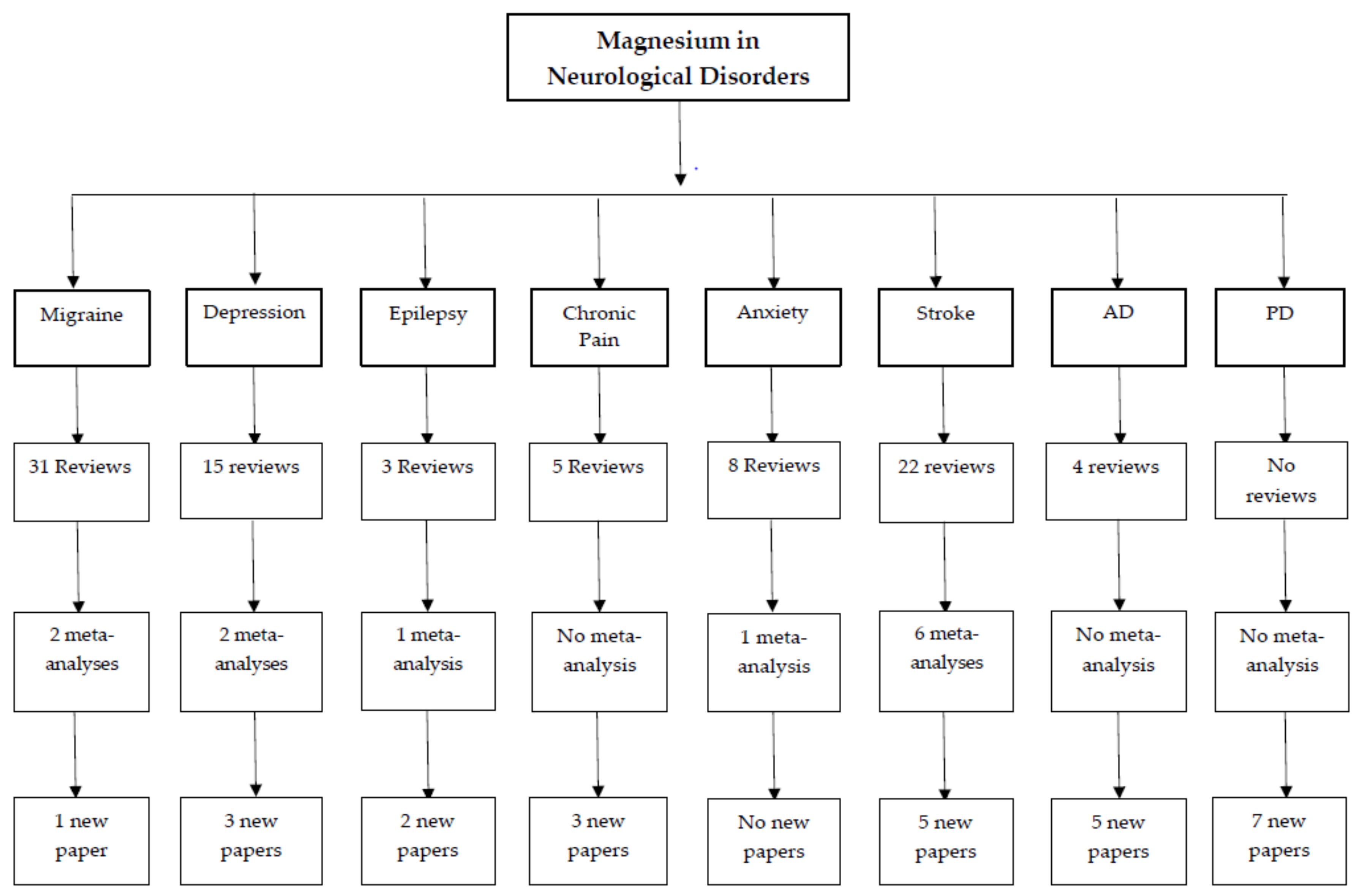
:1. Introduction
2. Materials and Methods
3. Results
3.1. Migraine
3.2. Chronic Pain
3.3. Anxiety and Depression
3.4. Epilepsy
3.5. Parkinson’s Disease
3.6. Alzheimer’s Disease
3.7. Stroke
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Grober, U.; Schmidt, J.; Kisters, K. Magnesium in prevention and therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Vink, R.; Nechifor, M. Magnesium in the Central Nervous System; University of Adelaide Press: Adelaide, Australia, 2011; p. 342. [Google Scholar]
- Stroebel, D.; Casado, M.; Paoletti, P. Triheteromeric nmda receptors: From structure to synaptic physiology. Curr. Opin. Physiol. 2018, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.F.; Ward, M.W.; Nicholls, D.G. Oxidative stress, mitochondrial function, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurochem. 1999, 72, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Cornejo-Cordova, E.; Verdaguer, E.; Soriano, F.X.; Binvignat, O.; Auladell, C.; Camins, A. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Clerc, P.; Young, C.A.; Bordt, E.A.; Grigore, A.M.; Fiskum, G.; Polster, B.M. Magnesium sulfate protects against the bioenergetic consequences of chronic glutamate receptor stimulation. PLoS ONE 2013, 8, e79982. [Google Scholar] [CrossRef] [PubMed]
- Lambuk, L.; Jafri, A.J.; Arfuzir, N.N.; Iezhitsa, I.; Agarwal, R.; Rozali, K.N.; Agarwal, P.; Bakar, N.S.; Kutty, M.K.; Yusof, A.P.; et al. Neuroprotective effect of magnesium acetyltaurate against NMDA-induced excitotoxicity in rat retina. Neurotox. Res. 2017, 31, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Kalia, S.K.; Salter, M.W. NMDA receptors in clinical neurology: Excitatory times ahead. Lancet Neurol. 2008, 7, 742–755. [Google Scholar] [CrossRef]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.; Loder, E.; Diamond, S.; Reed, M.L.; Bigal, M.E.; Lipton, R.B.; Group, A.A. Probable migraine in the United States: Results of the American migraine prevalence and prevention (AMPP) study. Cephalalgia 2007, 27, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.; Aminoff, D.A.G.P.; David, A.; Greenburg, D.; Roger, P.; Simon, R. Clinical Neurology; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Nattagh-Eshtivani, E.; Sani, M.A.; Dahri, M.; Ghalichi, F.; Ghavami, A.; Arjang, P.; Tarighat-Esfanjani, A. The role of nutrients in the pathogenesis and treatment of migraine headaches. Biomed. Pharmacother. 2018, 102, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of migraine: A disorder of sensory processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Charles, A. Glutamate and its receptors as therapeutic targets for migraine. Neurotherapeutics 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.; Ramadan, N.M. Mitochondria, magnesium and migraine. J. Neurol. Sci. 1995, 134, 9–14. [Google Scholar] [CrossRef]
- Peikert, A.; Wilimzig, C.; Köhne-Volland, R. Prophylaxis of migraine with oral magnesium: Results from a prospective, multi-center, placebo-controlled and double-blind randomized study. Cephalalgia 1996, 16, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Coata, G.; Firenze, C.; Morucci, P.; Abbritti, G.; Gallai, V. Serum and salivary magnesium levels in migraine and tension-type headache. Results in a group of adult patients. Cephalalgia 1992, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, N.; Halvorson, H.; Vande-Linde, A.; Levine, S.R.; Helpern, J.; Welch, K. Low brain magnesium in migraine. Headache J. Head Face Pain 1989, 29, 590–593. [Google Scholar] [CrossRef]
- Bigal, M.; Bordini, C.; Tepper, S.; Speciali, J. Intravenous magnesium sulphate in the acute treatment of migraine without aura and migraine with aura. A randomized, double-blind, placebo-controlled study. Cephalalgia 2002, 22, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Cete, Y.; Dora, B.; Ertan, C.; Ozdemir, C.; Oktay, C. A randomized prospective placebo-controlled study of intravenous magnesium sulphate vs. metoclopramide in the management of acute migraine attacks in the emergency department. Cephalalgia 2005, 25, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Corbo, J.; Esses, D.; Bijur, P.E.; Iannaccone, R.; Gallagher, E.J. Randomized clinical trial of intravenous magnesium sulfate as an adjunctive medication for emergency department treatment of migraine headache. Ann. Emerg. Med. 2001, 38, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.R.; Olson, C.M.; Shuler, K.B.; Gharib, S.F. Intravenous magnesium for acute benign headache in the emergency department: A randomized double-blind placebo-controlled trial. Can. J. Emerg. Med. 2004, 6, 327–332. [Google Scholar] [CrossRef]
- Ginder, S.; Oatman, B.; Pollack, M. A prospective study of i.v. magnesium and i.v. prochlorperazine in the treatment of headaches. J. Emerg. Med. 2000, 18, 311–315. [Google Scholar] [CrossRef]
- Choi, H.; Parmar, N. The use of intravenous magnesium sulphate for acute migraine: Meta-analysis of randomized controlled trials. Eur. J. Emerg. Med. 2014, 21, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Demirkaya, Ş.; Vural, O.; Dora, B.; Topçuoğlu, M.A. Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks. Headache J. Head Face Pain 2001, 41, 171–177. [Google Scholar] [CrossRef]
- Shahrami, A.; Assarzadegan, F.; Hatamabadi, H.R.; Asgarzadeh, M.; Sarehbandi, B.; Asgarzadeh, S. Comparison of therapeutic effects of magnesium sulfate vs. Dexamethasone/metoclopramide on alleviating acute migraine headache. J. Emerg. Med. 2015, 48, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, R. Clinical effects of ozagrel combined with magnesium for treating migraine. China Med. Eng. 2013, 21, 93. [Google Scholar]
- Tang, L.; Zhou, Y. Clinical effects of ozagrel combined with magnesium for treating migraine. J. Prac. Med. 2011, 27, 2531. [Google Scholar]
- Wang, Y. Clinical study of intravenous magnesium for treating migraine. J. Qiqihar Med. Coll. 2013, 31, 89. [Google Scholar]
- Xu, L.; XU, Y. Observation of the effect of intravenous magnesium combined with lidocaine for treating migraine. Med. Inf. 2010, 23, 2103. [Google Scholar]
- Maizels, M.; Blumenfeld, A.; Burchette, R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: A randomized trial. Headache J. Head Face Pain 2004, 44, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Observation of Clinical Effects of Magnesium Valproate Combined with Venlafaxine Hcl in 54 Individuals with Migraine. Available online: https://wenku.baidu.com/view/280201f404a1b0717fd5ddfc.html (accessed on 5 June 2018).
- Köseoglu, E.; Talaslıoglu, A.; Gönül, A.S.; Kula, M. The effects of magnesium prophylaxis in migraine without aura. Magnes. Res. 2008, 21, 101–108. [Google Scholar] [PubMed]
- Esfanjani, A.T.; Mahdavi, R.; Mameghani, M.E.; Talebi, M.; Nikniaz, Z.; Safaiyan, A. The effects of magnesium, l-carnitine, and concurrent cagnesium–l-carnitine supplementation in migraine prophylaxis. Biol. Trace Elem. Res. 2012, 150, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shan, H. Effects of magnesium sulphate, ergotamine, and flunarizine hydrochloride on treating migraine. Prev. Med. 2001, 12, 366–367. [Google Scholar]
- Yang, X.; Yang, J.; Yuan, R.; Xu, X.; Zhang, L. Randomized double blind controlled trial of magnesium valproate in migraine prophylaxis. China Hosp. Pharm. 2005, 25, 649–651. [Google Scholar]
- Bian, X.; Zhu, Y.; Xia, J.; Ai, H.; Guo, Q.; Lu, L.; Zhang, Q. Clinical observation on potassium magnesium asparate oral soluation combined with flinarizine capsule for migraine prophylaxis. J. Commun. Med. 2013, 11, 6–7. [Google Scholar]
- Lan, Q.; Yang, C. Clinical observation of magnesium propylvalerate on treating migraine. Youjiang Med. Coll. Natl. 1998, 21, 639–640. [Google Scholar]
- Tang, X.; Lin, W.; Zheng, X.; Liu, C. Observation of effects of nimodipine and magnesium sulfate combination for treating migraine. Qiqihar Med. Coll. 1998, 19, 5–6. [Google Scholar]
- Chiu, H.Y.; Yeh, T.H.; Huang, Y.C.; Chen, P.Y. Effects of intravenous and oral magnesium on reducing migraine: A meta-analysis of randomized controlled trials. Pain Phys. 2016, 19, E97–E112. [Google Scholar]
- Baratloo, A.; Mirbaha, S.; Delavar Kasmaei, H.; Payandemehr, P.; Elmaraezy, A.; Negida, A. Intravenous caffeine citrate vs. magnesium sulfate for reducing pain in patients with acute migraine headache; a prospective quasi-experimental study. Korean J. Pain 2017, 30, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Von Luckner, A.; Riederer, F. Magnesium in migraine prophylaxis—Is there an evidence-based rationale? A systematic review. Headache J. Head Face Pain 2018, 58, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Crofford, L.J. Chronic pain: Where the body meets the brain. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 167. [Google Scholar] [PubMed]
- McBeth, J.; Jones, K. Epidemiology of chronic musculoskeletal pain. Best Prac. Res. Clin. Rheumatol. 2007, 21, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Coderre, T.J.; Hagen, N.A. Targeting the N-methyl-d-aspartate receptor for chronic pain management: Preclinical animal studies, recent clinical experience and future research directions. J. Pain Symptom Manag. 2000, 20, 358–373. [Google Scholar] [CrossRef]
- Srebro, D.; Vuckovic, S.; Milovanovic, A.; Kosutic, J.; Savic Vujovic, K.; Prostran, M. Magnesium in pain research: State of the art. Curr. Med. Chem. 2017, 24, 424–434. [Google Scholar]
- Banerjee, S.; Jones, S. Magnesium as an Alternative or Adjunct to Opioids for Migraine and Chronic Pain: A Review of the Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2017. [Google Scholar]
- Fischer, S.G.; Collins, S.; Boogaard, S.; Loer, S.A.; Zuurmond, W.W.; Perez, R.S. Intravenous magnesium for chronic complex regional pain syndrome type 1 (CRPS-1). Pain Med. 2013, 14, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Van der Plas, A.A.; Schilder, J.C.; Marinus, J.; van Hilten, J.J. An explanatory study evaluating the muscle relaxant effects of intramuscular magnesium sulphate for dystonia in complex regional pain syndrome. J. Pain 2013, 14, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; Al-deeb, A. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia 2013, 68, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.A.; Zappa, S.; Minelli, C.; Bonardelli, S.; Lamberti, L.; Bisighini, L.; Zangrandi, M.; Turin, M.; Rizzo, F.; Rizzolo, A. Magnesium-oral supplementation to reduce pain in patients with severe peripheral arterial occlusive disease: The mag-paper randomised clinical trial protocol. BMJ Open 2015, 5, e009137. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P. The american college of rheumatology 1990 criteria for the classification of fibromyalgia. Arthr. Rheumatol. 1990, 33, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Bagis, S.; Karabiber, M.; As, I.; Tamer, L.; Erdogan, C.; Atalay, A. Is magnesium citrate treatment effective on pain, clinical parameters and functional status in patients with fibromyalgia? Rheumatol. Int. 2013, 33, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, G.; Guymer, E. Modulation of NMDA receptor activity in fibromyalgia. Biomedicines 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.E.; Flechas, J.D. Management of fibromyalgia: Rationale for the use of magnesium and malic acid. J. Nutr. Med. 1992, 3, 49–59. [Google Scholar] [CrossRef]
- Magaldi, M.; Moltoni, L.; Biasi, G.; Marcolongo, R. Role of intracellular calcium ions in the physiopathology of fibromyalgia syndrome. Boll. Della Soc. Ital. Biol. Sper. 2000, 76, 1–4. [Google Scholar]
- Eisinger, J.; Plantamura, A.; Ayavou, T. Glycolysis abnormalities in fibromyalgia. J. Am. Coll. Nutr. 1994, 13, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Eisinger, J.; Zakarian, H.; Pouly, E.; Plantamura, A.; Ayavou, T. Protein peroxidation, magnesium deficiency and fibromyalgia. Magnes. Res. 1996, 9, 313–316. [Google Scholar] [PubMed]
- Romano, T.J.; Stiller, J.W. Magnesium deficiency in fibromyalgia syndrome. J. Nutr. Med. 1994, 4, 165–167. [Google Scholar] [CrossRef]
- Clauw, D.; Ward, K.; Katz, P.; Rajan, S. Muscle intracellular magnesium levels correlate with pain tolerance in fibromyalgia (FM). Arthr. Rheum. 1994, 37, R29. [Google Scholar]
- Sakarya, S.T.; Akyol, Y.; Bedir, A.; Canturk, F. The relationship between serum antioxidant vitamins, magnesium levels, and clinical parameters in patients with primary fibromyalgia syndrome. Clin. Rheumatol. 2011, 30, 1039–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sendur, O.F.; Tastaban, E.; Turan, Y.; Ulman, C. The relationship between serum trace element levels and clinical parameters in patients with fibromyalgia. Rheumatol. Int. 2008, 28, 1117. [Google Scholar] [CrossRef] [PubMed]
- Kasim, A.A. Calcium, magnesium and phosphorous levels in serum of Iraqi women with fibromyalgia. Iraqi J. Pharm. Sci. 2017, 20, 34–37. [Google Scholar]
- Engen, D.J.; McAllister, S.J.; Whipple, M.O.; Cha, S.S.; Dion, L.J.; Vincent, A.; Bauer, B.A.; Wahner-Roedler, D.L. Effects of transdermal magnesium chloride on quality of life for patients with fibromyalgia: A feasibility study. J. Integr. Med. 2015, 13, 306–313. [Google Scholar] [CrossRef]
- Calleo, J.; Amspoker, A.B.; Marsh, L.; Kunik, M.E. Mental health diagnoses and health care utilization in persons with dementia, Parkinson’s disease, and stroke. J. Neuropsychiatry Clin. Neurosci. 2015, 27, e117–e121. [Google Scholar] [CrossRef] [PubMed]
- Tsatali, M.; Papaliagkas, V.; Damigos, D.; Mavreas, V.; Gouva, M.; Tsolaki, M. Depression and anxiety levels increase chronic musculoskeletal pain in patients with alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Cho, S.J.; Chung, Y.K.; Kim, J.M.; Chu, M.K. Combination of anxiety and depression is associated with an increased headache frequency in migraineurs: A population-based study. BMC Neurol. 2014, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Riaza Bermudo-Soriano, C.; Perez-Rodriguez, M.M.; Vaquero-Lorenzo, C.; Baca-Garcia, E. New perspectives in glutamate and anxiety. Pharmacol. Biochem. Behav. 2012, 100, 752–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niciu, M.J.; Ionescu, D.F.; Richards, E.M.; Zarate, C.A., Jr. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J. Neural Transm. 2014, 121, 907–924. [Google Scholar] [CrossRef] [PubMed]
- Pochwat, B.; Szewczyk, B.; Sowa-Kucma, M.; Siwek, A.; Doboszewska, U.; Piekoszewski, W.; Gruca, P.; Papp, M.; Nowak, G. Antidepressant-like activity of magnesium in the chronic mild stress model in rats: Alterations in the nmda receptor subunits. Int. J. Neuropsychopharmacol. 2014, 17, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Aguilar-Gaxiola, S.; Alonso, J.; Chatterji, S.; Lee, S.; Ormel, J.; Üstün, T.B.; Wang, P.S. The global burden of mental disorders: An update from the who world mental health (WMH) surveys. Epidemiol. Psychiatr. Sci. 2009, 18, 23–33. [Google Scholar] [CrossRef]
- Boyle, N.B.; Lawton, C.; Dye, L. The effects of magnesium supplementation on subjective anxiety and stress-a systematic review. Nutrients 2017, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Mocci, F.; Canalis, P.; Tomasi, P.; Casu, F.; Pettinato, S. The effect of noise on serum and urinary magnesium and catecholamines in humans. Occup. Med. 2001, 51, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Poleszak, E.; Szewczyk, B.; Kędzierska, E.; Wlaź, P.; Pilc, A.; Nowak, G. Antidepressant-and anxiolytic-like activity of magnesium in mice. Pharmacol. Biochem. Behav. 2004, 78, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Overland, S.; Stewart, R.; Tell, G.S.; Bjelland, I.; Mykletun, A. Association between magnesium intake and depression and anxiety in community-dwelling adults: The hordaland health study. Aust. N. Z. J. Psychiatry 2009, 43, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Ahmed, M.U.; Mitu, S.A.; Islam, M.S.; Rahman, G.M.; Qusar, M.S.; Hasnat, A. Comparative analysis of serum zinc, copper, manganese, iron, calcium, and magnesium level and complexity of interelement relations in generalized anxiety disorder patients. Biol. Trace Elem. Res. 2013, 154, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Gendle, M.H.; O’Hara, K.P. Oral magnesium supplementation and test anxiety in university undergraduates. J. Artic. Support Null Hypothesis 2015, 11, 21–30. [Google Scholar]
- Bourgeois, M. Rôle du magne-b6 dans les manifestations anxueuses en pratique medicale courante psychiatr. Pract. Med 1987, 39, 18–22. [Google Scholar]
- Scharbach, H. Anxiété et magné-b6. La Vie Médicale 1988, 69, 867–869. [Google Scholar]
- Caillard, V. Sanofi Internal Report Mab6-26, Paris, France, Unpublished work. 1992.
- Caillard, V. Sanofi Internal Report Mab6-32, Paris, France, Unpublished work. 1996.
- Cazaubiel, J.; Desor, D. Evaluation of the anti-stress effects of a fermented milk containing milk protein hydrolysate on healthy human subjects sensitive to the stress of everyday life. Proprietary data cited in scientific opinion of the panel on dietetic products, nutrition and allergies, No. 1924/20061. Eur. Food Saf. Auth. J. Unpubl. Work 2008, 905, 1–10. [Google Scholar]
- Hanus, M.; Lafon, J.; Mathieu, M. Double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of a fixed combination containing two plant extracts (Crataegus oxyacantha and Eschscholtzia californica) and magnesium in mild-to-moderate anxiety disorders. Curr. Med. Res. Opin. 2004, 20, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Borella, P.; Sances, G.; Fioroni, L.; Nappi, R.E.; Genazzani, A.R. Oral magnesium successfully relieves premenstrual mood changes. Obstetr. Gynecol. 1991, 78, 177–181. [Google Scholar]
- Walker, A.F.; De Souza, M.C.; Vickers, M.F.; Abeyasekera, S.; Collins, M.L.; Trinca, L.A. Magnesium supplementation alleviates premenstrual symptoms of fluid retention. J. Women Health 1998, 7, 1157–1165. [Google Scholar] [CrossRef]
- Walker, A.F.; Marakis, G.; Christie, S.; Byng, M. Mg citrate found more bioavailable than other mg preparations in a randomised, double-blind study. Magnes. Res. 2003, 16, 183–191. [Google Scholar] [PubMed]
- Khine, K.; Rosenstein, D.L.; Elin, R.J.; Niemela, J.E.; Schmidt, P.J.; Rubinow, D.R. Magnesium (MG) retention and mood effects after intravenous mg infusion in premenstrual dysphoric disorder. Biol. Psychiatry 2006, 59, 327–333. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.C.; Walker, A.F.; Robinson, P.A.; Bolland, K. A synergistic effect of a daily supplement for 1 month of 200 mg magnesium plus 50 mg vitamin b6 for the relief of anxiety-related premenstrual symptoms: A randomized, double-blind, crossover study. J. Women Health Gend. Based Med. 2000, 9, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, N.; Ebrahimi, E.; Valiani, M.; Tavakoli, N.; Yar, M.H. Evaluating the effect of magnesium and magnesium plus vitamin B6 supplement on the severity of premenstrual syndrome. Iran. J. Nurs. Midwifery Res. 2010, 15, 401. [Google Scholar] [PubMed]
- Fard, F.E.; Mirghafourvand, M.; Mohammad-Alizadeh Charandabi, S.; Farshbaf-Khalili, A.; Javadzadeh, Y.; Asgharian, H. Effects of zinc and magnesium supplements on postpartum depression and anxiety: A randomized controlled clinical trial. Women Health 2017, 57, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Vieira, K.F. Nutritional and herbal supplements for anxiety and anxiety-related disorders: Systematic review. Nutr. J. 2010, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Eby, G.A.; Eby, K.L. Magnesium for treatment-resistant depression: A review and hypothesis. Med. Hypotheses 2010, 74, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lv, J.; Wang, W.; Zhang, D. Dietary magnesium and calcium intake and risk of depression in the general population: A meta-analysis. Aust. N. Z. J. Psychiatry 2017, 51, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Rechenberg, K. Nutritional interventions in clinical depression. Clin. Psychol. Sci. 2016, 4, 144–162. [Google Scholar] [CrossRef]
- Paul, I.A. Antidepressant activity and calcium signaling cascades. Hum. Psychopharmacol. Clin. Exp. 2001, 16, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hollister, L.E.; Trevino, E.S.G. Calcium channel blockers in psychiatric disorders: A review of the literature. Can. J. Psychiatry 1999, 44, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Eby, G.A.; Eby, K.L. Rapid recovery from major depression using magnesium treatment. Med. Hypotheses 2006, 67, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Murck, H. Magnesium and affective disorders. Nutr. Neurosci. 2002, 5, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, M. Magnesium in major depression. Magnes. Res. 2009, 22, 163–166. [Google Scholar]
- Camardese, G.; De Risio, L.; Pizi, G.; Mattioli, B.; Buccelletti, F.; Serrani, R.; Leone, B.; Sgambato, A.; Bria, P.; Janiri, L. Plasma magnesium levels and treatment outcome in depressed patients. Nutr. Neurosci. 2012, 15, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Rodriguez, L.; Rodriguez-Moran, M.; Guerrero-Romero, F. Depressive symptoms and hypomagnesemia in older diabetic subjects. Arch. Med. Res. 2007, 38, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Hasey, G.M.; D’Alessandro, E.; Cooke, R.G.; Warsh, J.J. The interface between thyroid activity, magnesium, and depression: A pilot study. Biol. Psychiatry 1993, 33, 133–135. [Google Scholar] [CrossRef]
- Derom, M.-L.; Sayón-Orea, C.; Martínez-Ortega, J.M.; Martínez-González, M.A. Magnesium and depression: A systematic review. Nutr. Neurosci. 2013, 16, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Bhudia, S.K.; Cosgrove, D.M.; Naugle, R.I.; Rajeswaran, J.; Lam, B.K.; Walton, E.; Petrich, J.; Palumbo, R.C.; Gillinov, A.M.; Apperson-Hansen, C.; et al. Magnesium as a neuroprotectant in cardiac surgery: A randomized clinical trial. J. Thorac. Cardiovasc. Surg. 2006, 131, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.; Atlas, S.E.; Qadir, S.; Musselman, D.; Goldberg, S.; Woolger, J.M.; Corredor, R.; Abbas, M.H.; Arosemena, L.; Caccamo, S. Double-blind, randomized crossover study of intravenous infusion of magnesium sulfate versus 5% dextrose on depressive symptoms in adults with treatment-resistant depression. Psychiatry Clin. Neurosci. 2017, 71, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of magnesium supplementation in the treatment of depression: A randomized clinical trial. PLoS ONE 2017, 12, e0180067. [Google Scholar] [CrossRef] [PubMed]
- Rajizadeh, A.; Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Dehghani, A. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutrition 2017, 35, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Um, P.; Dickerman, B.; Liu, J. Zinc, magnesium, selenium and depression: A review of the evidence, potential mechanisms and implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.I.; Birnbaum, H.G.; Kidolezi, Y.; Qiu, Y.; Mallett, D.; Caleo, S. Economic burden of epilepsy among the privately insured in the US. Pharmacoeconomics 2010, 28, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Atlas: Country Resources for Neurological Disorders 2004: Results of a Collaborative Study of the World Health Organization and the World Federation of Neurology; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Metcalfe, A.; Jette, N. Medical and employment-related costs of epilepsy in the USA. Expert Rev. Pharmacoecon. Outcomes Res. 2010, 10, 645–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Alexopoulos, A.V. Pharmacoresistant epilepsy: From pathogenesis to current and emerging therapies. Cleve Clin. J. Med. 2010, 77, 457–567. [Google Scholar] [CrossRef] [PubMed]
- Yuen, A.W.; Sander, J.W. Can magnesium supplementation reduce seizures in people with epilepsy? A hypothesis. Epilepsy Res. 2012, 100, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, K.; McDowell, M.A.; Briefel, R.; Bischof, A.; Caughman, C.; Loria, C.; Johnson, C. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the united states: Third national health and nutrition examination survey, phase 1, 1988–1991. Adv. Data 1994, 258, 1–28. [Google Scholar]
- Ismail, Y.; Ismail, A.A.; Ismail, A.A. The underestimated problem of using serum magnesium measurements to exclude magnesium deficiency in adults; a health warning is needed for “normal” results. Clin. Chem. Lab. Med. 2010, 48, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Barker-Haliski, M.; White, H.S. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022863. [Google Scholar] [CrossRef] [PubMed]
- Coan, E.; Collingridge, G. Magnesium ions block an N-methyl-d-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci. Lett. 1985, 53, 21–26. [Google Scholar] [CrossRef]
- Isaev, D.; Ivanchick, G.; Khmyz, V.; Isaeva, E.; Savrasova, A.; Krishtal, O.; Holmes, G.L.; Maximyuk, O. Surface charge impact in low-magnesium model of seizure in rat hippocampus. J. Neurophysiol. 2011, 107, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Prasad, C.; Kobrzynski, M.; Campbell, C.; Filler, G. Seizures related to hypomagnesemia: A case series and review of the literature. Child Neurol. Open 2016, 3, 2329048X16674834. [Google Scholar] [CrossRef] [PubMed]
- Osborn, K.E.; Shytle, R.D.; Frontera, A.T.; Soble, J.R.; Schoenberg, M.R. Addressing potential role of magnesium dyshomeostasis to improve treatment efficacy for epilepsy: A reexamination of the literature. J. Clin. Pharmacol. 2016, 56, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, O.; Ajala, M.; Okubadejo, N.; Danesi, M.; Afonja, O. Plasma magnesium in adult nigerian patients with epilepsy. Niger. Postgrad. Med. J. 2003, 10, 234–237. [Google Scholar] [PubMed]
- Sinert, R.; Zehtabchi, S.; Desai, S.; Peacock, P.; Altura, B.; Altura, B. Serum ionized magnesium and calcium levels in adult patients with seizures. Scand. J. Clin. Lab. Investig. 2007, 67, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Manhas, A.S.; Gupta, V.K.; Bhatt, R. Serum magnesium levels in idiopathic epilepsy. J. Assoc. Phys. India 1994, 42, 456–457. [Google Scholar]
- Sood, A.; Handa, R.; Malhotra, R.; Gupta, B. Serum, CSF, RBC & urinary levels of magnesium & calcium in idiopathic generalised tonic clonic seizures. Indian J. Med. Res. 1993, 98, 152–154. [Google Scholar] [PubMed]
- Prebble, J. Primary infantile hypomagnesaemia: Report of two cases. J. Paediatr. Child Health 1995, 31, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Unachak, K.; Louthrenoo, O.; Katanyuwong, K. Primary hypomagnesemia in thai infants: A case report with 7 years follow-up and review of literature. J. Med. Assoc. Thai. 2002, 85, 1226–1231. [Google Scholar]
- Visudhiphan, P.; Visudtibhan, A.; Chiemchanya, S.; Khongkhatithum, C. Neonatal seizures and familial hypomagnesemia with secondary hypocalcemia. Pediatr. Neurol. 2005, 33, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Mahmoudi, M.; Meysamie, A.; Gharedaghi, M.; Zamponi, G.W.; Rezaei, N. Possible role of trace elements in epilepsy and febrile seizures: A meta-analysis. Nutr. Rev. 2015, 73, 760–779. [Google Scholar] [CrossRef] [PubMed]
- Fagan, C.; Phelan, D. Severe convulsant hypomagnesaemia and short bowel syndrome. Anaesth. Intens. Care 2001, 29, 281. [Google Scholar]
- Weisleder, P.; Tobin, J.A.; Kerrigan, J.F.; Bodensteiner, J.B. Hypomagnesemic seizures: Case report and presumed pathophysiology. J. Child Neurol. 2002, 17, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.F.; Khan, K.S.; Arnott, N. Magnesium sulphate in the treatment of eclampsia and pre-eclampsia: An overview of the evidence from randomised trials. BJOG Int. J. Obstet. Gynaecol. 1996, 103, 1085–1091. [Google Scholar] [CrossRef]
- Duley, L.; Gülmezoglu, A.M.; Henderson-Smart, D.J.; Chou, D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Libr. 2010, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Duley, L.; Henderson-Smart, D.J.; Walker, G.J.; Chou, D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Libr. 2010, 12, 127–186. [Google Scholar] [CrossRef] [PubMed]
- Kamate, M.; Singh, N.; Patil, S. Familial hypomagnesemia with secondary hypocalcemia mimicking neurodegenerative disorder. Indian Pediatr. 2015, 52, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Povalko, N.; Yatsuga, S.; Nishioka, J.; Kakuma, T.; Matsuishi, T.; Koga, Y. New trpm6 mutation and management of hypomagnesaemia with secondary hypocalcaemia. Brain Dev. 2015, 37, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Sassen, M.C.; Weber, S.; Pechmann, U.; Kusch, K.; Pelken, L.; Lotan, D.; Syrrou, M.; Prebble, J.J.; Cole, D.E. Novel TRPM6 mutations in 21 families with primary hypomagnesemia and secondary hypocalcemia. J. Am. Soc. Nephrol. 2005, 16, 3061–3069. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.A.; Braun, K.P.; Leijten, F.S.; van Nieuwenhuizen, O.; Wokke, J.H.; van den Bergh, W.M. Magnesium treatment for patients with refractory status epilepticus due to POLG1-mutations. J. Neurol. 2011, 258, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Gupta, A.; Baduni, N.; Vijfdar, H.; Sinha, S.; Jain, A. Refractory status epilepticus-magnesium as rescue therapy. Anaesth. Intens. Care 2010, 38, 962. [Google Scholar]
- Zou, L.P.; Wang, X.; Dong, C.H.; Chen, C.H.; Zhao, W.; Zhao, R.Y. Three-week combination treatment with acth + magnesium sulfate versus acth monotherapy for infantile spasms: A 24-week, randomized, open-label, follow-up study in China. Clin. Ther. 2010, 32, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Gorica, D.; Slavica, D.D.; Vladan, D.; Nebojsa, Z.; Curcic, D.; Rankovic, A.; Branimir, R.; Vladimir, J. Interictal ionized magnesium/total serum magnesium ratio in serbian population with drug resistant epilepsy-whether is severe epilepsy in fact brain injury? Neuropsychiatry 2017, 7, 629–636. [Google Scholar] [CrossRef]
- Forte, G.; Alimonti, A.; Violante, N.; Di Gregorio, M.; Senofonte, O.; Petrucci, F.; Sancesario, G.; Bocca, B. Calcium, copper, iron, magnesium, silicon and zinc content of hair in parkinson’s disease. J. Trace Elem. Med. Biol. 2005, 19, 195–201. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Ambrosi, G.; Cerri, S.; Blandini, F. A further update on the role of excitotoxicity in the pathogenesis of parkinson’s disease. J. Neural. Transm. 2014, 121, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Kurup, R.K.; Kurup, P.A. Hypothalamic digoxin-mediated model for Parkinson’s disease. Int. J. Neurosci. 2003, 113, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary intake of metals and risk of Parkinson’s disease: A case-control study in Japan. J. Neurol. Sci. 2011, 306, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.S.; Poryali, A.; Ames, A. Normal serum magnesium levels in Parkinson’s disease. Neurology 1964, 14, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Alimonti, A.; Senofonte, O.; Pino, A.; Violante, N.; Petrucci, F.; Sancesario, G.; Forte, G. Metal changes in csf and peripheral compartments of Parkinsonian patients. J. Neurol. Sci. 2006, 248, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Kihira, T.; Ota, K. Calcium, magnesium and aluminum concentrations in Parkinson’s disease. Neurotoxicology 1992, 13, 593–600. [Google Scholar] [PubMed]
- Uitti, R.J.; Rajput, A.H.; Rozdilsky, B.; Bickis, M.; Wollin, T.; Yuen, W.K. Regional metal concentrations in Parkinson’s disease, other chronic neurological diseases, and control brains. Can. J. Neurol. Sci. 1989, 16, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2018 alzheimer’s disease facts and figures. Alzheimer Dement. 2018, 14, 367–429. [Google Scholar]
- Zadori, D.; Veres, G.; Szalardy, L.; Klivenyi, P.; Vecsei, L. Alzheimer’s disease: Recent concepts on the relation of mitochondrial disturbances, excitotoxicity, neuroinflammation, and kynurenines. J. Alzheimers Dis. 2018, 62, 523–547. [Google Scholar] [CrossRef] [PubMed]
- Shatenstein, B.; Kergoat, M.-J.; Reid, I. Poor nutrient intakes during 1-year follow-up with community-dwelling older adults with early-stage Alzheimer dementia compared to cognitively intact matched controls. J. Am. Diet. Assoc. 2007, 107, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Zurlo, A.; Solmi, M.; Luchini, C.; Trevisan, C.; Bano, G.; Manzato, E.; Sergi, G.; Rylander, R. Magnesium status in Alzheimer’s disease: A systematic review. Am. J. Alzheimers Dis. Other Demen. 2016, 31, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Belvedere, M.; Di Bella, G.; Dominguez, L.J. Altered ionized magnesium levels in mild-to-moderate Alzheimer’s disease. Magnes. Res. 2011, 24, 115–121. [Google Scholar]
- Andrasi, E.; Igaz, S.; Molnár, Z.; Mako, S. Disturbances of magnesium concentrations in various brain areas in alzheimer’s disease. Magnes. Res. 2000, 13, 189–196. [Google Scholar] [PubMed]
- Glick, J.L. Dementias: The role of magnesium deficiency and an hypothesis concerning the pathogenesis of alzheimer’s disease. Med. Hypotheses 1990, 31, 211–225. [Google Scholar] [CrossRef]
- Basun, H.; Forssell, L.; Wetterberg, L.; Winblad, B. Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer’s disease. J. Neural Transm. Parkinson Dis. Dement. Sect. 1991, 3, 231–258. [Google Scholar]
- Boström, F.; Hansson, O.; Gerhardsson, L.; Lundh, T.; Minthon, L.; Stomrud, E.; Zetterberg, H.; Londos, E. Csf mg and Ca as diagnostic markers for dementia with lewy bodies. Neurobiol. Aging 2009, 30, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Fujiwara, S.; Arimoto, S.; Koide, H.; Fukuda, J.; Shimode, K.; Yamaguchi, S.; Okada, K.; Tsunematsu, T. Hair aluminium in normal aged and senile dementia of alzheimer type. Progress Clin. Biol. Res. 1989, 317, 1095–1109. [Google Scholar] [CrossRef]
- Shore, D.; Henkin, R.I.; Nelson, N.R.; Agarwal, R.P.; Wyatt, R.J. Hair and serum copper, zinc, calcium, and magnesium concentrations in alzheimer-type dementia. J. Am. Geriatr. Soc. 1984, 32, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Gustaw-Rothenberg, K.; Lerner, A.; Bonda, D.J.; Lee, H.-G.; Zhu, X.; Perry, G.; Smith, M.A. Biomarkers in Alzheimer’s disease: Past, present and future. Biomark. Med. 2010, 4, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Banerjee, B.; Bala, K.; Basu, M.; Chhillar, N. Polymorphism in cytochrome P450 2D6, glutathione S-transferases Pi 1 genes, and organochlorine pesticides in Alzheimer disease: A case–control study in north indian population. J. Geriatr. Psychiatry Neurol. 2014, 27, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Vural, H.; Demirin, H.; Kara, Y.; Eren, I.; Delibas, N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. J. Trace Elem. Med. Biol. 2010, 24, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Borella, P.; Giardino, A.; Neri, M.; Andermarker, E. Magnesium and potassium status in elderly subjects with and without dementia of the Alzheimer type. Magnes. Res. 1990, 3, 283–289. [Google Scholar] [PubMed]
- Brackenridge, C.; McDonald, C. The concentrations of magnesium and potassium in erythrocytes and plasma of geriatric patients with psychiatric disorders. Med. J. Aust. 1969, 2, 390. [Google Scholar] [PubMed]
- Lemke, M.R. Plasma magnesium decrease and altered calcium/magnesium ratio in severe dementia of the Alzheimer type. Biol. Psychiatry 1995, 37, 341–343. [Google Scholar] [CrossRef]
- Hozumi, I.; Hasegawa, T.; Honda, A.; Ozawa, K.; Hayashi, Y.; Hashimoto, K.; Yamada, M.; Koumura, A.; Sakurai, T.; Kimura, A. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J. Neurol. Sci. 2011, 303, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Çilliler, A.E.; Öztürk, Ş.; Özbakır, Ş. Serum magnesium level and clinical deterioration in alzheimer’s disease. Gerontology 2007, 53, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Koc, E.R.; Ilhan, A.; Ayturk, Z.; Acar, B.; Gurler, M.; Altuntas, A.; Karapirli, M.; Bodur, A.S. A comparison of hair and serum trace elements in patients with Alzheimer disease and healthy participants. Turk. J. Med. Sci. 2015, 45, 1034–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmus, I.M.; Strungaru, S.A.; Ciobica, A.; Nicoara, M.N.; Dobrin, R.; Plavan, G.; Stefanescu, C. Preliminary data on the interaction between some biometals and oxidative stress status in mild cognitive impairment and Alzheimer’s disease patients. Oxid. Med. Cell. Longev. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kieboom, B.C.T.; Licher, S.; Wolters, F.J.; Ikram, M.K.; Hoorn, E.J.; Zietse, R.; Stricker, B.H.; Ikram, M.A. Serum magnesium is associated with the risk of dementia. Neurology 2017, 89, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Kumar, R.; Sachdev, P.; Anstey, K.J. Dietary mineral intake and risk of mild cognitive impairment: The path through life project. Front. Aging Neurosci. 2014, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: The hisayama study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Majid, A. Neuroprotection in stroke: Past, present, and future. ISRN Neurol. 2014, 2014, 515716. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Laurant, P.; Touyz, R.M. Physiological and pathophysiological role of magnesium in the cardiovascular system: Implications in hypertension. J. Hypertens. 2000, 18, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Ohira, T.; Peacock, J.M.; Iso, H.; Chambless, L.E.; Rosamond, W.D.; Folsom, A.R. Serum and dietary magnesium and risk of ischemic stroke: The atherosclerosis risk in communities study. Am. J. Epidemiol. 2009, 169, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, S.N.; Jimenez, M.C.; Chiuve, S.E.; Spiegelman, D.; Willett, W.C.; Rexrode, K.M. Plasma magnesium and risk of ischemic stroke among women. Stroke 2014, 45, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Tsivgoulis, G.; Malhotra, K.; Houck, A.L.; Khorchid, Y.M.; Pandhi, A.; Inoa, V.; Alsherbini, K.; Alexandrov, A.V.; Arthur, A.S.; et al. Serum magnesium levels and outcomes in patients with acute spontaneous intracerebral hemorrhage. J. Am. Heart Assoc. 2018, 7, e008698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, J.; Del Gobbo, L.C.; Hruby, A.; Dai, Q.; Song, Y. Serum magnesium concentrations and all-cause, cardiovascular, and cancer mortality among U.S. Adults: Results from the NHANES I epidemiologic follow-up study. Clin. Nutr. 2017, 30304–30307. [Google Scholar] [CrossRef] [PubMed]
- Rosique-Esteban, N.; Guasch-Ferré, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Dietary magnesium and cardiovascular disease: A review with emphasis in epidemiological studies. Nutrients 2018, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Dietary magnesium intake and risk of stroke: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 2011, 95, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.-L.; Wang, Z.-M.; Zhou, B.; Tang, Z.-P.; Wang, S.-K. Magnesium intake and incidence of stroke: Meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, S.N.; Spiegelman, D.; Flint, A.J.; Willett, W.C.; Rexrode, K.M. Intakes of magnesium, potassium, and calcium and the risk of stroke among men. Int. J. Stroke 2015, 10, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Stampfer, M.J.; Manson, J.E.; Rexrode, K.; Hennekens, C.H.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke 1999, 30, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtanen, M.J.; Mars, M.; Männistö, S.; Pietinen, P.; Albanes, D.; Virtamo, J. Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch. Int. Med. 2008, 168, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.-C.; Yeh, W.-T.; Bai, C.-H.; Chen, H.-J.; Chuang, S.-Y.; Chang, H.-Y.; Lin, B.-F.; Chen, K.-J.; Pan, W.-H. Is ischemic stroke risk related to folate status or other nutrients correlated with folate intake? Stroke 2008, 39, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
- Leijenaar, J.F.; Dorhout Mees, S.M.; Algra, A.; van den Bergh, W.M.; Rinkel, G.J.; Group, M.-I.S. Effect of magnesium treatment and glucose levels on delayed cerebral ischemia in patients with subarachnoid hemorrhage: A substudy of the magnesium in aneurysmal subarachnoid haemorrhage trial (MASH-II). Int. J. Stroke 2015, 10 (Suppl. A100), 108–112. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Zhong, C.; Du, H.; Zhang, Y.; Zheng, D.; Wang, X.; Qiu, C.; Zhao, H.; Cao, Y.; Liu, C.F. Admission low magnesium level is associated with in-hospital mortality in acute ischemic stroke patients. Cerebrovasc. Dis. 2017, 44, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bayir, A.; Kara, H.; Ak, A.; Cander, B.; Kara, F. Magnesium sulfate in emergency department patients with hypertension. Biol. Trace Elem. Res. 2009, 128, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Liotta, E.M.; Prabhakaran, S.; Sangha, R.S.; Bush, R.A.; Long, A.E.; Trevick, S.A.; Potts, M.B.; Jahromi, B.S.; Kim, M.; Manno, E.M.; et al. Magnesium, hemostasis, and outcomes in patients with intracerebral hemorrhage. Neurology 2017, 89, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart disease and stroke statistics-2016 update: A report from the American heart association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Mees, S.M.D.; Algra, A.; Vandertop, W.P.; van Kooten, F.; Kuijsten, H.A.; Boiten, J.; van Oostenbrugge, R.J.; Salman, R.A.-S.; Lavados, P.M.; Rinkel, G.J. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): A randomised placebo-controlled trial. Lancet 2012, 380, 44–49. [Google Scholar] [CrossRef]
- Saver, J.L.; Starkman, S.; Eckstein, M.; Stratton, S.J.; Pratt, F.D.; Hamilton, S.; Conwit, R.; Liebeskind, D.S.; Sung, G.; Kramer, I. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N. Engl. J. Med. 2015, 372, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.M.; Finfer, S.; O’Connor, A.; Yarad, E.; Firth, R.; McCallister, R.; Harrington, T.; Steinfort, B.; Faulder, K.; Assaad, N. A randomised controlled trial of induced hypermagnesaemia following aneurysmal subarachnoid haemorrhage. Crit. Care Resusc. 2013, 15, 119. [Google Scholar] [PubMed]
- Van den Bergh, W.M.; van der Schaaf, I.; van Gijn, J. The spectrum of presentations of venous infarction caused by deep cerebral vein thrombosis. Neurology 2005, 65, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.K.C.; Poon, W.S.; Chan, M.T.; Boet, R.; Gin, T.; Ng, S.C.; Zee, B.C. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): A randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke 2010, 41, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Dorhout Mees, S.M.; Algra, A.; Wong, G.K.; Poon, W.S.; Bradford, C.M.; Saver, J.L.; Starkman, S.; Rinkel, G.J.; van den Bergh, W.M.; et al. Early magnesium treatment after aneurysmal subarachnoid hemorrhage: Individual patient data meta-analysis. Stroke 2015, 46, 3190–3193. [Google Scholar] [CrossRef] [PubMed]
- Veyna, R.S.; Seyfried, D.; Burke, D.G.; Zimmerman, C.; Mlynarek, M.; Nichols, V.; Marrocco, A.; Thomas, A.J.; Mitsias, P.D.; Malik, G.M. Magnesium sulfate therapy after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2002, 96, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Van Norden, A.; Van Den Bergh, W.; Rinkel, G. Dose evaluation for long-term magnesium treatment in aneurysmal subarachnoid haemorrhage. J. Clin. Pharm. Ther. 2005, 30, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-D.; Rimmer, J.H. Effects of exercise on quality of life in stroke survivors: A meta-analysis. Stroke 2011, 42, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.; Hughes, R.; Morgan, M.K. Magnesium: A useful adjunct in the prevention of cerebral vasospasm following aneurysmal subarachnoid haemorrhage. J. Clin. Neurosci. 2002, 9, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, H.; Kulakszoğlu, E.O.; Tucer, B.; Menkü, A.; Postalc, L.; Günald, Ö. Magnesium sulfate therapy for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg. Q. 2009, 19, 35–39. [Google Scholar] [CrossRef]
- Panahi, Y.; Mojtahedzadeh, M.; Najafi, A.; Ghaini, M.R.; Abdollahi, M.; Sharifzadeh, M.; Ahmadi, A.; Rajaee, S.M.; Sahebkar, A. The role of magnesium sulfate in the intensive care unit. EXCLI J. 2017, 16, 464. [Google Scholar] [PubMed]
- Lampl, Y.; Gilad, R.; Geva, D.; Eshel, Y.; Sadeh, M. Intravenous administration of magnesium sulfate in acute stroke: A randomized double-blind study. Clin. Neuropharmacol. 2001, 24, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.E.; Chaudhry, S.A.; Grigoryan, M.; Tekle, W.G.; Qureshi, A.I. National trends in utilization and outcomes of endovascular treatment of acute ischemic stroke patients in the mechanical thrombectomy era. Stroke 2012, 43, 3012–3017. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.N.; Pan, Y.; Muengtaweeponsa, S.; Geller, T.J.; Cruz-Flores, S. Cannabis-related stroke: Case series and review of literature. J. Stroke Cerebrovasc. Dis. 2012, 21, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Afshari, D.; Moradian, N.; Rezaei, M. Evaluation of the intravenous magnesium sulfate effect in clinical improvement of patients with acute ischemic stroke. Clin. Neurol. Neurosurg. 2013, 115, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Lai, Y.H.; Yeh, W.T.; Chen, J.R.; Jeng, J.S.; Bai, C.H.; Lin, R.T.; Lee, T.H.; Chang, K.C.; Lin, H.J.; et al. Intake of potassium- and magnesium-enriched salt improves functional outcome after stroke: A randomized, multicenter, double-blind controlled trial. Am. J. Clin. Nutr. 2017, 106, 1267–1273. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. https://doi.org/10.3390/nu10060730
Kirkland AE, Sarlo GL, Holton KF. The Role of Magnesium in Neurological Disorders. Nutrients. 2018; 10(6):730. https://doi.org/10.3390/nu10060730
Chicago/Turabian StyleKirkland, Anna E., Gabrielle L. Sarlo, and Kathleen F. Holton. 2018. "The Role of Magnesium in Neurological Disorders" Nutrients 10, no. 6: 730. https://doi.org/10.3390/nu10060730
APA StyleKirkland, A. E., Sarlo, G. L., & Holton, K. F. (2018). The Role of Magnesium in Neurological Disorders. Nutrients, 10(6), 730. https://doi.org/10.3390/nu10060730




