1. Introduction
Linoleic acid (LA) and α-linolenic acid (ALA) are considered essential and can be converted into long-chain polyunsaturated fatty acid (LC-PUFA) metabolites that are responsible for important biological processes [
1,
2]. In humans, the enzymatic conversion from ALA to n-3 LC-PUFA such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) is ranging from 4 to less than 0.05% [
3]. It is well recognized that n-3 LC-PUFA are essential for normal growth and development [
4,
5,
6]. To maintain adequate levels, humans needs to source dietary n-3 fatty acids from animal foods and in preference from fish and marine products [
7].
n-3 LC-PUFA are integral part of cell membranes and provide the substrates for the production of signaling molecules that regulate blood clotting, contraction and relaxation of arterial walls, and inflammation [
7,
8] and immune response [
6]. Likely, due to these effects, n-3 LC-PUFA have been shown to help prevent heart disease and stroke, may also help to reduce eczema and rheumatoid arthritis, and play protective roles in other conditions such as cancer [
9,
10,
11,
12]. DHA is highly abundant in the brain and retina and it is important to ensure optimum neural and visual functions [
13].
Most dietary lipids are present in foods as triacylglycerols (TAGs) or in small proportions as monoacylglycerol (MAG), diacylglycerol (DAG) or phospholipids (PL). TAGs are hydrolyzed by lipolytic enzymes into free fatty acids and
sn-2-monoacylglycerols (
sn-2-MAG) mainly by the
sn-1,3 specific pancreatic lipases in the upper part of the gastrointestinal tract. Free fatty acids and
sn-2-MAG are absorbed by enterocytes and re-esterified to form TAG, cholesteryl esters and PL (mainly phosphatidylcholine), which are assembled into chylomicrons and secreted into lymphatic circulation. The metabolic fates and hydrolysis rates depend on the chain length, nature of the fatty acid in the TAG molecule and stereospecific location on the TAG. Indeed, there is evidence that the positional specificity has an effect on the uptake and impact of particular FA bioavailability [
1,
14,
15,
16].
Ensuring optimal absorption of n-3 LC-PUFA by optimizing their galenic form has become an important part of nutritional and therapeutic strategies. As a consequence, numerous studies have assessed the absorption, bioavailability and accretion into various tissues of different forms of n-3 LC-PUFA (mainly EPA and DHA) such as structured TAG, PL, free fatty acids, ethyl esters (EE), MAG, and DAG [
14,
15,
17,
18,
19,
20]. Intestinal absorption of DHA and EPA given as EE was reported to be lower than when provided as TAG or free fatty acids and different results were reported when related to lipoprotein studies [
21,
22,
23,
24]. Others report differences in the absorption of DHA and EPA when either esterified at the
sn-2 or at the
sn-1,3 positions of TAG [
14,
15,
18,
25,
26]. We reported MAG are potent dietary carriers of DHA and EPA by providing superior bioavailability under impaired absorption conditions compared to conventional dietary TAG [
19,
27].
However, the absorption and incorporation of DHA in tissues when provided as PL-DHA and MAG-DHA have never been compared to TAG-DHA in a single design experiment. In the present study, the incorporation of DHA in circulatory lipids (plasma and erythrocytes) has been investigated longitudinally over 60 days. Retina and brain region tissues were also analyzed to assess the benefit of using such alternative DHA carriers on DHA accretion in these tissues.
4. Discussion
During the 8-week study under the experimental diets all animals grew as expected. The difference observed in food intake in the PL-DHA group was reflected in the body weight gain (
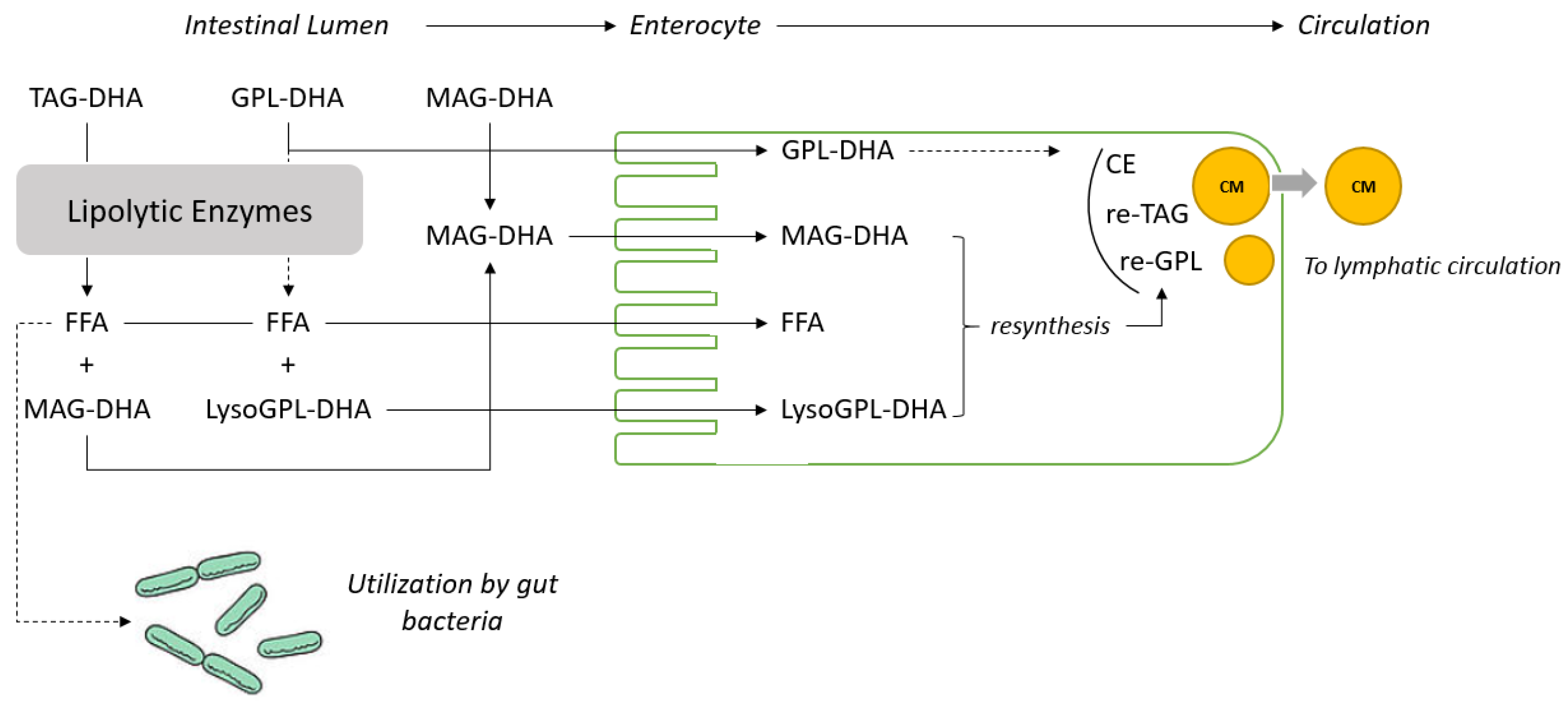
Table 3). To adjust for the difference in food intake, a parametric statistical model has been used. In addition, the accretion of DHA in different important organs such as adipose tissue was not measured and circulatory lipids (erythrocytes and plasma) were considered in the present study as surrogate markers to assess the potency of the different dietary DHA carriers. Nevertheless, the results obtained for the TAG-DHA group in this study and the literature available on the digestion, absorption and metabolism of TAG, MAG and PL give good insight to understand the fate of the fatty acids provided by these different dietary carriers as represented in
Figure 1.
We previously demonstrated in animal and human model conditions using lipase inhibitors that n-3 LC-PUFAs as
sn-1(3)-MAG can be directly absorbed by enterocytes and do not require the action of pancreatic lipases [
19,
27]. This hypothesis is further supported by the data obtained using DHA recovered from lymphatic cannulated rats fed with TAG-DHA, DAG-DHA, MAG-DHA and EE-DHA [
20]. Analysis of the lymphatic recovery of DHA from TAG-DHA, DAG-DHA, MAG-DHA and EE-DHA confirmed that the level of DHA absorbed is much higher when provided as MAG [
20]. It can therefore be concluded that MAG are channeled very efficiently from the intestinal lumen to the enterocyte as displayed in
Figure 2. In the present study, significantly higher levels of DHA in erythrocyte lipids were observed in animals fed with MAG-DHA and PL-DHA compared with animals fed with TAG-DHA (
Table 4). These results strongly suggest that PL-DHA enriched oil may also require minimal digestion prior to absorption (
Figure 1).
The evolution of the concentration of DHA, for all groups, in plasma and erythrocytes is consistent with data reported elsewhere showing that dietary supplementation with DHA leads to a progressive increase of the concentration of DHA in circulatory lipids until it reaches a steady state concentration [
1]. It has been demonstrated that the DHA level reached at steady states in circulatory lipids depends on the concentration of DHA in the diet [
1]. The differences observed in circulatory lipids were larger than the differences in concentration of DHA in the diets suggesting that the level reached at steady state depends not only on the level of DHA supplemented but also on the nature of the dietary carrier used. To explain such differences, it is necessary to revisit the basics of the digestion of dietary lipids such as TAG and take in consideration recent progress in understanding the lipid metabolism of the gut microbiota.
TAG are the main source of dietary lipids and TAG digestion by pancreatic lipase and colipase requires the emulsification of TAG in the presence of bile salts to generate the aqueous interface required for the enzyme to function [
17]. The process of emulsification is triggered by the moderate hydrolysis of bile PL that is catalyzed by pancreatic phospholipase A2 [
31]. The Lyso-PL formed from bile PL are facilitating the emulsification process of the dietary TAG. However, it does not imply that dietary PL need to be quantitatively hydrolyzed before being absorbed as PL can spontaneously form micelles due to their amphiphilic nature. It has been reported that fatty acids linked to Lyso-PL are preferentially incorporated and channeled by the HDL [
32]. MAG and free fatty acids in a 1:2 ratio formed by the partial lipolysis of dietary TAG are absorbed and re-esterified to form lipid droplets that are excreted as chylomicrons into the lymphatic system (see
Figure 2 for schematic representation). The level of absorption of dietary lipids is commonly measured using the so called “apparent digestibility” method which is calculated as follow: [(lipid intake–fatty acid excretion in feces)/(fatty acid intake) × 100]. The results obtained under physiological conditions with this method are extremely high (>95%) and suggest that the absorption of the lipolytic products formed during the digestion of dietary TAG is quantitative. The apparent digestibility can be lowered under maldigestion conditions induced for example by inhibition of the lipase function or lipid malabsorption. In these conditions, lipids are excreted with the consecutive apparition of steatorrhea [
29]. The apparent digestibility of n-3 LC-PUFA from fish oil (TAG) and krill oil (PL) was compared in rat models and results were found to be identical [
33] while the n-3 LC-PUFA deposition was higher in animals fed with the krill oil diet. Others reported that incorporation in infant piglets of DHA from PL-DHA in plasma phospholipids as provided by egg phospholipids was significantly higher (c.a. 50%) than TAG-DHA provided by fungal oil [
34]. In accordance with literature data, we found that the incorporation of DHA in plasma and erythrocyte lipids was higher with MAG-DHA and PL-DHA compared to TAG-DHA, even if corrected for the bias related to the level of DHA provided by the TAG-DHA diet (
Table 4 and
Table 5). Therefore, if the apparent digestibility data reported in literature are similar for PL-DHA and TAG-DHA but their level of circulatory lipids differs, it suggests that part of the free fatty acids released from TAG are not absorbed nor excreted.
Gut microbiota “metabolism” is extremely active and the lipid metabolism of model gut bacteria such as
Escherichia coli has been characterized in detail [
35]. It is well established that
E. coli is able to use various type of fatty acids as a source of energy [
35].
E. coli can uptake free fatty acids from the intestinal lumen and transport them into the cytosol via a transport/acyl-activation mechanism involving an outer membrane protein, FadL, and inner membrane-associated FadD [acyl-CoA synthase (fatty acid-CoA ligase)]. Fatty acids that are absorbed by
E. coli are beta-oxidized resulting in the formation of acetyl coA [
35]. It seems that other bacteria such as
Lactobacillaceae are able to metabolize fatty acids as well [
36]. Therefore, we can hypothesize that part of the free fatty acids released from TAG during the hydrolysis process catalyzed by the lipase and colipase can be up-taken and utilized by the gut microbiota as schematically represented in
Figure 1.
It can be estimated that the gut microbiota utilize 5–15% of free fatty acids released from TAG as represented; assuming that the quantification of DHA in enterocyte and plasma lipids is representative of the DHA level absorbed and that the level of free fatty acids excreted in feces under physiological condition is lower than 5% (
Figure 2). Precise evaluation of the level of utilization of free fatty acids released from the digestion of lipids by gut microbiota needs to be further demonstrated in dedicated experiments. However, it suggests that the assessment of the “apparent digestibility”, which provides in most of the cases a very high value, might not provide a quantitative estimation of the dietary fatty acid absorbed.
Together with bioavailability, several reports have documented different efficacy and incorporation of DHA in tissues after feeding PL and TAG [
33,
34,
37,
38]. DHA supplementation has shown effects on attention-deficit/hyperactivity disorders and hyperactivity in children [
39,
40]. Higher levels of DHA in brain tissue accretion were found in term baboons [
41] and mice [
42] when provided as PL compared to TAG. Most studies have examined the alteration of fatty acid composition by analyzing the whole brain. However, specific differences in phosphoglycerides in neonatal rats or mice have been only identified in certain brain regions such as frontal cortex and hippocampus [
43,
44,
45]. Thus, it appeared more informative to investigate the fatty acid composition of specific brain regions in order to establish relationships with the studied conditions. Three brain regions were sampled in this study: cortex, hippocampus and hypothalamus with the aim to establish the fatty acids composition of each region with the three different DHA carriers supplemented. The region with the highest DHA levels was the cortex, followed closely by the retina.
These results agree with those observed in rats by [
43,
44,
45] who found more DHA in frontal cortex than in the striatum. Similar increments were observed for all DHA treatments when compared with control. Plasma fatty acids content has been highly related to tissue fatty acids status and cognitive functions [
46,
47], which is reflected in the results we obtained in retina, hippocampus and hypothalamus. In contrast with plasma and erythrocyte lipids, the level of DHA in neural tissues of adult animals is high and it seems that it cannot be increased in a dose dependent manner over a certain level. Therefore, we did not observe major differences in the level of DHA found in these neural tissues when using different dietary carrier of DHA after 60 days of treatment (see
Table 6). However, testing the hypothesis under different conditions such as depleted adults or neonatal animal models might provide interesting insight on the impact of the dietary carriers of DHA on the incorporation in DHA in neural tissues as previously observed for arachidonic acid [
41].