A Vitamin E-Enriched Antioxidant Diet Interferes with the Acute Adaptation of the Liver to Physical Exercise in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diets and Exercise Protocol
2.2. Metabolites and Glucocorticoid Receptor Activity
2.3. RNA Isolation, Quantitative PCR and Transcriptome Analysis
2.4. Data Analysis
3. Results
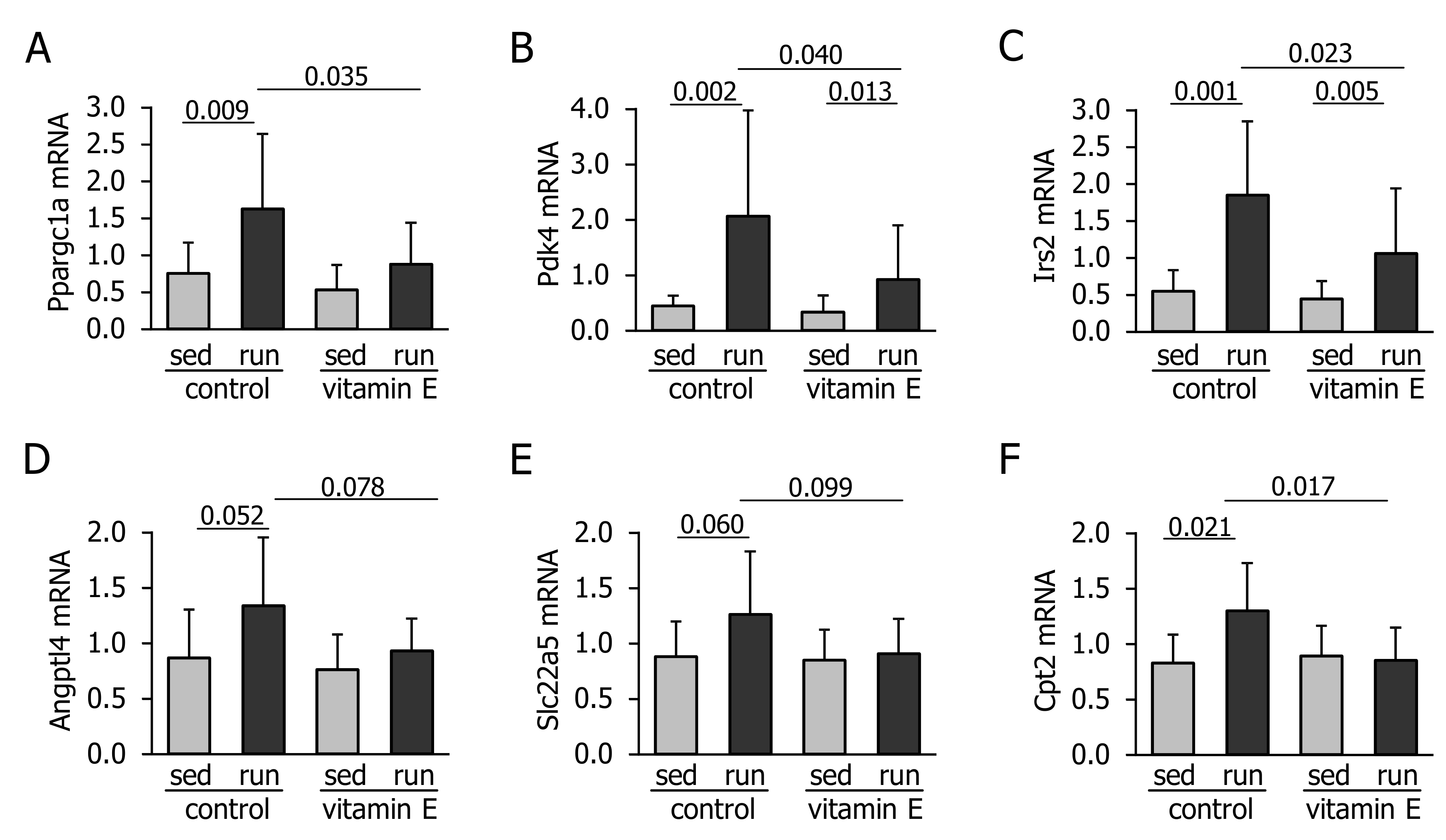
3.1. Vitamin E Interferes with Exercise-Induced Increase in Plasma FFA and Expression of Key Metabolic Regulators in the Liver
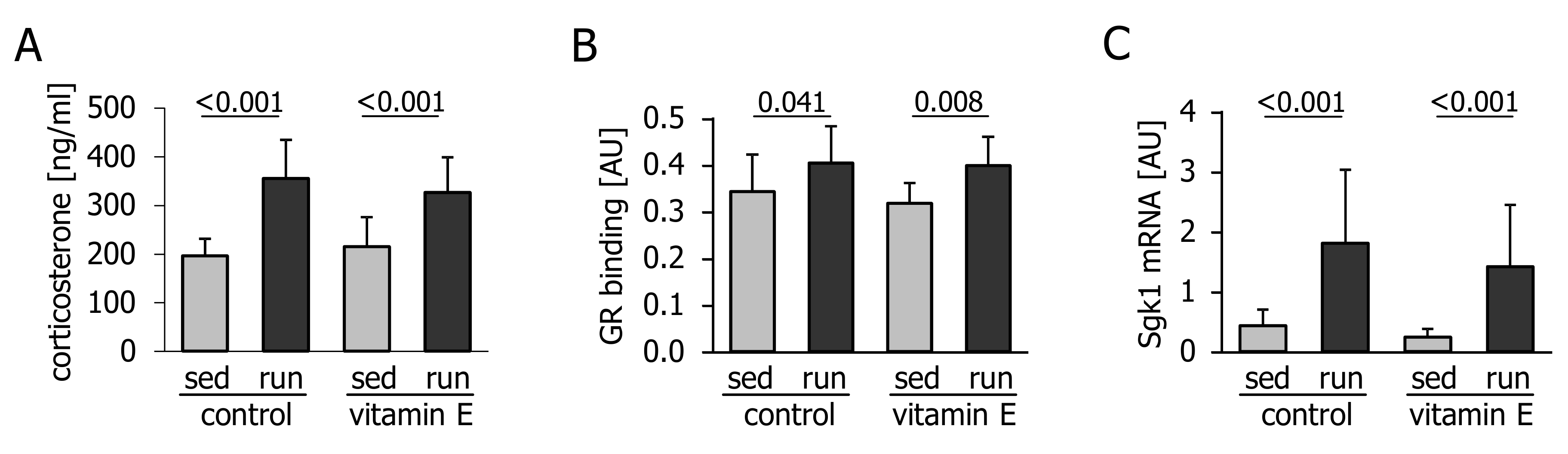
3.2. Corticosterone Levels and Glucocorticoid Signalling are not Affected by Vitamin E
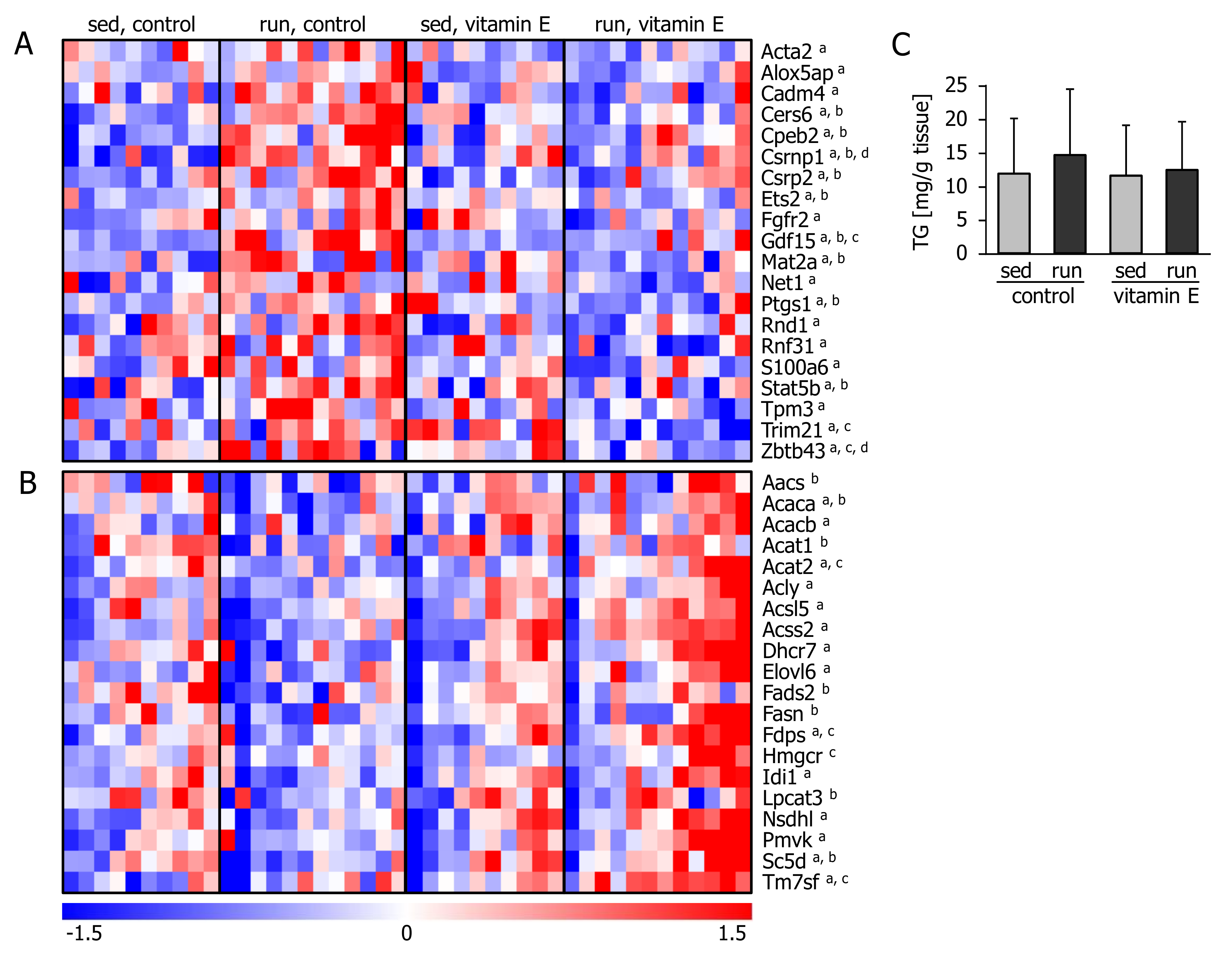
3.3. Vitamin E Alters the Hepatic Transcriptional Response Related to Lipid and Cholesterol Synthesis and Inflammation
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jakicic, J.M.; Rogers, R.J.; Davis, K.K.; Collins, K.A. Role of Physical Activity and Exercise in Treating Patients with Overweight and Obesity. Clin. Chem. 2018, 64, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.A.; Gariani, K.; Oldani, G.; Delaune, V.; Morel, P.; Toso, C. Exercise-based Interventions for Nonalcoholic Fatty Liver Disease: A Meta-analysis and Meta-regression. Clin. Gastroenterol. Hepatol. 2016, 14, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Williams, A.S.; Wasserman, D.H. Exercise and the Regulation of Hepatic Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J.; Johnson, N. Effects of physical activity upon the liver. Eur. J. Appl. Physiol. 2015, 115, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Niess, A.M.; Simon, P. Response and adaptation of skeletal muscle to exercise—The role of reactive oxygen species. Front. Biosci. 2007, 12, 4826–4838. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.P.; Bracciotti, G.; Falsini, S. Spin-trappers and vitamin E prolong endurance to muscle fatigue in mice. Free Radic. Biol. Med. 1990, 8, 9–13. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Ostgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind randomized controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Strobel, N.A.; Peake, J.M.; Matsumoto, A.; Marsh, S.A.; Coombes, J.S.; Wadley, G.D. Antioxidant Supplementation Reduces Skeletal Muscle Mitochondrial Biogenesis. Med. Sci. Sports Exerc. 2010, 43, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.-M.; Azzi, A. Non-antioxidant activities of vitamin E. Curr. Med. Chem. 2004, 11, 1113–1133. [Google Scholar] [CrossRef] [PubMed]
- Tsiakitzis, K.; Kourounakis, A.P.; Tani, E.; Rekka, E.A.; Kourounakis, P.N. Stress and active oxygen species—Effect of alpha-tocopherol on stress response. Arch. Pharm. (Weinheim) 2005, 338, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, A.; Kojima, H.; Ohtani, T.; Hayashi, K. Vitamin E reduces glucocorticoid-induced oxidative stress in rat skeletal muscle. J. Nutr. Sci. Vitaminol. (Tokyo) 1998, 44, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P.; Hiscock, N.J.; Penkowa, M.; Basu, S.; Vessby, B.; Kallner, A.; Sjoberg, L.B.; Pedersen, B.K. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J. Physiol. 2004, 558, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Higashida, K.; Kim, S.H.; Higuchi, M.; Holloszy, J.O.; Han, D.-H. Normal adaptations to exercise despite protection against oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E779–E784. [Google Scholar] [CrossRef] [PubMed]
- Yfanti, C.; Nielsen, A.R.; Akerström, T.; Nielsen, S.; Rose, A.J.; Richter, E.A.; Lykkesfeldt, J.; Fischer, C.P.; Pedersen, B.K. Effect of antioxidant supplementation on insulin sensitivity in response to endurance exercise training. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E761–E770. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Ajani, U.A.; Mokdad, A.H. Brief communication: The prevalence of high intake of vitamin E from the use of supplements among U.S. adults. Ann. Intern. Med. 2005, 143, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Hoene, M.; Weigert, C. The stress response of the liver to physical exercise. Exerc. Immunol. Rev. 2010, 16, 163–183. [Google Scholar] [PubMed]
- Pillon Barcelos, R.; Freire Royes, L.F.; Gonzalez-Gallego, J.; Bresciani, G. Oxidative stress and inflammation: Liver responses and adaptations to acute and regular exercise. Free Radic. Res. 2017, 51, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Hoene, M.; Lehmann, R.; Hennige, A.M.; Pohl, A.K.; Häring, H.U.; Schleicher, E.D.; Weigert, C. Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. J. Physiol. 2009, 587, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Hoene, M.; Li, J.; Li, Y.; Runge, H.; Zhao, X.; Häring, H.-U.; Lehmann, R.; Xu, G.; Weigert, C. Muscle and liver-specific alterations in lipid and acylcarnitine metabolism after a single bout of exercise in mice. Sci. Rep. 2016, 6, 22218. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Arai, H. Molecular mechanisms of vitamin E transport. Annu. Rev. Nutr. 1999, 19, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mizrachi, C.; Weng, S.; Feng, C.; Finck, B.N.; Knutsen, R.H.; Leone, T.C.; Coleman, T.; Mecham, R.P.; Kelly, D.P.; Semenkovich, C.F. Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat. Med. 2003, 9, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Koliwad, S.K.; Kuo, T.; Shipp, L.E.; Gray, N.E.; Backhed, F.; So, A.Y.-L.; Farese, R.V.; Wang, J.-C. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J. Biol. Chem. 2009, 284, 25593–25601. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Harris, C.A.; Wang, J.-C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, G.; Moehring, J.; Huebbe, P.; Lodge, J.K. Gene-regulatory activity of alpha-tocopherol. Molecules 2010, 15, 1746–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhm, A.; Hoffmann, C.; Irmler, M.; Schneeweiss, P.; Schnauder, G.; Sailer, C.; Schmid, V.; Hudemann, J.; Machann, J.; Schick, F.; et al. TGF-β Contributes to Impaired Exercise Response by Suppression of Mitochondrial Key Regulators in Skeletal Muscle. Diabetes 2016, 65, 2849–2861. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. BioTechniques 2003, 34, 374–378. [Google Scholar] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Zhao, X.; Irmler, M.; Liu, X.; Hoene, M.; Scheler, M.; Li, Y.; Beckers, J.; Hrabĕ de Angelis, M.; Häring, H.-U.; et al. Type 2 diabetes alters metabolic and transcriptional signatures of glucose and amino acid metabolism during exercise and recovery. Diabetologia 2015, 58, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Pégorier, J.-P.; Le May, C.; Girard, J. Control of gene expression by fatty acids. J. Nutr. 2004, 134, 2444S–2449S. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-C. Phospholipid transfer protein: Its impact on lipoprotein homeostasis and atheroslcerosis. J. Lipid Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Djurhuus, C.B.; Gravholt, C.H.; Nielsen, S.; Mengel, A.; Christiansen, J.S.; Schmitz, O.E.; Møller, N. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E172–E177. [Google Scholar] [CrossRef] [PubMed]
- Jhala, U.S.; Canettieri, G.; Screaton, R.A.; Kulkarni, R.N.; Krajewski, S.; Reed, J.; Walker, J.; Lin, X.; White, M.; Montminy, M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003, 17, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Buler, M.; Aatsinki, S.-M.; Skoumal, R.; Komka, Z.; Tóth, M.; Kerkelä, R.; Georgiadi, A.; Kersten, S.; Hakkola, J. Energy-sensing factors coactivator peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) and AMP-activated protein kinase control expression of inflammatory mediators in liver: Induction of interleukin 1 receptor antagonist. J. Biol. Chem. 2012, 287, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Jialal, I. Anti-inflammatory effects of alpha-tocopherol. Ann. N. Y. Acad. Sci. 2004, 1031, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Special feature for the Olympics: Effects of exercise on the immune system: Exercise and cytokines. Immunol. Cell Biol. 2000, 78, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.; Hoene, M.; Lehmann, R.; Ellingsgaard, H.; Hennige, A.M.; Pohl, A.K.; Häring, H.U.; Schleicher, E.D.; Weigert, C. IL-6 deficiency in mice neither impairs induction of metabolic genes in the liver nor affects blood glucose levels during fasting and moderately intense exercise. Diabetologia 2010, 53, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Napolitano, G.; Barone, D.; Di Meo, S. Effect of training and vitamin E administration on rat liver oxidative metabolism. Free Radic. Res. 2014, 48, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.A.; Baker, D.H.; Yu, X.X.; Novakofski, J.; Oscai, L.; Ji, L.L. Effects of acute exercise on hepatic lipogenic enzymes in fasted and refed rats. J. Appl. Physiol. 1995, 79, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Bilan, P.J.; Fink, L.N.; Klip, A. Cross-talk between skeletal muscle and immune cells: Muscle-derived mediators and metabolic implications. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E453–E465. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Mooren, F.-C.; Pilat, C. The Immunomodulatory Effects of Physical Activity. Curr. Pharm. Des. 2016, 22, 3730–3748. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Febbraio, M.A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014, 35, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Huber, Y.; Gehrke, N.; Biedenbach, J.; Helmig, S.; Simon, P.; Straub, B.K.; Bergheim, I.; Huber, T.; Schuppan, D.; Galle, P.R.; et al. Voluntary distance running prevents TNF-mediated liver injury in mice through alterations of the intrahepatic immune milieu. Cell Death Dis. 2017, 8, e2893. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.E.; Vettor, R.; Rossato, M. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth? Antioxidants 2018, 7, 12. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Galli, F. Vitamin E: A vitamin still awaiting the detection of its biological function. Mol. Nutr. Food Res. 2010, 54, 583–587. [Google Scholar] [CrossRef] [PubMed]

| Upstream Regulator | Molecule Type | Activation z-Score | p-Value of Overlap | Predicted State |
|---|---|---|---|---|
| SCAP | Other | 3.58 | <0.001 | Activated |
| SREBF2 | Transcription regulator | 3.27 | <0.001 | Activated |
| SREBF1 | Transcription regulator | 2.71 | <0.001 | Activated |
| TSC2 | Other | 2.45 | 0.004 | Activated |
| Nr1h (LXR) | Group | 2.40 | 0.016 | Activated |
| PPARD | Ligand-dependent nuclear receptor | 2.23 | 0.006 | Activated |
| MLXIPL (ChREBP) | Transcription regulator | 2.22 | <0.001 | Activated |
| miR-145-5p 1 | Mature microRNA | 2.20 | <0.001 | Activated |
| FAS | Transmembrane receptor | 2.18 | 0.004 | Activated |
| INHA | Growth factor | 2.00 | 0.049 | Activated |
| ATP7B | Transporter | 2.00 | 0.002 | Activated |
| TLR7 | Transmembrane receptor | −2.62 | 0.004 | Inhibited |
| Interferon alpha | Group | −2.61 | 0.031 | Inhibited |
| TGFB1 | Growth factor | −2.59 | 0.002 | Inhibited |
| TICAM1 | Other | −2.43 | 0.011 | Inhibited |
| SP1 | Transcription regulator | −2.43 | 0.033 | Inhibited |
| APP | Other | −2.40 | 0.024 | Inhibited |
| IFNB1 | Cytokine | −2.40 | 0.013 | Inhibited |
| INSIG1 | Other | −2.39 | <0.001 | Inhibited |
| SRF | Transcription regulator | −2.37 | 0.002 | Inhibited |
| Vegf | Group | −2.36 | 0.035 | Inhibited |
| POR | Enzyme | −2.35 | <0.001 | Inhibited |
| MAP4K4 | Kinase | −2.24 | 0.005 | Inhibited |
| Ifn | Group | −2.20 | 0.021 | Inhibited |
| CREB1 | Transcription regulator | −2.17 | 0.008 | Inhibited |
| TGM2 | Enzyme | −2.16 | <0.001 | Inhibited |
| IFNG | Cytokine | −2.15 | <0.001 | Inhibited |
| MGEA5 | Enzyme | −2.11 | 0.025 | Inhibited |
| CSF2 (GM-CSF) | Cytokine | −2.07 | 0.018 | Inhibited |
| OSM | Cytokine | −2.04 | 0.025 | Inhibited |
| SAMSN1 | Other | −2.00 | 0.032 | Inhibited |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoene, M.; Irmler, M.; Beckers, J.; Hrabě de Angelis, M.; Häring, H.-U.; Weigert, C. A Vitamin E-Enriched Antioxidant Diet Interferes with the Acute Adaptation of the Liver to Physical Exercise in Mice. Nutrients 2018, 10, 547. https://doi.org/10.3390/nu10050547
Hoene M, Irmler M, Beckers J, Hrabě de Angelis M, Häring H-U, Weigert C. A Vitamin E-Enriched Antioxidant Diet Interferes with the Acute Adaptation of the Liver to Physical Exercise in Mice. Nutrients. 2018; 10(5):547. https://doi.org/10.3390/nu10050547
Chicago/Turabian StyleHoene, Miriam, Martin Irmler, Johannes Beckers, Martin Hrabě de Angelis, Hans-Ulrich Häring, and Cora Weigert. 2018. "A Vitamin E-Enriched Antioxidant Diet Interferes with the Acute Adaptation of the Liver to Physical Exercise in Mice" Nutrients 10, no. 5: 547. https://doi.org/10.3390/nu10050547





