The Effect of Depressive Symptoms on the Association between Gluten-Free Diet Adherence and Symptoms in Celiac Disease: Analysis of a Patient Powered Research Network
Abstract
:1. Introduction
2. Patients and Methods
2.1. Inclusion Criteria
2.2. Data Collection
2.3. Data Analysis
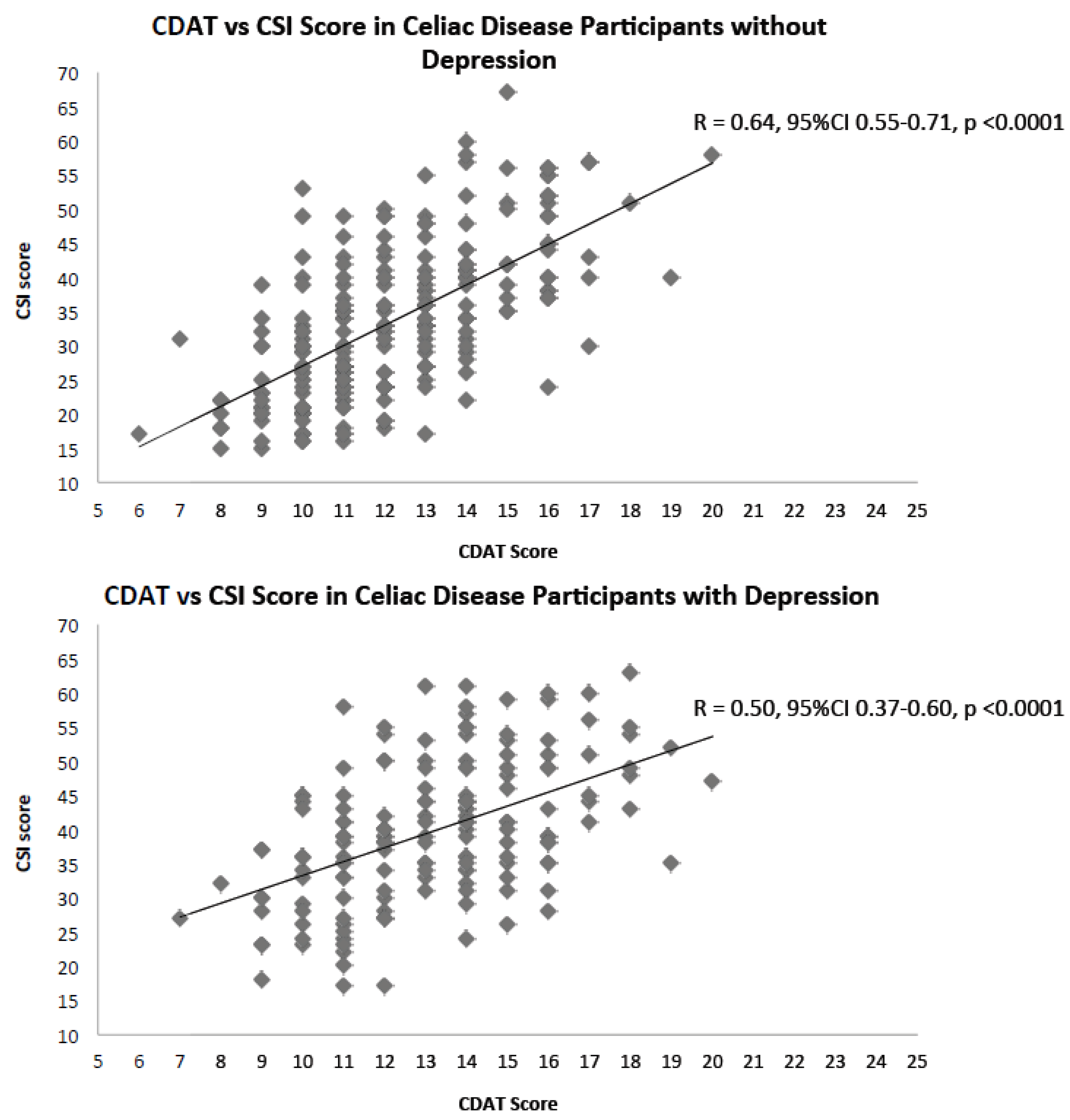
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rubio-Tapia, A.; Ludvigsson, J.F.; Brantner, T.L.; Murray, J.A.; Everhart, J.E. The prevalence of celiac disease in the United States. Am. J. Gastroenterol. 2012, 107, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Ludvigsson, J.F.; Green, P.H. Celiac disease and non-celiac gluten sensitivity. BMJ 2015, 351, h4347. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Reutfors, J.; Osby, U.; Ekbom, A.; Montgomery, S.M. Coeliac disease and risk of mood disorders—A general population-based cohort study. J. Affect. Disord. 2007, 99, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Gerdes, L.U. Meta-analysis on anxiety and depression in adult celiac disease. Acta Psychiatr. Scand. 2012, 125, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.R.; Eaton, W.W.; Cascella, N.G.; Fasano, A.; Kelly, D.L. Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity. Psychiatr. Q. 2012, 83, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Zingone, F.; Swift, G.L.; Card, T.R.; Sanders, D.S.; Ludvigsson, J.F.; Bai, J.C. Psychological morbidity of celiac disease: A review of the literature. United Eur. Gastroenterol. J. 2015, 3, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Van Hees, N.J.; Van der Does, W.; Giltay, E.J. Coeliac disease, diet adherence and depressive symptoms. J. Psychosom. Res. 2013, 74, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; Stefanini, G.F.; Capristo, E.; Caputo, F.; Gasbarrini, A.; Gasbarrini, G. Anxiety and depression in adult untreated celiac subjects and in patients affected by inflammatory bowel disease: A personality “trait” or a reactive illness? Hepatogastroenterology 1996, 43, 1513–1517. [Google Scholar] [PubMed]
- Carta, M.G.; Conti, A.; Lecca, F.; Sancassiani, F.; Cossu, G.; Carruxi, R.; Boccone, A.; Cadoni, M.; Pisanu, A.; Francesca, M.; et al. The Burden of Depressive and Bipolar Disorders in Celiac Disease. Clin. Pract. Epidemiol. Ment. Health 2015, 11, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Hardoy, M.C.; Boi, M.F.; Mariotti, S.; Carpiniello, B.; Usai, P. Association between panic disorder, major depressive disorder and celiac disease: A possible role of thyroid autoimmunity. J. Psychosom. Res. 2002, 53, 789–793. [Google Scholar] [CrossRef]
- Addolorato, G.; Di Giuda, D.; De Rossi, G.; Valenza, V.; Domenicali, M.; Caputo, F.; Gasbarrini, A.; Capristo, E.; Gasbarrini, G. Regional cerebral hypoperfusion in patients with celiac disease. Am. J. Med. 2004, 116, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Garud, S.; Leffler, D.; Dennis, M.; Edwards-George, J.; Saryan, D.; Sheth, S.; Schuppan, D.; Jamma, S.; Kelly, C.P. Interaction between psychiatric and autoimmune disorders in coeliac disease patients in the Northeastern United States. Aliment. Pharmacol. Ther. 2009, 29, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Janke, K.H.; Klump, B.; Gregor, M.; Hinz, A. Anxiety and depression in adult patients with celiac disease on a gluten-free diet. World J. Gastroenterol. 2010, 16, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Fera, T.; Cascio, B.; Angelini, G.; Martini, S.; Guidetti, C.S. Affective disorders and quality of life in adult coeliac disease patients on a gluten-free diet. Eur. J. Gastroenterol. Hepatol. 2003, 15, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Hernanz, A.; Polanco, I. Plasma precursor amino acids of central nervous system monoamines in children with coeliac disease. Gut 1991, 32, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Sedvall, G. Improvement in central monoamine metabolism in adult coeliac patients starting a gluten-free diet. Psychol. Med. 1983, 13, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Nachman, F.; del Campo, M.P.; González, A.; Corzo, L.; Vázquez, H.; Sfoggia, C.; Smecuol, E.; Sánchez, M.I.; Niveloni, S.; Sugai, E.; et al. Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Dig. Liver Dis. 2010, 42, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Baysoy, G.; Gencoglan, S.; Uluca, U. Effects of Gluten-Free Diet on Quality of Life and Depression in Children with Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Borghini, R.; Di Tola, M.; Salvi, E.; Isonne, C.; Puzzono, M.; Marino, M.; Donato, G.; Picarelli, A. Impact of gluten-free diet on quality of life in celiac patients. Acta Gastroenterol. Belg. 2016, 79, 447–453. [Google Scholar] [PubMed]
- Addolorato, G.; Capristo, E.; Ghittoni, G.; Valeri, C.; Mascianà, R.; Ancona, C.; Gasbarrini, G. Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: A longitudinal study. Scand. J. Gastroenterol. 2001, 36, 502–506. [Google Scholar] [CrossRef] [PubMed]
- DiMatteo, M.R.; Lepper, H.S.; Croghan, T.W. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch. Intern. Med. 2000, 160, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, K.; Marques, M.M. The relationship between gluten free diet adherence and depressive symptoms in adults with coeliac disease: A systematic review with meta-analysis. Appetite 2018, 120, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; De Lorenzi, G.; Abenavoli, L.; Leggio, L.; Capristo, E.; Gasbarrini, G. Psychological support counselling improves gluten-free diet compliance in coeliac patients with affective disorders. Aliment. Pharmacol. Ther. 2004, 20, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A.; American College of Gastroenterology. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed]
- Rostom, A.; Murray, J.A.; Kagnoff, M.F. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology 2006, 131, 1981–2002. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Dennis, M.; Edwards George, J.; Jamma, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A validated disease-specific symptom index for adults with celiac disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Dennis, M.; Edwards George, J.B.; Jamma, S.; Magge, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin. Gastroenterol. Hepatol. 2009, 7, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Pilkonis, P.A.; Yu, L.; Dodds, N.E.; Johnston, K.L.; Maihoefer, C.C.; Lawrence, S.M. Validation of the depression item bank from the Patient-Reported Outcomes Measurement Information System (PROMIS) in a three-month observational study. J. Psychiatr. Res. 2014, 56, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Brooks/Cole Pub. Co.: Pacific Grove, CA, USA, 1996. [Google Scholar]
| Characteristic | Study Population (n = 519) | |
|---|---|---|
| Gender | ||
| Male | 74 (14%) | |
| Female | 445 (86%) | |
| Age (mean ± SD) | 40.9 (±16.7) | |
| Race/Ethnicity (n = 509) | ||
| Black/African American | 3 (0.6%) | |
| Latino/Hispanic | 8 (1.6%) | |
| White | 469 (92.1%) | |
| Other (including more than 1) | 29 (5.7%) | |
| Highest education level (n = 207) | ||
| Less than a college degree | 60 (29%) | |
| College degree or equivalent | 85 (41.1%) | |
| Master’s degree or degree beyond Bachelor’s degree | 49 (23.7%) | |
| Doctorate degree | 13 (6.3%) | |
| Currently working (n = 209) | ||
| Yes | 149 (71.3%) | |
| No—on disability | 5 (2.4%) | |
| No—retired | 24 (11.5%) | |
| No—other | 24 (11.5%) | |
| Degree of Strict Gluten Free Diet Adherence (n = 516) | ||
| Always | 453 (87.3%) | |
| Often | 51 (9.8%) | |
| Sometimes | 9 (1.7%) | |
| Rarely | 2 (0.4%) | |
| Never | 1 (0.2%) | |
| Skipped/Missing | 3 (0.6%) | |
| Length of time from symptom onset to Celiac Disease diagnosis | ||
| <5 years | 356 (68.6%) | |
| 5–15 years | 67 (12.9%) | |
| >15 years | 38 (7.3%) | |
| Don’t Know/missing | 58 (11.2%) | |
| Depressed (n = 519) | ||
| Yes | Very Much | 35 (6.7%) |
| Quite A bit | 65 (12.5%) | |
| Somewhat | 139 (26.8%) | |
| No | A little bit | 157 (30.3%) |
| Not at all | 125 (23.7%) | |
| Celiac Dietary Adherence Test (n = 519) | ||
| Mean (±SD) | 12.8 (±2.5) | |
| First Quartile | 3 | |
| Median | 13 | |
| Third Quartile | 14 | |
| Interquartile Range | 11 | |
| Celiac Symptom Index (n = 392) | ||
| Mean (±SD) | 36.1 (±11.2) | |
| First Quartile | 27 | |
| Median | 36 | |
| Third Quartile | 43 | |
| Interquartile Range | 16 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joelson, A.M.; Geller, M.G.; Zylberberg, H.M.; Green, P.H.R.; Lebwohl, B. The Effect of Depressive Symptoms on the Association between Gluten-Free Diet Adherence and Symptoms in Celiac Disease: Analysis of a Patient Powered Research Network. Nutrients 2018, 10, 538. https://doi.org/10.3390/nu10050538
Joelson AM, Geller MG, Zylberberg HM, Green PHR, Lebwohl B. The Effect of Depressive Symptoms on the Association between Gluten-Free Diet Adherence and Symptoms in Celiac Disease: Analysis of a Patient Powered Research Network. Nutrients. 2018; 10(5):538. https://doi.org/10.3390/nu10050538
Chicago/Turabian StyleJoelson, Andrew M., Marilyn G. Geller, Haley M. Zylberberg, Peter H. R. Green, and Benjamin Lebwohl. 2018. "The Effect of Depressive Symptoms on the Association between Gluten-Free Diet Adherence and Symptoms in Celiac Disease: Analysis of a Patient Powered Research Network" Nutrients 10, no. 5: 538. https://doi.org/10.3390/nu10050538





