Dose-Response Relationship between Serum Retinol Levels and Survival in Patients with Colorectal Cancer: Results from the DACHS Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Data Collection
2.3. Follow-Up
2.4. Outcomes
2.5. Laboratory Measurements
2.6. Covariates Assessment
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Serum Concentrations of Retinol and 25(OH)D3
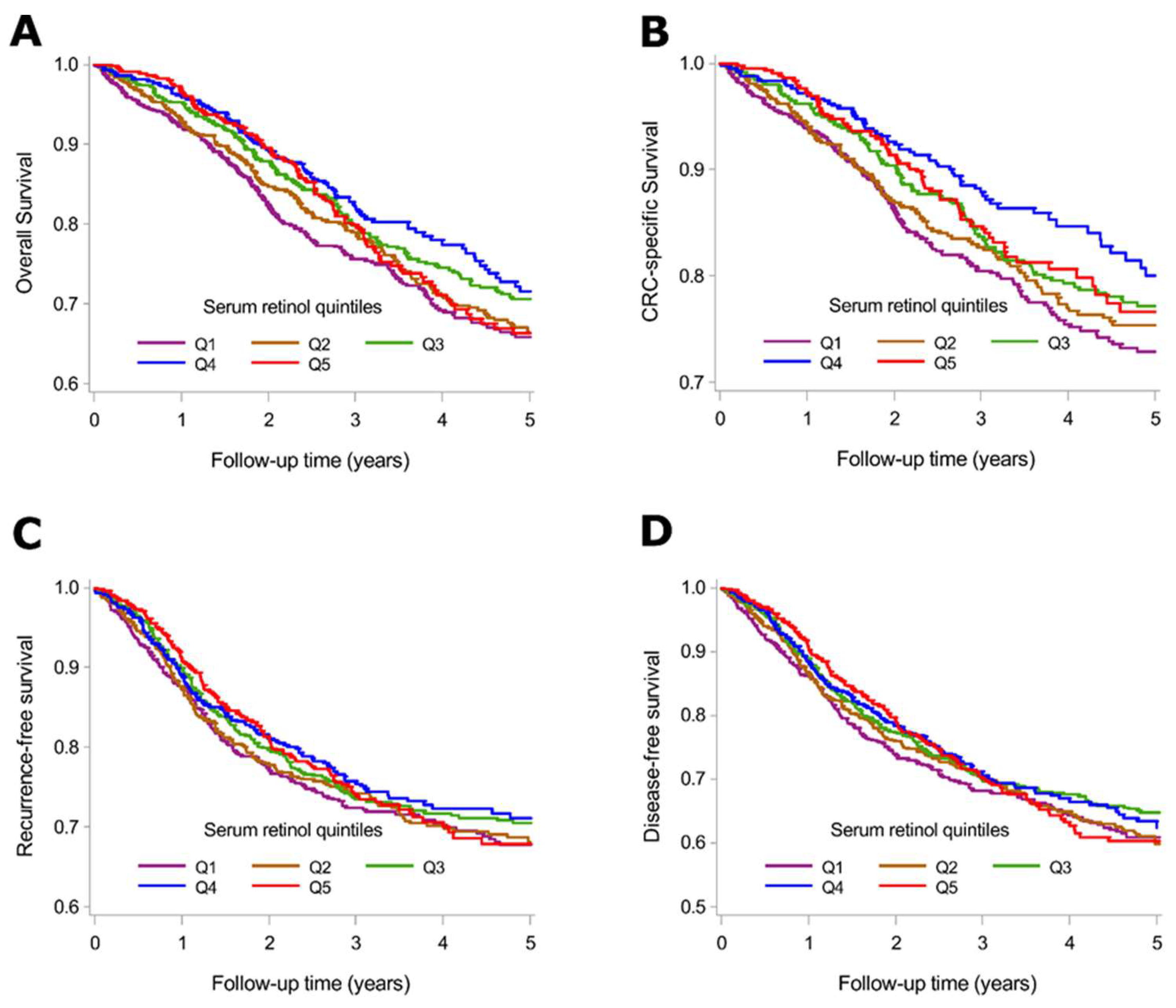
3.3. Serum Retinol Levels and Survival
3.4. Joint Asociations of Serum Retinol and 25(OH)D3 Levels with Survival Outcomes
4. Discussion
4.1. Magnitude of Retinol Deficiency
4.2. Retinol Status and CRC Survival
4.3. Associations with CRC Prognosis Compared to Other Health-Related Outcomes
4.4. Chemopreventive Role of Retinoid and Vitamin D
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C. What is vitamin a and why do we need it? Commun. Eye Health 2013, 26, 65. [Google Scholar]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lip. Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Applegate, C.C.; Lane, M.A. Role of retinoids in the prevention and treatment of colorectal cancer. World J. Gastrointest. Oncol. 2015, 7, 184–203. [Google Scholar] [CrossRef] [PubMed]
- Altucci, L.; Gronemeyer, H. The promise of retinoids to fight against cancer. Nat. Rev. Cancer 2001, 1, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Siddikuzzaman; Guruvayoorappan, C.; Berlin Grace, V.M. All trans retinoic acid and cancer. Immunopharm. Immunotoxicol. 2011, 33, 241–249. [Google Scholar]
- Niles, R.M. Vitamin a and cancer. Nutrition 2000, 16, 573–576. [Google Scholar] [CrossRef]
- Doldo, E.; Costanza, G.; Agostinelli, S.; Tarquini, C.; Ferlosio, A.; Arcuri, G.; Passeri, D.; Scioli, M.G.; Orlandi, A. Vitamin A, cancer treatment and prevention: The new role of cellular retinol binding proteins. BioMed Res. Int. 2015, 2015, 624627. [Google Scholar] [CrossRef] [PubMed]
- Penny, H.L.; Prestwood, T.R.; Bhattacharya, N.; Sun, F.; Kenkel, J.A.; Davidson, M.G.; Shen, L.; Zuniga, L.A.; Seeley, E.S.; Pai, R.; et al. Restoring retinoic acid attenuates intestinal inflammation and tumorigenesis in apcmin/+ mice. Cancer Immunol. Res. 2016, 4, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Melichar, B.; Krcmova, L.; Kalabova, H.; Holeckova, P.; Kasparova, M.; Plisek, J.; Hyspler, R.; Studentova, H.; Solichova, D. Serum retinol, alpha-tocopherol and systemic inflammatory response in metastatic colorectal carcinoma patients treated with combination chemotherapy and cetuximab. J. Nutr. Sci. Vitaminol. 2010, 56, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Cooney, R.V.; Chai, W.; Franke, A.A.; Wilkens, L.R.; Kolonel, L.N.; Le Marchand, L. C-reactive protein, lipid-soluble micronutrients, and survival in colorectal cancer patients. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.Y.; Crozier, J.E.; Talwar, D.; O’Reilly, D.S.; McKee, R.F.; Horgan, P.G.; McMillan, D.C. Vitamin antioxidants, lipid peroxidation, tumour stage, the systemic inflammatory response and survival in patients with colorectal cancer. Int. J. Cancer 2008, 123, 2460–2464. [Google Scholar] [CrossRef] [PubMed]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin d signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Lipid soluble vitamins in gene regulation. BioFactors (Oxf. Engl.) 1999, 10, 91–97. [Google Scholar] [CrossRef]
- Brenner, H.; Chang-Claude, J.; Seiler, C.M.; Rickert, A.; Hoffmeister, M. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann. Int. Med. 2011, 154, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Chang-Claude, J.; Jansen, L.; Knebel, P.; Stock, C.; Hoffmeister, M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology 2014, 146, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.R.; Jansen, L.; Walter, V.; Kloor, M.; Roth, W.; Blaker, H.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Associations of red and processed meat with survival after colorectal cancer and differences according to timing of dietary assessment. Am. J. Clin. Nutr. 2016, 103, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Walter, V.; Jansen, L.; Ulrich, A.; Roth, W.; Blaker, H.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Alcohol consumption and survival of colorectal cancer patients: A population-based study from germany. Am. J. Clin. Nutr. 2016, 103, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Maalmi, H.; Walter, V.; Jansen, L.; Chang-Claude, J.; Owen, R.W.; Ulrich, A.; Schottker, B.; Hoffmeister, M.; Brenner, H. Relationship of very low serum 25-hydroxyvitamin d3 levels with long-term survival in a large cohort of colorectal cancer patients from germany. Eur. J. Epidemiol. 2017, 32, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Phinney, K.W. Development of a standard reference material for Vitamin D in serum. Am. J. Clin. Nutr. 2008, 88, 511s–512s. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Loberiza, F.R.; Klein, J.P.; Zhang, M.J. A sas macro for estimation of direct adjusted survival curves based on a stratified cox regression model. Comput. Methods Programs Biomed. 2007, 88, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Ballew, C.; Bowman, B.A.; Sowell, A.L.; Gillespie, C. Serum retinol distributions in residents of the united states: Third national health and nutrition examination survey, 1988–1994. Am. J. Clin. Nutr. 2001, 73, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, C.; Ballew, C.; Bowman, B.A.; Donehoo, R.; Serdula, M.K. Intraindividual variation in serum retinol concentrations among participants in the third national health and nutrition examination survey, 1988–1994. Am. J. Clin. Nutr. 2004, 79, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of Vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin d from the institute of medicine: What clinicians need to know. J. Clin. Endocrinol. Metabol. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Absorption of vitamin a and carotenoids by the enterocyte: Focus on transport proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef] [PubMed]
- Beisel, W.R. Infection-induced depression of serum retinol—A component of the acute phase response or a consequence? Am. J. Clin. Nutr. 1998, 68, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Rosales, F.J.; Ritter, S.J.; Zolfaghari, R.; Smith, J.E.; Ross, A.C. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mrna in the liver and kidneys of vitamin a-sufficient rats. J. Lip. Res. 1996, 37, 962–971. [Google Scholar]
- Mayland, C.; Allen, K.R.; Degg, T.J.; Bennet, M. Micronutrient concentrations in patients with malignant disease: Effect of the inflammatory response. Ann. Clin. Biochem. 2004, 41, 138–141. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C.; Talwar, D.; Sattar, N.; Underwood, M.; O’Reilly, D.S.; McArdle, C. The relationship between reduced vitamin antioxidant concentrations and the systemic inflammatory response in patients with common solid tumours. Clin. Nutr. (Edinb. Scotl.) 2002, 21, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Mata-Granados, J.M.; Cuenca-Acevedo, R.; Luque de Castro, M.D.; Sosa, M.; Quesada-Gomez, J.M. Vitamin d deficiency and high serum levels of vitamin a increase the risk of osteoporosis evaluated by quantitative ultrasound measurements (qus) in postmenopausal spanish women. Clin. Biochem. 2010, 43, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Michaelsson, K.; Lithell, H.; Vessby, B.; Melhus, H. Serum retinol levels and the risk of fracture. N. Engl. J. Med. 2003, 348, 287–294. [Google Scholar] [CrossRef] [PubMed]
- The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L., Jr.; Omenn, G.S.; Valanis, B.; Williams, J.H., Jr. The beta-carotene and retinol efficacy trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, F.; Bolhassani, A.; Khavari, A.; Bathaie, S.Z.; Naji, T.; Bidgoli, S.A. Retinoids and their biological effects against cancer. Int. Immunopharmacol. 2014, 18, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, M.; Di Majo, D.; La Guardia, M.; Aiello, S.; Crescimannno, M.; Flandina, C.; Tumminello, F.M.; Leto, G. Vitamin d in cancer chemoprevention. Pharm. Biol. 2015, 53, 1399–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.H.; Ghosn, C.; Hinchman, C.; Forbes, C.; Wang, J.; Snider, N.; Cordrey, A.; Zhao, Y.; Chandraratna, R.A. Adenomatous polyposis coli (apc)-independent regulation of beta-catenin degradation via a retinoid x receptor-mediated pathway. J. Biol. Chem. 2003, 278, 29954–29962. [Google Scholar] [CrossRef] [PubMed]
- Melhus, H.; Michaelsson, K.; Kindmark, A.; Bergstrom, R.; Holmberg, L.; Mallmin, H.; Wolk, A.; Ljunghall, S. Excessive dietary intake of vitamin a is associated with reduced bone mineral density and increased risk for hip fracture. Ann. Int. Med. 1998, 129, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Furr, H.C.; Amedee-Manesme, O.; Clifford, A.J.; Bergen, H.R., 3rd; Jones, A.D.; Anderson, D.P.; Olson, J.A. Vitamin a concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin a and by biopsy in generally healthy adult humans. Am. J. Clin. Nutr. 1989, 49, 713–716. [Google Scholar] [CrossRef] [PubMed]

| N (%) | Serum Retinol [µmol/L] | |||
|---|---|---|---|---|
| Characteristics | Mean (SD) | p-Value * | ||
| Sex | Men | 1731 (60%) | 2.4 (1.6) | 0.0310 |
| Women | 1177 (40%) | 2.3 (1.3) | ||
| Age at diagnosis (years) | 30–59 | 579 (20%) | 2.4 (1.4) | 0.0470 |
| 60–69 | 942 (32%) | 2.3 (1.4) | ||
| 70–79 | 949 (33%) | 2.4 (1.7) | ||
| 80+ | 438 (15%) | 2.2 (1.3) | ||
| Cancer stage at diagnosis (UICC) | I | 641 (22%) | 2.6 (1.6) | <0.0001 |
| II | 893 (31%) | 2.4 (1.7) | ||
| III | 940 (32%) | 2.3 (1.3) | ||
| IV | 425 (15%) | 1.9 (1.4) | ||
| History of diabetes | Yes | 536 (19%) | 2.3 (1.5) | 0.0013 |
| No | 2353 (81%) | 2.6 (1.5) | ||
| History of hypertension | Yes | 1480 (52%) | 2.4 (1.7) | 0.0006 |
| No | 1392 (48%) | 2.2 (1.2) | ||
| History of cardiovascular diseases | Yes | 721 (25%) | 2.4 (1.5) | 0.0737 |
| No | 2181 (75%) | 2.3 (1.5) | ||
| Smoking | Never | 1324 (46%) | 2.3 (1.3) | 0.0019 |
| Former | 1138 (39%) | 2.5 (1.8) | ||
| Current | 440 (15%) | 2.2 (1.3) | ||
| Alcohol consumption 1 | None | 852 (30%) | 2.3 (1.3) | 0.1114 |
| Low | 1286 (45%) | 2.4 (1.4) | ||
| High | 729 (25%) | 2.3 (1.8) | ||
| Physical activity 2 | Low | 950 (33%) | 2.4 (1.5) | 0.0767 |
| Moderate | 949 (33%) | 2.3 (1.7) | ||
| High | 949 (33%) | 2.3 (1.4) | ||
| Red meat | High: >once/week | 2081 (72%) | 2.4 (1.6) | 0.0643 |
| Low: ≤once/week | 822 (28%) | 2.3 (1.3) | ||
| Fish | High: >once/week | 494 (17%) | 2.4 (1.9) | 0.1076 |
| Low: ≤once/week | 2408 (83%) | 2.3 (1.4) | ||
| Milk | High: ≥once/day | 727 (25%) | 2.4 (1.3) | 0.1424 |
| Moderate: ≥once/week | 620 (22%) | 2.3 (1.8) | ||
| Low: <once/week | 1549 (53%) | 2.3 (1.5) | ||
| Vegetables | High: ≥once/day | 451 (16%) | 2.2 (1.5) | 0.0067 |
| Moderate: ≥once/week | 1986 (68%) | 2.3 (1.3) | ||
| Low: <once/week | 465 (16%) | 2.5 (2.1) | ||
| Salad | High: ≥once/day | 406 (14%) | 2.6 (2.1) | 0.0892 |
| Moderate: ≥once/week | 2389 (82%) | 2.3 (1.4) | ||
| Low: <once/week | 107 (4%) | 2.1 (1.4) | ||
| Fruit | High: ≥once/day | 1855 (64%) | 2.4 (1.5) | 0.0040 |
| Moderate: ≥once/week | 900 (31%) | 2.3 (1.5) | ||
| Low: <once/week | 141 (5%) | 2.3 (1.6) | ||
| Serum 25(OH)D3 (nmol/L) 3 | Deficient (<30) | 1725 (59%) | 2.1 (1.5) | <0.0001 |
| Insufficient (30-<50) | 721 (25%) | 2.6 (1.7) | ||
| Sufficient (≥50) | 462 (16%) | 2.8 (1.5) | ||
| Serum retinol (µmol/L) | Low (<1) | 397 (14%) | 0.7 (0.2) | <0.0001 |
| Normal (1–3) | 1772 (61%) | 1.9 (0.5) | ||
| High (>3) | 739 (25%) | 4.2 (1.6) | ||
| Serum Retinol (µmol/L) by Quintiles | |||||
|---|---|---|---|---|---|
| Quintile 1 (<1.2) | Quintile 2 (1.2 < 1.8) | Quintile 3 (1.8 ≤ 2.4) | Quintile 4–5 (≥2.4) | p-Trend | |
| Overall survival a | |||||
| No. at risk | 534 | 542 | 550 | 1102 | |
| No. of events | 214 | 151 | 135 | 219 | |
| Model 1, HR (95% CI) * | 1.92 (1.60–2.31) | 1.31 (1.07–1.60) | 1.10 (0.89–1.36) | Reference | <0.0001 |
| Model 2, HR (95% CI) ** | 1.56 (1.28–1.90) | 1.30 (1.05–1.61) | 1.04 (0.84–1.30) | Reference | <0.0001 |
| Model 3, HR (95% CI) *** | 1.46 (1.19–1.78) | 1.25 (1.01–1.55) | 1.03 (0.82–1.28) | Reference | 0.0003 |
| CRC-specific survival a | |||||
| No. at risk | 534 | 542 | 550 | 1102 | |
| No. of events | 169 | 114 | 100 | 139 | |
| Model 1, HR (95% CI) * | 2.44 (1.96–3.03) | 1.54 (1.21–1.96) | 1.35 (1.05–1.73) | Reference | <0.0001 |
| Model 2, HR (95% CI) ** | 1.80 (1.43–2.28) | 1.54 (1.20–1.99) | 1.23 (0.94–1.60) | Reference | <0.0001 |
| Model 3, HR (95% CI) *** | 1.69 (1.33–2.15) | 1.48 (1.15–1.92) | 1.21 (0.93–1.58) | Reference | <0.0001 |
| Recurrence-free survival b | |||||
| No. at risk | 520 | 523 | 537 | 1067 | |
| No. of events | 187 | 142 | 132 | 218 | |
| Model 1, HR (95% CI) * | 1.72 (1.42–2.08) | 1.26 (1.02–1.55) | 1.20 (0.97–1.48) | Reference | <0.0001 |
| Model 2, HR (95% CI) ** | 1.25 (1.02–1.54) | 1.15 (0.92–1.43) | 1.08 (0.86–1.34) | Reference | 0.0265 |
| Model 3, HR (95% CI) *** | 1.20 (0.97–1.47) | 1.12 (0.90–1.39) | 1.07 (0.86–1.33) | Reference | 0.0790 |
| Disease-free survival b | |||||
| No. at risk | 520 | 523 | 537 | 1067 | |
| No. of events | 229 | 176 | 162 | 282 | |
| Model 1, HR (95% CI) * | 1.60 (1.35–1.89) | 1.19 (0.99–1.43) | 1.09 (0.90–1.32) | Reference | <0.0001 |
| Model 2, HR (95% CI) ** | 1.25 (1.04–1.49) | 1.11 (0.91–1.34) | 1.01 (0.83–1.23) | Reference | 0.0188 |
| Model 3, HR (95% CI) *** | 1.18 (0.98–1.42) | 1.07 (0.88–1.30) | 1.00 (0.82–1.22) | Reference | 0.0865 |
| Serum 25(OH)D3, nmol/L | Serum Retinol Levels, µmol/L | ||||||
|---|---|---|---|---|---|---|---|
| <1.2 | 1.2 ≤ 2.4 | ≥2.4 | |||||
| N at risk/N Events | HR (95% CI) | N at Risk/N Events | HR (95% CI) | N at Risk/N Events | HR (95% CI) | ||
| Overall survival a | <30 | 386/170 | 1.76 (1.23–2.50) | 675/198 | 1.31 (0.93–1.85) | 543/120 | 1.09 (0.75–1.57) |
| 30 ≤ 50 | 101/30 | 1.15 (0.71–1.85) | 265/54 | 1.09 (0.72–1.65) | 315/54 | 0.90 (0.59–1.36) | |
| ≥50 | 38/13 | 1.65 (0.87–3.13) | 168/39 | 0.96 (0.61–1.50) | 237/41 | Reference | |
| CRC-specific survival a | <30 | 386/132 | 2.06 (1.32–3.21) | 675/148 | 1.55 (1.00–2.40) | 543/76 | 1.12 (0.70–1.78) |
| 30 ≤ 50 | 101/24 | 1.31 (0.74–2.32) | 265/42 | 1.46 (0.88–2.41) | 315/34 | 0.86 (0.50–1.45) | |
| ≥50 | 38/12 | 2.10 (1.02–4.31) | 168/29 | 1.11 (0.64–1.91) | 237/25 | Reference | |
| Recurrence-free survival b | <30 | 376/149 | 1.29 (0.89–1.86) | 655/191 | 1.07 (0.75–1.53) | 526/119 | 0.92 (0.63–1.34) |
| 30 ≤ 50 | 98/25 | 0.67 (0.40–1.12) | 255/50 | 0.89 (0.58–1.36) | 304/57 | 0.76 (0.50–1.16) | |
| ≥50 | 37/12 | 1.24 (0.64–2.42) | 165/37 | 0.84 (0.53–1.33) | 231/39 | Reference | |
| Disease-free survival b | <30 | 376/185 | 1.25 (0.91–1.72) | 655/237 | 1.02 (0.75–1.38) | 526/154 | 0.90 (0.65–1.24) |
| 30 ≤ 50 | 98/30 | 0.67 (0.42–1.05) | 255/60 | 0.77 (0.53–1.11) | 304/71 | 0.74 (0.52–1.06) | |
| ≥50 | 37/13 | 1.07 (0.57–1.99) | 165/45 | 0.76 (0.51–1.13) | 231/54 | Reference | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maalmi, H.; Walter, V.; Jansen, L.; Owen, R.W.; Ulrich, A.; Schöttker, B.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Dose-Response Relationship between Serum Retinol Levels and Survival in Patients with Colorectal Cancer: Results from the DACHS Study. Nutrients 2018, 10, 510. https://doi.org/10.3390/nu10040510
Maalmi H, Walter V, Jansen L, Owen RW, Ulrich A, Schöttker B, Chang-Claude J, Hoffmeister M, Brenner H. Dose-Response Relationship between Serum Retinol Levels and Survival in Patients with Colorectal Cancer: Results from the DACHS Study. Nutrients. 2018; 10(4):510. https://doi.org/10.3390/nu10040510
Chicago/Turabian StyleMaalmi, Haifa, Viola Walter, Lina Jansen, Robert W. Owen, Alexis Ulrich, Ben Schöttker, Jenny Chang-Claude, Michael Hoffmeister, and Hermann Brenner. 2018. "Dose-Response Relationship between Serum Retinol Levels and Survival in Patients with Colorectal Cancer: Results from the DACHS Study" Nutrients 10, no. 4: 510. https://doi.org/10.3390/nu10040510





