Meeting Vitamin D Requirements in White Caucasians at UK Latitudes: Providing a Choice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Underlying Data and Rationale
2.2. Defining the End Summer Target Levels of 25(OH)D
2.3. Relating a Change in Circulating 25(OH)D to Sun Exposure
2.4. Ensuring Health Risks from UVR Exposure Are Minimised
2.5. Translating from the Irradiation Cabinet to Real Life and from the Vertical to the Horizontal
2.6. Calculating Whether the End-Summer Target Can Be Achieved with Daily Sun Exposure for a Range of Exposure Scenarios
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.D.; Mughal, M.Z.; Adams, J.E.; Wilkinson, J.; Berry, J.L.; Edwards, L.; Kift, R.; Marjanovic, E.; Vail, A.; Webb, A.R.; et al. Sun exposure behavior, seasonal vitamin D deficiency and relationship to bone health in adolescents. J. Clin. Endocrinol. Metab. 2016, 101, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- SACN Vitamin D and Health, 2016. Crown Copyright. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 21 February 2018).
- Public Health England (PHE). Ultraviolet Radiation and Vitamin D: The Effects on Health. 2017. Available online: https://www.gov.uk/government/publications/ultraviolet-radiation-and-vitamin-d-the-effects-on-health (accessed on 26 March 2018).
- Bouillon, R.; Bischoff-Ferrari, H.; Willett, W. Vitamin D and health: Perspectives from mice and man. J. Bone Miner. Res. 2008, 23, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.; Irving, K.; Gregory, J.; Bates, C.J.; Prentice, A.; Perks, J.; Swan, G.; Farron, M. The National Diet and Nutrition Survey: Adults Aged 19 to 64 Years. Volume 3. Vitamin and Mineral Intakes and Urinary Analytes; The Stationery Office: London, UK, 2003; ISBN 0-11-621568-2. [Google Scholar]
- Department of Health. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom; Report on Health and Social Subjects: 41; HMSO: London, UK, 1991; ISBN 978-0113213979.
- Department of Health. Nutrition and Bone Health: With Particular Reference to Calcium and Vitamin D; Report on Health and Social Subject: 49; The Stationery Office: London, UK, 1998.
- Webb, A.R.; Holick, M.F. The role of sunlight in the cutaneous production of vitamin D3. In Annual Review of Nutrition; Olson, R.E., Ed.; Annual Reviews Inc.: Palo Alto, CA, USA, 1988; Volume 8, pp. 375–399. ISBN 0-8243-2808-6. [Google Scholar]
- Webb, A.R.; Kline, L.W.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D synthesis in human skin. J. Clin. Endocrinol. Metabol. 1988, 67, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Kift, R.; Durkin, M.T.; O’brien, S.J.; Vail, A.; Berry, J.L.; Rhodes, L.E. The role of sunlight exposure in determining the vitamin D status of the UK white Caucasian adult population. Br. J. Dermatol. 2010, 163, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Bates, B.; Lennox, A.; Prentic, A.; Bates, C.; Page, P.; Nicholson, S.; Swan, G. The National Diet and Nutrition Survey: Results from Years 1, 2, 3 and 4 (Combined) of the Rolling Programme (2008/2009–2011/2012); The Stationary Office: London, UK, 2014; ISBN 978-1910535332. [Google Scholar]
- Hypponen, E.; Power, C. Hypovitaminosis D in British adults at age 45 years: Nationwide cohort study of dietary and lifestyle predictors. Am. J. Clin. Nutr. 2007, 85, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Mavroeidi, A.; Fraser, W.D.; Darling, A.L.; Black, J.; Aucott, L.; O’Neill, F.; Hart, K.; Berry, J.L.; Lanham-New, S.A.; et al. Sunlight and dietary contributions to the seasonal vitamin D status of cohorts of healthy postmenopausal women living at northerly latitudes: A major cause for concern? Osteoporos. Int. 2011, 22, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Radiation IARC: Lyon, France, 2012; Volume 100D, pp. 1–68. ISBN 978-92 832-1321-5. [Google Scholar]
- Ashwell, M.; Stone, E.M.; Stolte, H.; Cashman, K.D.; Macdonald, H.; Lanham-New, S.; Hiom, S.; Webb, A.; Fraser, D. UK Food Standards Agency workshop report: An investigation of the relative contributions of diet and sunlight to vitamin D status. Br. J. Nutr. 2010, 104, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.E.; Webb, A.R.; Fraser, H.; Kift, R.; Durkin, M.; Vail, A.; Berry, J.L. Recommended Summer Sunlight Exposure Levels Can Produce Sufficient (>20 ng mL−1) but Not the Proposed Optimal (>32 ng mL−1) 25(OH)D Levels at UK Latitudes. J. Investig. Dermatol. 2010, 130, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Kazantzidis, A.; Smedley, A.R.D.; Kift, R.C.; Rimmer, J.S.; Berry, J.L.; Rhodes, L.E.; Webb, A.R. Modeling approach to determine how much UV radiation is available across the UK and Ireland for health risk and benefit studies. Photochem. Photobiol. Sci. 2015, 14, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-16394-1. [Google Scholar]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin type I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- CIE 174:20. Action Spectrum for the Production of Previtamin D3 in Human Skin; CIE: Vienna, Austria, 2006; ISBN 978 3 901906 50 3. [Google Scholar]
- Diffey, B.L.; Jansen, C.T.; Urbach, F.; Wulf, H.C. The standard erythema dose: A new photobiological concept. Photodermatol. Photoimmunol. Photomed. 1997, 13, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Kift, R.; Berry, J.L.; Rhodes, L.E. The vitamin D debate: Translating controlled experiments into reality for human sun exposure times. Photochem. Photobiol. 2011, 87, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Engelsen, O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem. Photobiol. 2006, 82, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 28 February 2018).
- Carrasco-Hernandez, R.; Smedley, A.R.D.; Webb, A.R. Using urban canyon geometries obtained from Google Street View for atmospheric studies: Potential applications in the calculation of street level total shortwave irradiances. Energy Build. 2014, 86, 340–348. [Google Scholar] [CrossRef]
- National Radiological Protection Board. Health Effects from Ultraviolet Radiation; Report of an Advisory Group on Non-Ionising Radiation; NRPB: Didcot, UK, 2002; Volume 13.
- Holick, M.F.; Jenkins, M. The UV Advantage; iBooks, Inc.: New York, NY, USA, 2003; ISBN 978-1596879003. [Google Scholar]
- Dowdy, J.C.; Sayre, R.M.; Holick, M.F. Holick’s rule and vitamin D from sunlight. J. Steroid Biochem. Mol. Biol. 2010, 121, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Felton, S.J.; Cooke, M.S.; Kift, R.; Berry, J.L.; Webb, A.R.; Lam, P.M.; de Gruijl, F.R.; Vail, A.; Rhodes, L.E. Concurrent beneficial (vitamin D production) and hazardous (cutaneous DNA damage) impact of repeated low-level summer sunlight exposures. Br. J. Dermatol. 2016, 175, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Chadwick, C.A.; Harrison, G.I.; Hawk, J.L.; Nikaido, O.; Potten, C.S. The in situ repair kinetics of epidermal thymine dimers and 6-4 photoproducts in human skin types I and II. J. Investig. Dermatol. 1996, 106, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Bränström, R.; Kasparian, N.A.; Chang, Y.M.; Affleck, P.; Tibben, A.; Aspinwall, L.G.; Azizi, E.; Baron-Epel, O.; Battistuzzi, L.; Bergman, W.; et al. Predictors of sun protection behaviors and severe sunburn in an international online study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Maclaughlin, J.A.; Holick, M.F. Aging decreases the capacity human skin to produce vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Kazantzidis, A.; Kift, R.C.; Farrar, M.D.; Wilkinson, J.; Rhodes, L.E. Colour Counts: Sunlight and skin type as drivers of vitamin D deficiency at UK latitudes. Nutrients 2018, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- NICE Guideline. Sunlight Exposure: Risks and Benefits. 2016. Available online: https://www.nice.org.uk/guidance/ng34/resources/sunlight-exposure-risks-and-benefits-1837392363205 (accessed on 20 February 2018).
- O’Neill, C.M.; Kazantzidis, A.; Ryan, M.J.; Barber, N.; Sempos, C.T.; Durazo-Arvizu, R.A.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal Changes in vitamin D-effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D. Nutrients 2016, 8, 533. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Type Volunteers | Relevant Measures | Input |
|---|---|---|---|
| Webb et al. 2010 [11] | Observation Adult white Caucasian (n = 109; median age 44 (range 20–60) years; 78%F, 22%M) | Monthly 25(OH)D | September maximum 25(OH)D February minimum 25(OH)D Winter rate of 25(OH)D spend |
| Rhodes et al. 2010 [17] | Intervention Adult white Caucasian (n = 109; median age 35 (range 20–60) years; 68%F, 32%M) | 25(OH)D response to 6 weeks of regular simulated sun | Increase in 25(OH)D for unit exposure to sunlight (skin types I–IV *) |
| Kazantzidis et al. 2015 [18] | Climate modelling n/a | Detailed UVR climatology of UK, validated against ground based measurements | Available UVR on 1° latitude by 1° longitude grid includes altitude and all weather 2003–2012 |
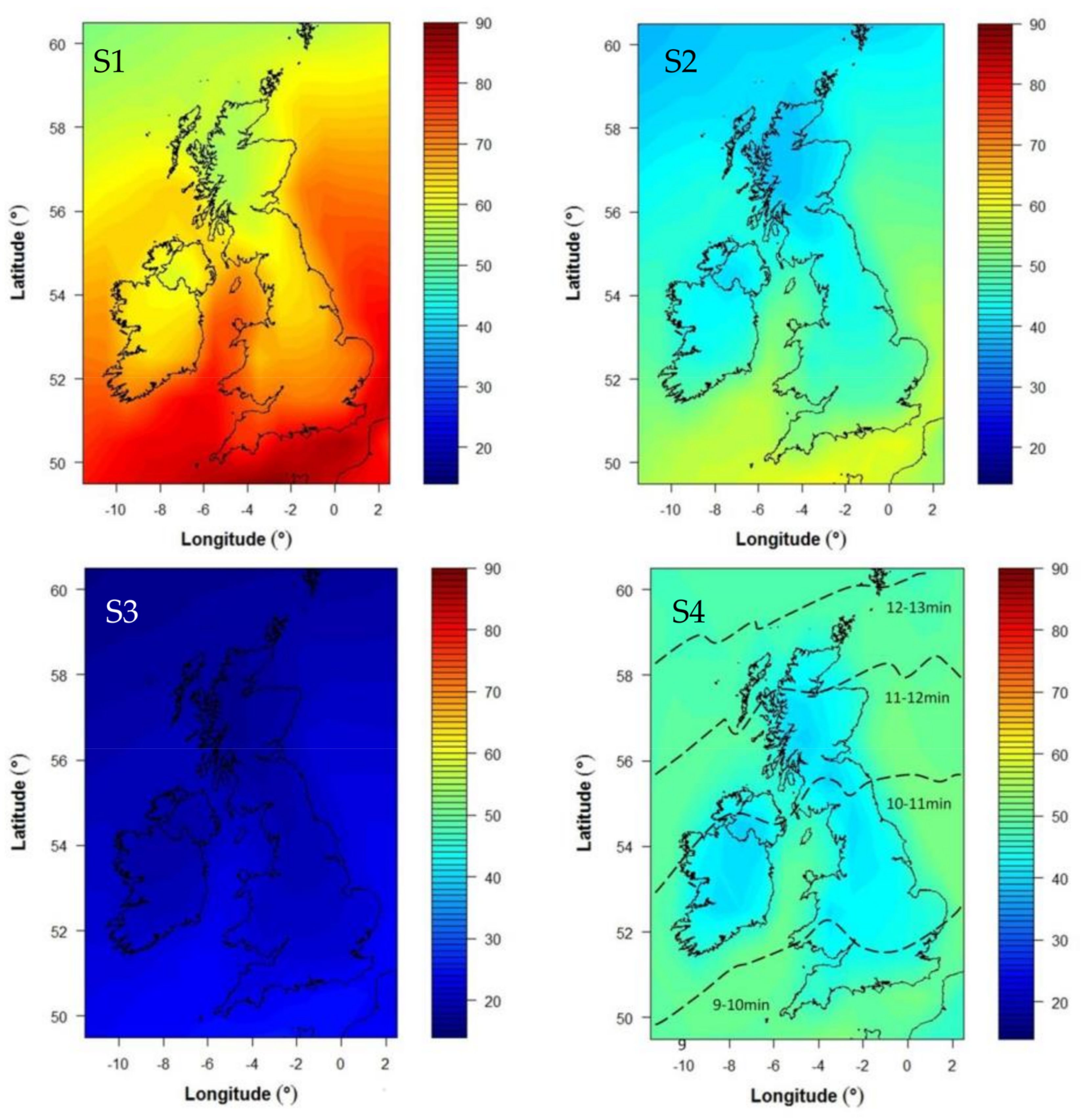
| Scenario | Skin Area | Months | Time |
|---|---|---|---|
| S1 | 35% (face, hands, forearms, lower legs) | March–September | Constant |
| S2 | 35% (face, hands, forearms, lower legs) | June–August | Constant |
| 10% (face and hands) | March–May, September | ||
| S3 | 10% (face and hands) | March–September | Constant |
| S4 | 35% (face, hands, forearms, lower legs) | June–August | Varies with latitude (equivalent to 1 SED) * |
| Method Step | Result |
|---|---|
| End summer month | September |
| End summer 25OHD target, A + (nmol/L) | 80.5 |
| Monthly 25OHD spend, B (nmol/L/month) | 6.25 |
| Summer dose required, C (SED) | 38 * |
| Acceptable daily dose (SED) | 1 |
| Time for fixed daily dose (S1–3), D (minutes) | 9 |
| Time range (S4) for daily dose of 1 SED at noon in June. Time (minutes) varies with latitude from S England to N Scotland | 9–13 |
| S1: E > C 35% skin area March–September | Y |
| S2: E > C 10% skin area March–May + September plus 35% skin area June–August | Y ** |
| S3: E > C 10% skin area all summer | N |
| S4: E > C 35% skin area, June–August, D adjusted for latitude to give 1 SED | Y |
| Exposures are assumed to occur during normal lunchtime hours (approximately 12–2 pm during British Summer Time, which is one hour either side of the solar noon). |
| Exposure should occur every day * during the months from March to September. If a day is missed, double exposure time should not be pursued the next day. If there is a wish to compensate, more skin area could be exposed but for the same short time. |
| Exposure should be in an open place if possible and in direct sun when available (i.e., without seeking shade for this short period). |
| Exposed skin should be unprotected (no sunscreen, make up, or clothing e.g., tights) |
| During the months June–August, about 1/3 of skin area should be exposed. This is equivalent to face, hands, forearms, and lower legs, but areas are interchangeable so if the face is protected then upper arms or upper chest might be exposed instead. |
| During the remaining cooler months, only hands and face (or equivalent) need to be exposed although larger areas would be an advantage when appropriate. |
| White-skinned people need a daily 9 min exposure. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webb, A.R.; Kazantzidis, A.; Kift, R.C.; Farrar, M.D.; Wilkinson, J.; Rhodes, L.E. Meeting Vitamin D Requirements in White Caucasians at UK Latitudes: Providing a Choice. Nutrients 2018, 10, 497. https://doi.org/10.3390/nu10040497
Webb AR, Kazantzidis A, Kift RC, Farrar MD, Wilkinson J, Rhodes LE. Meeting Vitamin D Requirements in White Caucasians at UK Latitudes: Providing a Choice. Nutrients. 2018; 10(4):497. https://doi.org/10.3390/nu10040497
Chicago/Turabian StyleWebb, Ann R., Andreas Kazantzidis, Richard C. Kift, Mark D. Farrar, Jack Wilkinson, and Lesley E. Rhodes. 2018. "Meeting Vitamin D Requirements in White Caucasians at UK Latitudes: Providing a Choice" Nutrients 10, no. 4: 497. https://doi.org/10.3390/nu10040497






