Effects of Intragastric Administration of Tryptophan on the Blood Glucose Response to a Nutrient Drink and Energy Intake, in Lean and Obese Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Intragastric Infusions
2.3. Protocol
2.4. Measurements
2.4.1. Blood Glucose and Plasma Hormone Analysis
2.4.2. Gastric Emptying
2.4.3. Appetite Perceptions, GI Symptoms and Food Intake
2.5. Data and Statistical Analyses
3. Results
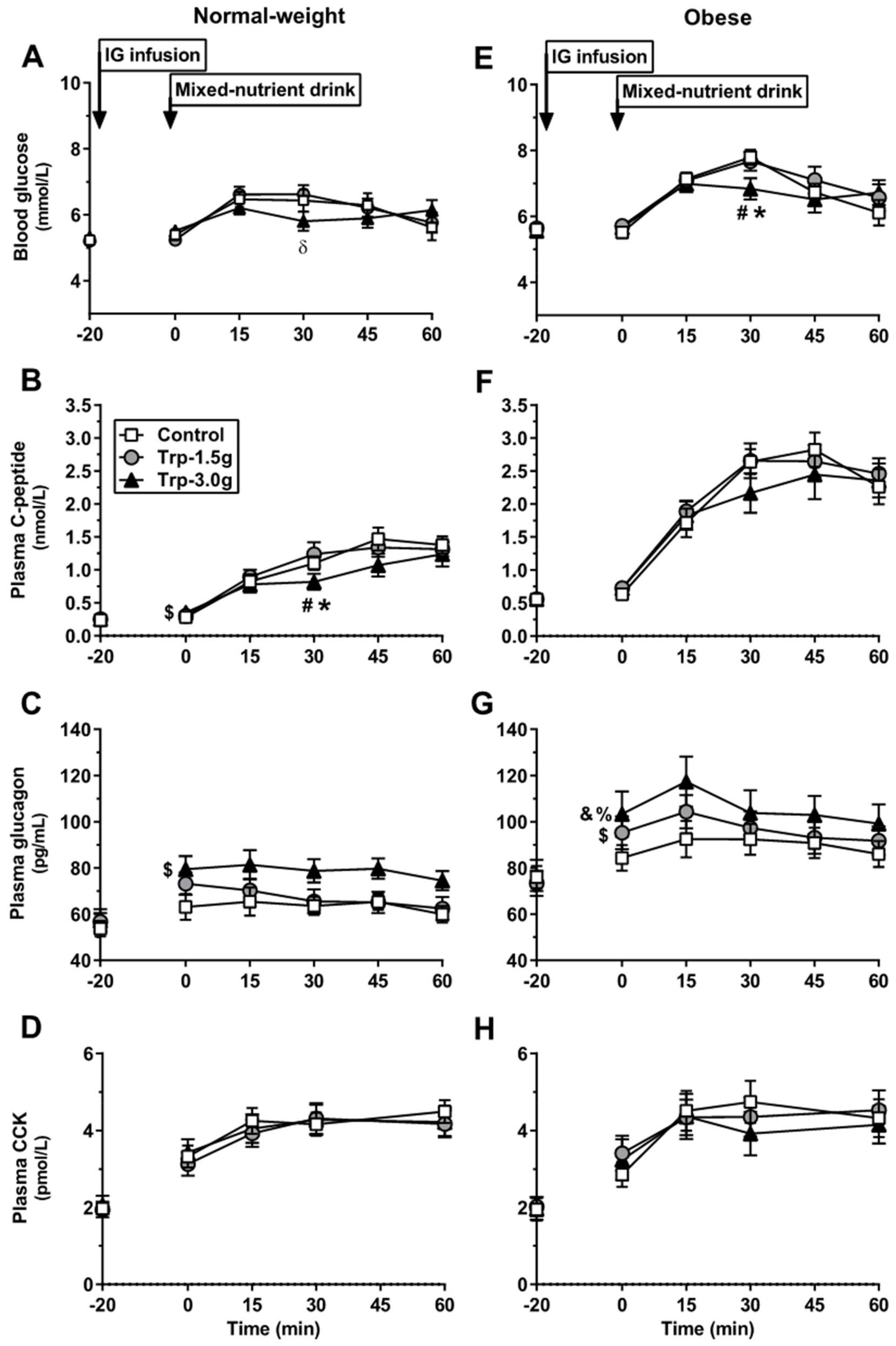
3.1. Normal-Weight Subjects
3.1.1. Blood Glucose
3.1.2. C-Peptide
3.1.3. Glucagon
3.1.4. CCK
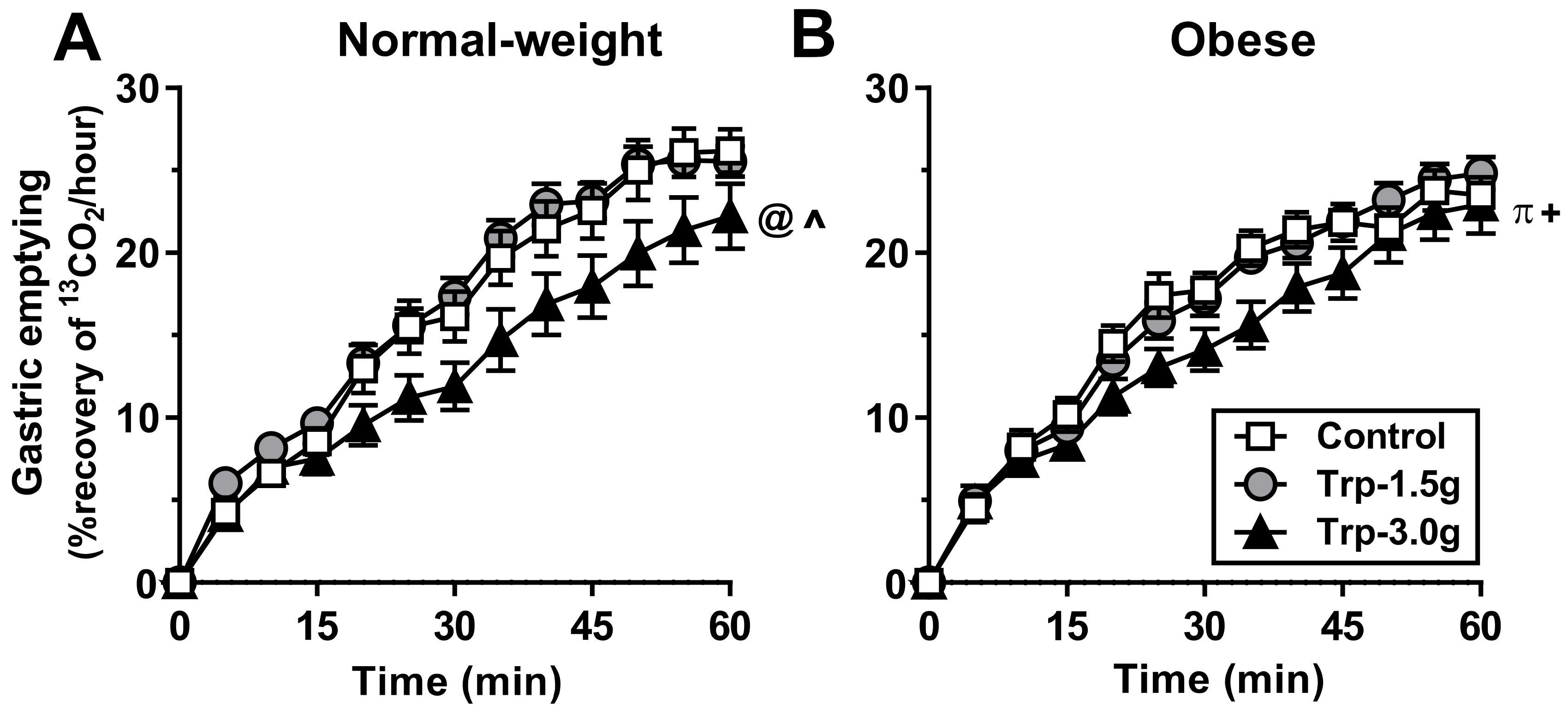
3.1.5. Gastric Emptying
3.1.6. Plasma Tryptophan
3.1.7. Appetite Perceptions, GI Symptoms and Food Intake
3.2. Obese Subjects
3.2.1. Blood Glucose
3.2.2. C-Peptide
3.2.3. Glucagon
3.2.4. CCK
3.2.5. Gastric Emptying
3.2.6. Plasma Tryptophan
3.2.7. Appetite Perceptions, GI Symptoms and Food Intake
3.3. Comparison between Obese and Normal-Weight Subject
3.4. Relationships among Energy Intake and Blood Glucose with Gastric Emptying, Hormones and Tryptophan Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kissileff, H.R.; Pi-Sunyer, F.X.; Thornton, J.; Smith, G.P. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am. J. Clin. Nutr. 1981, 34, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Beglinger, C.; Degen, L.; Matzinger, D.; D’Amato, M.; Drewe, J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1149–R1154. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Edelbroek, M.A.L.; Wishart, J.M.; Straathof, J.W. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993, 36, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Seimon, R.V.; Brennan, I.M.; Russo, A.; Little, T.J.; Jones, K.L.; Standfield, S.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E294–E300. [Google Scholar] [CrossRef] [PubMed]
- Marathe, C.S.; Horowitz, M.; Trahair, L.G.; Wishart, J.M.; Bound, M.; Lange, K.; Rayner, C.K.; Jones, K.L. Relationships of early and late glycemic responses with gastric emptying during an oral glucose tolerance test. J. Clin. Endocrinol. Metab. 2015, 100, 3565–3571. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Horowitz, M.; Carney, B.I.; Wishart, J.M.; Guha, S.; Green, L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J. Nucl. Med. 1996, 37, 1643–1648. [Google Scholar] [PubMed]
- Phillips, W.T.; Schwartz, J.G.; McMahan, C.A. Reduced postprandial blood glucose levels in recently diagnosed non-insulin-dependent diabetics secondary to pharmacologically induced delayed gastric emptying. Dig. Dis. Sci. 1993, 38, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.N. A possible relation between the regulation of gastric emptying and food intake. Am. J. Physiol. 1980, 239, G1–G4. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.T.; Luscombe-Marsh, N.D.; Saies, A.A.; Little, T.J.; Standfield, S.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 2013, 98, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Smout, A.J.; Wishart, J.; Pilichiewicz, A.N.; Rades, T.; Chapman, I.M.; Feinle-Bisset, C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R524–R533. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Luscombe-Marsh, N.D.; Little, T.J.; Standfield, S.; Otto, B.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal infusion of l-tryptophan on ad libitum eating, antropyloroduodenal motility, glycemia, insulinemia, and gut peptide secretion in healthy men. J. Clin. Endocrinol. Metab. 2014, 99, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Gerspach, A.C.; Hafliger, S.; Meili, J.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Beglinger, C.; Wolnerhanssen, B. Effect of l-tryptophan and l-leucine on gut hormone secretion, appetite feelings and gastric emptying rates in lean and non-diabetic obese participants: A randomized, double-blind, parallel-group trial. PLoS ONE 2016, 11, e0166758. [Google Scholar] [CrossRef] [PubMed]
- Carney, B.I.; Jones, K.L.; Horowitz, M.; Sun, W.M.; Hebbard, G.; Edelbroek, M.A. Stereospecific effects of tryptophan on gastric emptying and hunger in humans. J. Gastroenterol. Hepatol. 1994, 9, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Edelbroek, M.; Sun, W.M.; Horowitz, M.; Dent, J.; Smout, A.; Akkermans, L. Stereospecific effects of intraduodenal tryptophan on pyloric and duodenal motility in humans. Scand. J. Gastroenterol. 1994, 29, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Hrboticky, N.; Leiter, L.A.; Anderson, G.H. Effects of l-tryptophan on short term food intake in lean men. Nutr. Res. 1985, 5, 595–607. [Google Scholar] [CrossRef]
- Caballero, B.; Finer, N.; Wurtman, R.J. Plasma amino acids and insulin levels in obesity: Response to carbohydrate intake and tryptophan supplements. Metabolism 1988, 37, 672–676. [Google Scholar] [CrossRef]
- Jones, A.G.; Hattersley, A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 2013, 30, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Feinle-Bisset, C.; Fitzgerald, P.C.; Standfield, S.; Horowitz, M.; Clifton, P.M.; Luscombe-Marsh, N.D. Comparative effects of intraduodenal whey protein hydrolysate on antropyloroduodenal motility, gut hormones, glycemia, appetite, and energy intake in lean and obese men. Am. J. Clin. Nutr. 2015, 102, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Randomization.com. Available online: www.randomization.com (accessed on 7 April 2018).
- ANZCTR. Available online: www.anzctr.org.au.
- Mossi, S.; Meyer-Wyss, B.; Beglinger, C.; Schwizer, W.; Fried, M.; Ajami, A.; Brignoli, R. Gastric emptying of liquid meals measured noninvasively in humans with [13C]acetate breath test. Dig. Dis. Sci. 1994, 39, 107S–109S. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.S.; Brennan, I.M.; Little, T.J.; Gentilcore, D.; Hausken, T.; Jones, K.L.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Reproducibility of energy intake, gastric emptying, blood glucose, plasma insulin and cholecystokinin responses in healthy young males. Br. J. Nutr. 2009, 101, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, A.; Peracchi, M.; Conte, D.; Fraquelli, M.; Porrini, M. Physical state of meal affects gastric emptying, cholecystokinin release and satiety. Br. J. Nutr. 1998, 80, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Harder, U.; Koletzko, B.; Peissner, W. Quantification of 22 plasma amino acids combining derivatization and ion-pair LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Gucciardi, A.; Pirillo, P.; Di Gangi, I.M.; Naturale, M.; Giordano, G. A rapid UPLC-MS/MS method for simultaneous separation of 48 acylcarnitines in dried blood spots and plasma useful as a second-tier test for expanded newborn screening. Anal. Bioanal. Chem. 2012, 404, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.A.; Sturm, K.; MacIntosh, C.G.; Feinle, C.; Horowitz, M.; Chapman, I.M. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur. J. Clin. Nutr. 2004, 58, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Landrock, M.F.; Ullrich, S.S.; Standfield, S.; Otto, B.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal infusion of the branched-chain amino acid leucine on ad libitum eating, gut motor and hormone functions, and glycemia in healthy men. Am. J. Clin. Nutr. 2015, 102, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Trahair, L.G.; Horowitz, M.; Marathe, C.S.; Lange, K.; Standfield, S.; Rayner, C.K.; Jones, K.L. Impact of gastric emptying to the glycemic and insulinemic responses to a 75-g oral glucose load in older subjects with normal and impaired glucose tolerance. Physiol. Rep. 2014, 2, e12204. [Google Scholar] [CrossRef] [PubMed]
- Jordi, J.; Herzog, B.; Lutz, T.A.; Verrey, F. Novel antidiabetic nutrients identified by in vivo screening for gastric secretion and emptying regulation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R869–R878. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.R.; Woolson, R.F.; Cooke, A.R. Osmolyte and tryptophan receptors controlling gastric emptying in the dog. Am. J. Physiol. 1976, 231, 848–853. [Google Scholar] [PubMed]
- Kuwata, H.; Iwasaki, M.; Shimizu, S.; Minami, K.; Maeda, H.; Seino, S.; Nakada, K.; Nosaka, C.; Murotani, K.; Kurose, T.; et al. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: A randomised, controlled crossover, exploratory trial. Diabetologia 2016, 59, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.C., Jr.; Fajans, S.S.; Conn, J.W.; Knopf, R.F.; Rull, J. Stimulation of insulin secretion by amino acids. J. Clin. Investig. 1966, 45, 1487–1502. [Google Scholar] [CrossRef] [PubMed]
- Little, T.J.; Pilichiewicz, A.N.; Russo, A.; Phillips, L.; Jones, K.L.; Nauck, M.A.; Wishart, J.; Horowitz, M.; Feinle-Bisset, C. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: Relationships with postprandial glycemic and insulinemic responses. J. Clin. Endocrinol. Metab. 2006, 91, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Linnebjerg, H.; Park, S.; Kothare, P.A.; Trautmann, M.E.; Mace, K.; Fineman, M.; Wilding, I.; Nauck, M.; Horowitz, M. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul. Pept. 2008, 151, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Pfeiffer, C.; Steinstrasser, A.; Becker, R.H.; Rutten, H.; Ruus, P.; Horowitz, M. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes—Relationship to postprandial glycemia. Regul. Pept. 2013, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.S.; Fitzgerald, P.C.; Nkamba, I.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Intragastric lysine lowers the circulating glucose and insulin responses to a mixed-nutrient drink without slowing gastric emptying in healthy adults. J. Nutr. 2017, 147, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.S.; Fitzgerald, P.C.; Schober, G.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Intragastric administration of leucine or isoleucine lowers the blood glucose response to a mixed-nutrient drink by different mechanisms in healthy, lean volunteers. Am. J. Clin. Nutr. 2016, 104, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Hedo, J.A.; Villanueva, M.L.; Marco, J. Elevation of plasma glucose and glucagon after tryptophan ingestion in man. Metabolism 1977, 26, 1131–1134. [Google Scholar] [CrossRef]
- Salter, M.; Pogson, C.I. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem. J. 1985, 229, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Brennan, I.M.; Feltrin, K.L.; Nair, N.S.; Hausken, T.; Little, T.J.; Gentilcore, D.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Feinle-Bisset, C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G602–G610. [Google Scholar] [CrossRef] [PubMed]

| Normal-Weight | Obese | |||||
|---|---|---|---|---|---|---|
| Control | Trp-1.5g | Trp-3.0g | Control | Trp-1.5g | Trp3.0 g | |
| Blood glucose (mmol/L*min) | 369 ± 14 | 373 ± 11 | 360 ± 10 | 411 ± 10 | 418 ± 14 | 397 ± 15 |
| Plasma C-peptide (nmol/L*min) | 64 ± 6 | 63 ± 6 | 53 ± 8 *,θ | 129 ± 10 | 132 ± 11 | 120 ± 14 |
| Plasma glucagon (pg/mL*min) | 3862 ± 268 | 4021 ± 281 | 4740 ± 308 ^,@ | 5413 ± 387 | 5824 ± 408 | 6382 ± 553 δ,θ |
| Plasma CCK (pmol/L*min) | 249 ± 16 | 242 ± 20 | 244 ± 22 | 260 ± 29 | 256 ± 28 | 252 ± 31 |
| Gastric emptying (%recovery of 13CO2/hour*min) | 960 ± 68 | 1005 ± 46 | 768 ± 75 ^,@ | 969 ± 47 | 986 ± 38 | 832 ± 55 π,+ |
| Plasma tryptophan (µmol/L*min) | 4457 ± 138 | 17,264 ± 1368 ^ | 27,652 ± 2042 ^,@ | 4643 ± 163 | 13,442 ± 841 ^ | 20,816 ± 1914 ^,@ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullrich, S.S.; Fitzgerald, P.C.E.; Giesbertz, P.; Steinert, R.E.; Horowitz, M.; Feinle-Bisset, C. Effects of Intragastric Administration of Tryptophan on the Blood Glucose Response to a Nutrient Drink and Energy Intake, in Lean and Obese Men. Nutrients 2018, 10, 463. https://doi.org/10.3390/nu10040463
Ullrich SS, Fitzgerald PCE, Giesbertz P, Steinert RE, Horowitz M, Feinle-Bisset C. Effects of Intragastric Administration of Tryptophan on the Blood Glucose Response to a Nutrient Drink and Energy Intake, in Lean and Obese Men. Nutrients. 2018; 10(4):463. https://doi.org/10.3390/nu10040463
Chicago/Turabian StyleUllrich, Sina S., Penelope C. E. Fitzgerald, Pieter Giesbertz, Robert E. Steinert, Michael Horowitz, and Christine Feinle-Bisset. 2018. "Effects of Intragastric Administration of Tryptophan on the Blood Glucose Response to a Nutrient Drink and Energy Intake, in Lean and Obese Men" Nutrients 10, no. 4: 463. https://doi.org/10.3390/nu10040463




