Development of Colorectal-Targeted Dietary Supplement Tablets Containing Natural Purple Rice Bran Oil as a Colorectal Chemopreventive
Abstract
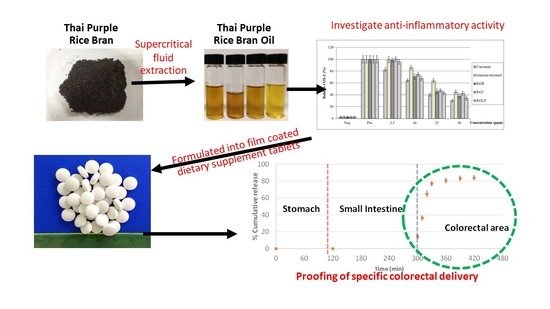
:1. Introduction
2. Material and Methods
2.1. Chemicals and Materials
2.2. Preparation of Natural Purple Rice Bran Oil (NPRBO)
2.3. Determination of the γ-Oryzanol Content
2.4. Determination of the Tocotrienol and Tocopherol Content
2.5. Determination of Anti-Inflammatory Activities
2.6. Determination of Nitric Oxide and iNOS Production
2.7. Determination of COX-2 Production
2.8. Preparation of NPRBO Core Tablets
2.9. Coating of NPRBO Tablets
2.10. In Vitro Gastrointestinal γ-Oryzanol Release Study
2.11. Analysis of the γ-Oryzanol Content
2.12. Statistical Analysis
3. Results and Discussion
3.1. Determination of the Tocotrienol, Tocopherol, and γ-Oryzanol Contents
3.2. Investigation of the Anti-Inflammatory Activity of NPRBO Via Nitric Oxide and COX-2 Inhibition
3.3. In Vitro Release Proof of Concept for the NPRBO Colorectal Delivery Platform
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cunningham, D.; Atkin, W.; Lenz, H.-J.; Lynch, H.T.; Minsky, B.; Nordlinger, B.; Starling, N. Colorectal cancer. Lancet 2010, 375, 1030–1047. [Google Scholar] [CrossRef]
- Emilsson, L.; Holme, Ø.; Bretthauer, M.; Cook, N.R.; Buring, J.E.; Løberg, M.; Adami, H.O.; Sesso, H.D.; Gaziano, M.J.; Kalager, M. Systematic review with meta-analysis: The comparative effectiveness of aspirin vs. Screening for colorectal cancer prevention. Aliment. Pharmacol. Ther. 2017, 45, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.; Squires, H.; Carroll, C.; Papaioannou, D.; Booth, A.; Logan, R.; Maguire, C.; Hind, D.; Tappenden, P. Chemoprevention of Colorectal Cancer: Systematic Review and Economic Evaluation. Health Technol. Assess. 2010, 14, 1–206. [Google Scholar] [CrossRef] [PubMed]
- Chotimarkorn, C.; Benjakul, S.; Silalai, N. Antioxidant components and properties of five long-grained rice bran extracts from commercial available cultivars in Thailand. Food Chem. 2008, 111, 636–641. [Google Scholar] [CrossRef]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.J.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1163–1170. [Google Scholar]
- Nam, S.H.; Choi, S.P.; Kang, M.Y.; Kozukue, N.; Friedman, M. Antioxidative, antimutagenic, and anticarcinogenic activities of rice bran extracts in chemical and cell assays. J. Agric. Food Chem. 2005, 53, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Srisala, S.; Chunhabundit, R.; Kongkachuichai, R.; Jittorntrum, B.; Visetpanit, Y. Effects of bran extracts from thai molecular breeding rices on growth and apoptosis in human promyelocytic leukemia cells. Thai J. Toxicol. 2009, 24, 81–91. [Google Scholar]
- Nagasaka, R.; Chotimarkorn, C.; Shafiqul, I.M.; Hori, M.; Ozaki, H.; Ushio, H. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem. Biophys. Res. Commun. 2007, 358, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Verschoyle, R.D.; Greaves, P.; Cai, H.; Edwards, R.E.; Steward, W.P.; Gescher, A.J. Evaluation of the cancer chemopreventive efficacy of rice bran in genetic mouse models of breast, prostate and intestinal carcinogenesis. Br. J. Cancer 2007, 96, 248. [Google Scholar] [CrossRef] [PubMed]
- Saenjum, C.; Chaiyasut, C.; Chansakaow, S.; Suttajit, M.; Sirithunyalug, B. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from thai purple rice bran. J. Med. Plants Res. 2012, 6, 1070–1077. [Google Scholar]
- Tsushimoto, G.; Shibahara, T.; Awogi, T.; Kaneko, E.; Sutou, S.; Yamamoto, K.; Shirakawa, H. DNA-damaging, mutagenic, clastogenic and cell-cell communication inhibitory properties of γ-oryzanol. J. Toxicol. Sci. 1991, 16, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, M.; Shimizu, Y.; Takahashi, T.; Otaka, T.; Kimura, S.; Kadowaki, H.; Uda, F.; Miwa, T. Carcinogenicity study of γ-oryzanol in F344 rats. Food Chem. Toxicol. 1992, 30, 41–48. [Google Scholar] [CrossRef]
- Malekian, F.; Rao, R.M.; Prinyawiwatkul, W.; Marshall, W.E.; Windhauser, M.; Ahmedna, M. Lipase and Lipoxygenase Activity, Functionality, and Nutrient Losses in Rice Bran During Storage; Bulletin Number 870; Louisiana Agricultural Experiment Station: Baton Rouge, LA, USA, January 2000; pp. 1–68. [Google Scholar]
- Jesus, S.P.; Grimaldi, R.; Hense, H. Recovery of γ-oryzanol from rice bran oil byproduct using supercritical fluid extraction. J. Supercrit. Fluid. 2010, 55, 149–155. [Google Scholar] [CrossRef]
- Xu, Z.; Godber, J.S. Purification and identification of components of γ-oryzanol in rice bran oil. J. Agric. Food Chem. 1999, 47, 2724–2728. [Google Scholar] [CrossRef] [PubMed]
- Pengkumsri, N.; Chaiyasut, C.; Sivamaruthi, B.S.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Chaiyasut, K.; Kesika, P. The influence of extraction methods on composition and antioxidant properties of rice bran oil. Food Sci. Technol. 2015, 35, 493–501. [Google Scholar] [CrossRef]
- Hong, C.H.; Hur, S.K.; Oh, O.J.; Kim, S.S.; Nam, K.A.; Lee, S.K. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J. Ethnopharmacol. 2002, 83, 153–159. [Google Scholar] [CrossRef]
- Hu, C.; Zawistowski, J.; Ling, W.; Kitts, D.D. Black rice (Oryza sativa l. Indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems. J. Agric. Food Chem. 2003, 51, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Tuntipopipat, S.; Muangnoi, C.; Failla, M.L. Anti-inflammatory activities of extracts of thai spices and herbs with lipopolysaccharide-activated RAW 264.7 murine macrophages. J. Med. Food 2009, 12, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Sreejayan; Rao, M.N.A. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jomha, N.M.; Elliott, J.A.W.; Law, G.K.; McGann, L.E. Evaluation of chondrocyte survival in situ using WST-1 and membrane integrity stains. Cell Tissue Bank. 2007, 8, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Colucci, R.; Blandizzi, C.; Ghisu, N.; Florio, T.; Del Tacca, M. Somatostatin inhibits colon cancer cell growth through cyclooxygenase-2 downregulation. Br. J. Pharmacol. 2008, 155, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-J.; Liu, P.-L.; Ng, L.-T. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol. Nutr. Food Res. 2008, 52, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.P.; Kim, S.P.; Kang, M.Y.; Nam, S.H.; Friedman, M. Protective effects of black rice bran against chemically-induced inflammation of mouse skin. J. Agric. Food Chem. 2010, 58, 10007–10015. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Picinich, S.C.; Yang, Z.; Zhao, Y.; Suh, N.; Kong, A.-N.; Yang, C.S. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2010, 31, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Cooney, R.V.; Franke, A.A.; Harwood, P.J.; Hatch-Pigott, V.; Custer, L.J.; Mordan, L.J. Gamma-tocopherol detoxification of nitrogen dioxide: Superiority to alpha-tocopherol. Proc. Natl. Acad. Sci. USA 1993, 90, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Buecher, B.; Bouancheau, D.; Broquet, A.; Bezieau, S.; Denis, M.G.; Bonnet, C.; Heymann, M.F.; Jarry, A.; Galmiche, J.P.; Blottiere, H.M. Growth inhibitory effect of celecoxib and rofecoxib on human colorectal carcinoma cell lines. Anticancer Res. 2005, 25, 225–233. [Google Scholar] [PubMed]
- Elder, D.J.E.; Halton, D.E.; Crew, T.E.; Paraskeva, C. Apoptosis induction and cyclooxygenase-2 regulation in human colorectal adenoma and carcinoma cell lines by the cyclooxygenase-2-selective non-steroidal anti-inflammatory drug NS-398. Int. J. Cancer 2000, 86, 553–560. [Google Scholar] [CrossRef]
- Gupta, R.A.; DuBois, R.N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer 2001, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Shirode, A.B.; Sylvester, P.W. Synergistic anticancer effects of combined γ-tocotrienol and celecoxib treatment are associated with suppression in akt and NF-κB signaling. Biomed. Pharmacother. 2010, 64, 327–332. [Google Scholar] [CrossRef] [PubMed]
- [1216] TABLET FRIABILITY. In 2017 U.S. Pharmacopoeia-National Formulary [USP 40 NF 35]; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2016; Volume 1, p. 1749.
- Leopold, C.S. Coated dosage forms for colon-specific drug delivery. Pharm. Sci. Technol. Today 1999, 2, 197–204. [Google Scholar] [CrossRef]
- Rubinstein, A. Gastrointestinal anatomy, physiology and permeation pathways. In Enhancement in Drug Delivery; CRC Press Tylor & Francis Group: London, UK, 2006; p. 3. [Google Scholar]
- Martinez, M.; Amidon, G.; Clarke, L.; Jones, W.W.; Mitra, A.; Riviere, J. Applying the biopharmaceutics classification system to veterinary pharmaceutical products: Part ii. Physiological considerations. Adv. Drug Deliv. Rev. 2002, 54, 825–850. [Google Scholar] [CrossRef]



| Samples | Components (mg/g NPRBO) | ||||||
|---|---|---|---|---|---|---|---|
| δ-Tocotrienol | γ-Tocotrienol | α-Tocotrienol | δ-Tocopherol | γ-Tocopherol | α-Tocopherol | γ-Oryzanol | |
| Khao’ Gam Boung | 0.09 ± 0.02 b | 1.42 ± 0.08 a,b | 0.18 ± 0.02 b | 0.09 ± 0.02 b | 1.03 ± 0.08 a | 0.35 ± 0.03 c,d | 9.73 ± 0.47 a,b |
| Khao’ Gam Thor | 0.10 ± 0.02 a,b | 1.33 ± 0.09 b | 0.17 ± 0.02 b | 0.09 ± 0.02 b | 0.95 ± 0.07 b | 0.32 ± 0.03 d | 9.32 ± 0.52 b |
| Khao’ Gam Leum-Phua | 0.13 ± 0.03 a | 1.57 ± 0.10 a | 0.22 ± 0.03 a | 0.12 ± 0.02 a | 1.06 ± 0.08 a | 0.48 ± 0.03 a | 9.88 ± 0.58 a,b |
| Khao’ Gam Pah E-Kaw | 0.10 ± 0.02 a,b | 1.46 ± 0.09 a,b | 0.23 ± 0.03 a | 0.11 ± 0.03 a,b | 0.98 ± 0.07 a,b | 0.43 ± 0.04 a,b | 10.11 ± 0.48 a |
| Khao’ Niaw Dam | 0.09 ± 0.02 b | 1.44 ± 0.07 a,b | 0.20 ± 0.02 a,b | 0.10 ± 0.02 a,b | 1.00 ± 0.06 a,b | 0.38 ± 0.04 b,c | 9.68 ± 0.45 a,b |
| Samples | 50% Inhibition Concentration (µg/mL) | |||
|---|---|---|---|---|
| Nitric Oxide | iNOS | COX-2 | ||
| HCT 116 | HT-29 | |||
| Khao’ Gam Boung | 19.46 ± 1.19 d | 24.62 ± 1.55 b,c | 22.24 ± 1.22 b | 21.08 ± 0.84 c |
| Khao’ Gam Thor | 22.28 ± 1.12 e | 27.46 ± 1.68 c,d | 23.27 ± 0.64 b,c | 22.18 ± 0.72 c,d |
| Khao’ Gam Leum-Phua | 15.19 ± 0.75 b | 22.72 ± 1.47 b | 21.08 ± 0.87 b | 18.73 ± 0.76 b |
| Khao’ Gam Pah E-Kaw | 17.02 ± 0.84 b,c | 24.08 ± 1.32 b,c | 22.48 ± 0.63 b | 21.26 ± 0.82 c |
| Khao’ Niaw Dam | 18.67 ± 0.79 c,d | 25.25 ± 1.30 b,c | 27.39 ± 1.02 d | 26.75 ± 0.92 e |
| Gamma-oryzanol | 35.36 ± 1.56 f | 29.52 ± 1.42 d | 35.75 ± 1.28 e | 33.47 ± 0.86 f |
| Curcumin | 12.52 ± 0.63 a | 15.55 ± 1.34 a | 16.14 ± 0.65 a | 14.29 ± 0.58 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirithunyalug, B.; Saenjum, C.; Charumanee, S.; Sivamaruthi, B.S.; Chaiyasut, C.; Sirithunyalug, J.; Tipduangta, P. Development of Colorectal-Targeted Dietary Supplement Tablets Containing Natural Purple Rice Bran Oil as a Colorectal Chemopreventive. Nutrients 2018, 10, 444. https://doi.org/10.3390/nu10040444
Sirithunyalug B, Saenjum C, Charumanee S, Sivamaruthi BS, Chaiyasut C, Sirithunyalug J, Tipduangta P. Development of Colorectal-Targeted Dietary Supplement Tablets Containing Natural Purple Rice Bran Oil as a Colorectal Chemopreventive. Nutrients. 2018; 10(4):444. https://doi.org/10.3390/nu10040444
Chicago/Turabian StyleSirithunyalug, Busaban, Chalermpong Saenjum, Suporn Charumanee, Bhagavathi Sundaram Sivamaruthi, Chaiyavat Chaiyasut, Jakkapan Sirithunyalug, and Pratchaya Tipduangta. 2018. "Development of Colorectal-Targeted Dietary Supplement Tablets Containing Natural Purple Rice Bran Oil as a Colorectal Chemopreventive" Nutrients 10, no. 4: 444. https://doi.org/10.3390/nu10040444
APA StyleSirithunyalug, B., Saenjum, C., Charumanee, S., Sivamaruthi, B. S., Chaiyasut, C., Sirithunyalug, J., & Tipduangta, P. (2018). Development of Colorectal-Targeted Dietary Supplement Tablets Containing Natural Purple Rice Bran Oil as a Colorectal Chemopreventive. Nutrients, 10(4), 444. https://doi.org/10.3390/nu10040444