Pepsin Egg White Hydrolysate Improves Glucose Metabolism Complications Related to Metabolic Syndrome in Zucker Fatty Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Egg White Hydrolysates
2.2. Animal Study
2.3. Evaluation of Mechanical Sensitivity: Von Frey Hair Test
2.4. Blood and Organ Collection
2.5. Plasma Analytical Procedures: Glucose and Insulin Concentration
2.6. Pancreatic Islet Isolation and Determination of Insulin Secretion
2.7. Pancreas Histopathological Analysis
2.8. Statistical Analysis
3. Results
3.1. Tactile Allodynia
3.2. Plasma Glucose, Plasma Insulin, and Indexes of Insulin Resistance
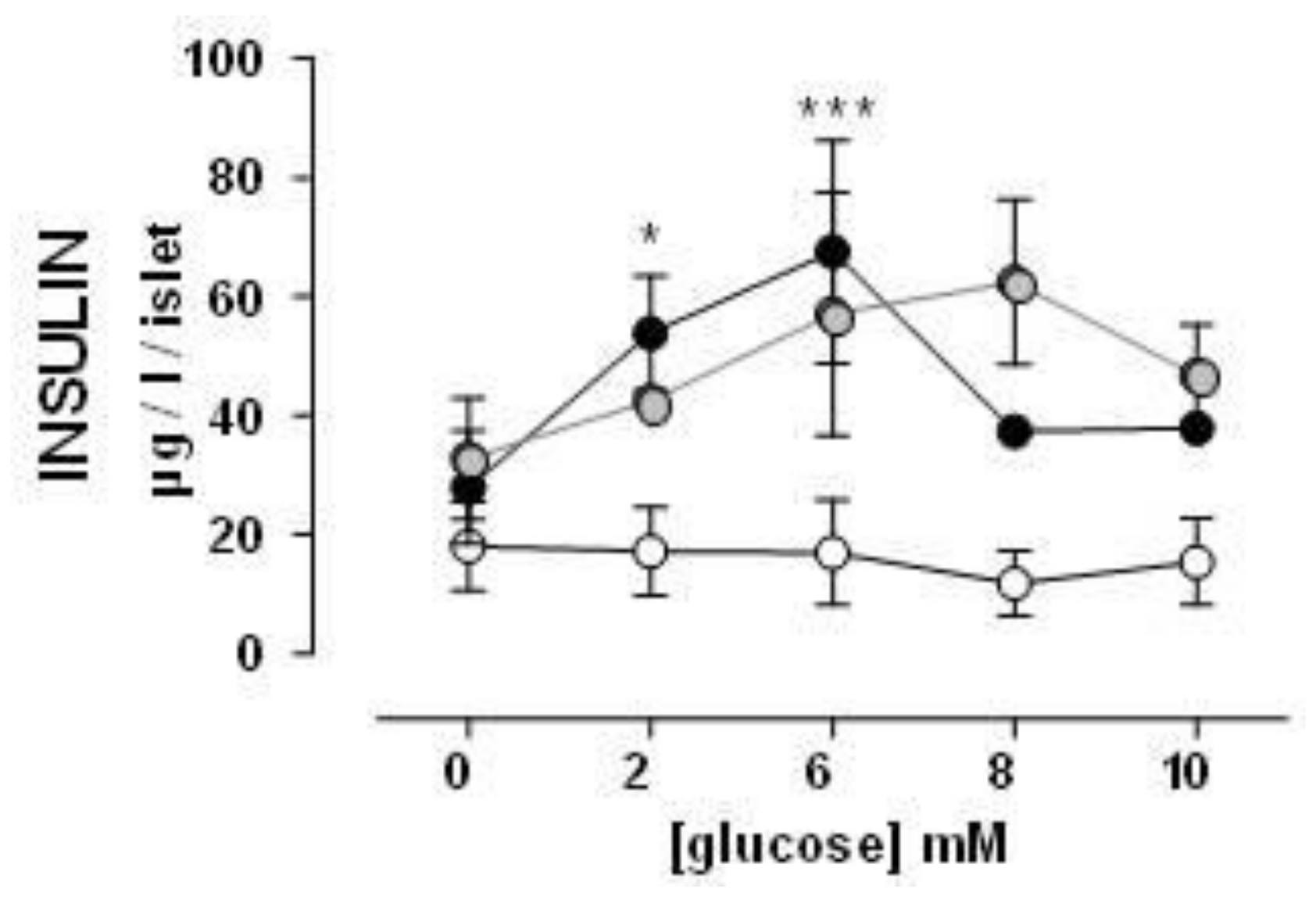
3.3. Insulin Secretion by Isolated Pancreas Islets
3.4. Pancreas Histology
4. Discussion
5. Conclusions
6. Patents
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aguilar-Salinas, C.A.; Rojas, R.; Gómez-Pérez, F.J.; Mehta, R.; Franco, A.; Olaiz, G.; Rull, J.A. The metabolic syndrome: A concept hard to define. Arch. Med. Res. 2005, 36, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Lee, J.H.; Lim, S.Y.; Ha, H.S.; Kwon, H.S.; Park, Y.M.; Lee, W.C.; Kang, M.I.; Yim, H.W.; Yoon, K.H.; et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J. Diabetes Investig. 2013, 4, 334–343. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Iglesias, A.; Pajuelo, D.; Quesada, H.; Díaz, S.; Bladé, C.; Arola, L.; Salvadó, M.J.; Mulero, M. Grape seed proanthocyanidin extract improves the hepatic glutathione metabolism in obese Zucker rats. Mol. Nutr. Food Res. 2014, 58, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Miguel, M. Experimental rat models to study the metabolic syndrome. Br. J. Nutr. 2009, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Susuceptibility of milk protein-derived peptides to dipeptidyl peptidase IV (DPP-IV) hydrolysis. Food Chem. 2014, 145, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Moughan, P.J.; Rutherfurd, S.M.; Montoya, C.A.; Dave, L.A. Food-derived bioactive peptides—A new paradigm. Nutr. Res. Rev. 2014, 27, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Recio, I.; Gómez-Ruiz, J.A.; Ramos, M.; López-Fandiño, R. Angiotensin I-converting enzyme inhibitory activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Miguel, M.; Bartolomé, B.; López-Fandiño, R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Álvarez, Y.; López-Fandiño, R.; Alonso, M.J.; Salaices, M. Vasodilator effects of peptides derived from egg white proteins. Regul. Pept 2007, 140, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Manso, M.; Aleixandre, A.; Alonso, M.J.; Salaices, M.; López-Fandiño, R. Vascular effects, angiotensin I-converting enzyme (ACE)-inhibitory activity, and antihypertensive properties of peptides derived from egg White. J. Agric. Food Chem. 2007, 55, 10615–10621. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; López-Fandiño, R.; Ramos, M.; Aleixandre, A. Short-term effect of egg-white hydrolysate products on the arterial blood pressure of hypertensive rats. Br. J. Nutr. 2005, 94, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; López-Fandiño, R.; Ramos, M.; Aleixandre, A. Long-term intake of egg white hydrolysate attenuates the development of hypertension in spontaneously hypertensive rats. Life Sci. 2006, 78, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- Manso, M.A.; Miguel, M.; Even, J.; Hernández, R.; Aleixandre, A.; López-Fandiño, R. Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chem. 2008, 109, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Liu, J.; Chen, F. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012, 135, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Landheer, S.; van Gilst, W.H.M.; van Amerongen, A.; Hammes, H.P.; Henning, R.H.; Deelman, L.E.; Buikema, H. Attenuation of Renovascular Damage in Zucker Diabetic Fatty Rat by NWT-03, an Egg Protein Hydrolysate with ACE- and DPP4-Inhibitory Activity. PLoS ONE 2012, 7, e46781. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Rimón, M.; López-Expósito, I.; López-Fandiño, R.; Miguel, M. Egg white hydrolysates with in vitro biological multi-activities to control complications associated to the metabolic syndrome. Eur. Food Res. Technol. 2016, 242, 61–69. [Google Scholar] [CrossRef]
- Garcés-Rimón, M.; González, C.; Uranga, J.A.; López-Miranda, V.; López-Fandiño, R.; Miguel, M. Pepsin egg white hydrolysate ameliorates obesity-related oxidative stress, inflammation and steatosis in Zucker Fatty Rats. PLoS ONE 2016, 11, e0151193. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Rafacho, A.; Roma, L.P.; Taboga, S.R.; Boschero, A.C.; Bosqueiro, J.R. Dexamethasone-induced insulin resistance is associated with increased connexin 36 mRNA and protein expression in pancreatic rat islets. Can. J. Physiol. Pharmacol. 2007, 85, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Lupachyk, S.; Watcho, P.; Obrosov, A.A.; Stavniichuk, R.; Obrosova, I.G. Endoplasmic reticulum stress contributes to prediabetic peripheral neuropathy. Exp. Neurol. 2013, 247, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Oltman, C.L.; Davidson, E.P.; Coppey, L.J.; Kleinschmidt, T.L.; Lund, D.D.; Adebara, E.T.; Yorek, M.A. Vascular and neural dysfunction in Zucker diabetic fatty rats: A difficult condition to reverse. Diabetes Obes. Metab. 2007, 10, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Oana, F.; Takeda, H.; Hayakawa, K.; Matsuzawa, A.; Akahane, S.; Isaji, M.; Akahane, M. Physiological difference between obese (fa/fa) zucker rats and lean zucker rats concerning adiponectin. Metabolism 2005, 54, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Bong, H.Y.; Kim, J.Y.; Jeong, H.I.; Moon, M.S.; Kim, J.; Kwon, O. Effects of corn gluten hydrolyzates, branched chain amino acids, and leucine on body weight reduction in obese rats induced by a high fat diet. Nutr. Res. Pract. 2010, 4, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.F.; Li, G.Z.; Peng, H.B.; Zhang, F.; Chen, Y.; Li, Y. Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl. Physiol. Nutr. Metab. 2010, 35, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Justo, M.L.; Rodriguez-Rodriguez, R.; Claro, C.M.; Alvarez de Sotomayor, M.; Parrado, J.; Herrera, M.D. Water-soluble rice bran enzymatic extract attenuates dyslipidemia, hypertension and insulin resistance in obese Zucker rats. Eur. J. Nutr. 2013, 52, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Nolan, C.J. Islet beta cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Somesh, B.P.; Verma, M.K.; Sadasivuni, M.K.; Mammen-Oommen, A.; Biswas, S.; Shilpa, P.C.; Reddy, A.K.; Yateesh, A.N.; Pallavi, P.M.; Nethra, S.; et al. Chronic glucolipotoxic conditions in pancreatic islets impair insulin secretion due to dysregulated calcium dynamics, glucose responsiveness and mitochondrial activity. BMC Cell Biol. 2013, 1, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Augstein, P.; Salzsieder, E. Morphology of pancreatic islets: A time course of pre-diabetes in Zucker fatty rats. Methods Mol. Biol. 2009, 560, 159–189. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Rashid, I.B.; Kojima, K.; Shoji, M.; Tanabe, J.; Tamasawa, N.; Suda, T.; Yasujima, M. Time course of pain sensation in rat models of insulin resistance, type 2 diabetes, and exogenous hyperinsulinaemia. Diabetes Metab. Res. Rev. 2008, 24, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Roane, D.S.; Porter, J.R. Nociception and opioid-induced analgesia in lean (Fa/-) and obese (fa/fa) Zucker rats. Physiol. Behav. 1986, 38, 215–218. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Xia, R.; Reynolds, E.; Banerjee, M.; Rothberg, A.E.; Burant, C.F.; Villegas-Umana, E.; Pop-Busui, R.; Feldman, E.L. Association Between Metabolic Syndrome Components and Polyneuropathy in an Obese Population. JAMA Neurol. 2016, 73, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Vera, G.; López-Miranda, V.; Herradón, E.; Martín, M.I.; Abalo, R. Characterization of cannabinoid-induced relief of neuropathic pain in rat models of type 1 and type 2 diabetes. Pharmacol. Biochem. Behav. 2012, 102, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Brussee, V.; Guo, G.; Dong, Y.; Cheng, C.; Martinez, J.A.; Smith, D.; Glazner, G.W.; Fernyhough, P.; Zochodne, D.W. Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes 2008, 57, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Shevalye, H.; Watcho, P.; Stavniichuk, R.; Dyukova, E.; Lupachyk, S.; Obrosova, I.G. Metanx alleviates multiple manifestations of peripheral neuropathy and increases intraepidermal nerve fiber density in Zucker diabetic fatty rats. Diabetes 2012, 61, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.G.; Spindola, H.; Longato, G.; Pintado, M.E.; Malcata, F.X. Antinociceptive and anti-inflammatory effects of novel dietary protein hydrolysate produced from whey by proteases of Cynara cardunculus. Int. Dairy J. 2013, 32, 156–162. [Google Scholar] [CrossRef]



| ZLR | ZFR | ZFR + HEW1 | ZFR + HEW2 | |
|---|---|---|---|---|
| Glucose (mg/dL) | 76.6 ± 3.4 | 85.9 ± 2.6 | 89.2 ± 1.7 | 93.2 ± 2.0 |
| Insulin (ng/mL) | 6.43 ± 1.0 | 25.7 ± 4.8 * | 14.3 ± 2.1 # | 18.4 ± 3.1 |
| HOMA-IR | 24.5 ± 2.5 | 139.8 ± 20.5 * | 79.5 ± 11.5 # | 106.4 ± 17.4 |
| HOMA-β | 146.1 ± 39.2 | 415.7 ± 65.7 * | 199.6 ± 35.7 # | 234.7 ± 42.8 |
| QUICKI | 0.37 ± 0.006 | 0.29 ± 0.007 * | 0.31 ± 0.007 # | 0.30 ± 0.006 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcés-Rimón, M.; González, C.; Vera, G.; Uranga, J.-A.; López-Fandiño, R.; López-Miranda, V.; Miguel, M. Pepsin Egg White Hydrolysate Improves Glucose Metabolism Complications Related to Metabolic Syndrome in Zucker Fatty Rats. Nutrients 2018, 10, 441. https://doi.org/10.3390/nu10040441
Garcés-Rimón M, González C, Vera G, Uranga J-A, López-Fandiño R, López-Miranda V, Miguel M. Pepsin Egg White Hydrolysate Improves Glucose Metabolism Complications Related to Metabolic Syndrome in Zucker Fatty Rats. Nutrients. 2018; 10(4):441. https://doi.org/10.3390/nu10040441
Chicago/Turabian StyleGarcés-Rimón, Marta, Cristina González, Gema Vera, José-A. Uranga, Rosina López-Fandiño, Visitación López-Miranda, and Marta Miguel. 2018. "Pepsin Egg White Hydrolysate Improves Glucose Metabolism Complications Related to Metabolic Syndrome in Zucker Fatty Rats" Nutrients 10, no. 4: 441. https://doi.org/10.3390/nu10040441





