Eight-Week Consumption of High-Sucrose Diet Has a Pro-Oxidant Effect and Alters the Function of the Salivary Glands of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochemical Analysis
2.2. Salivary and Plasma Antioxidants
2.3. Salivary and Plasma Oxidative Damage Products
2.4. Salivary and Plasma Inflammation and ROS Production
2.5. Histological Examination
2.6. Statistical Analysis
3. Results
3.1. General Characteristics
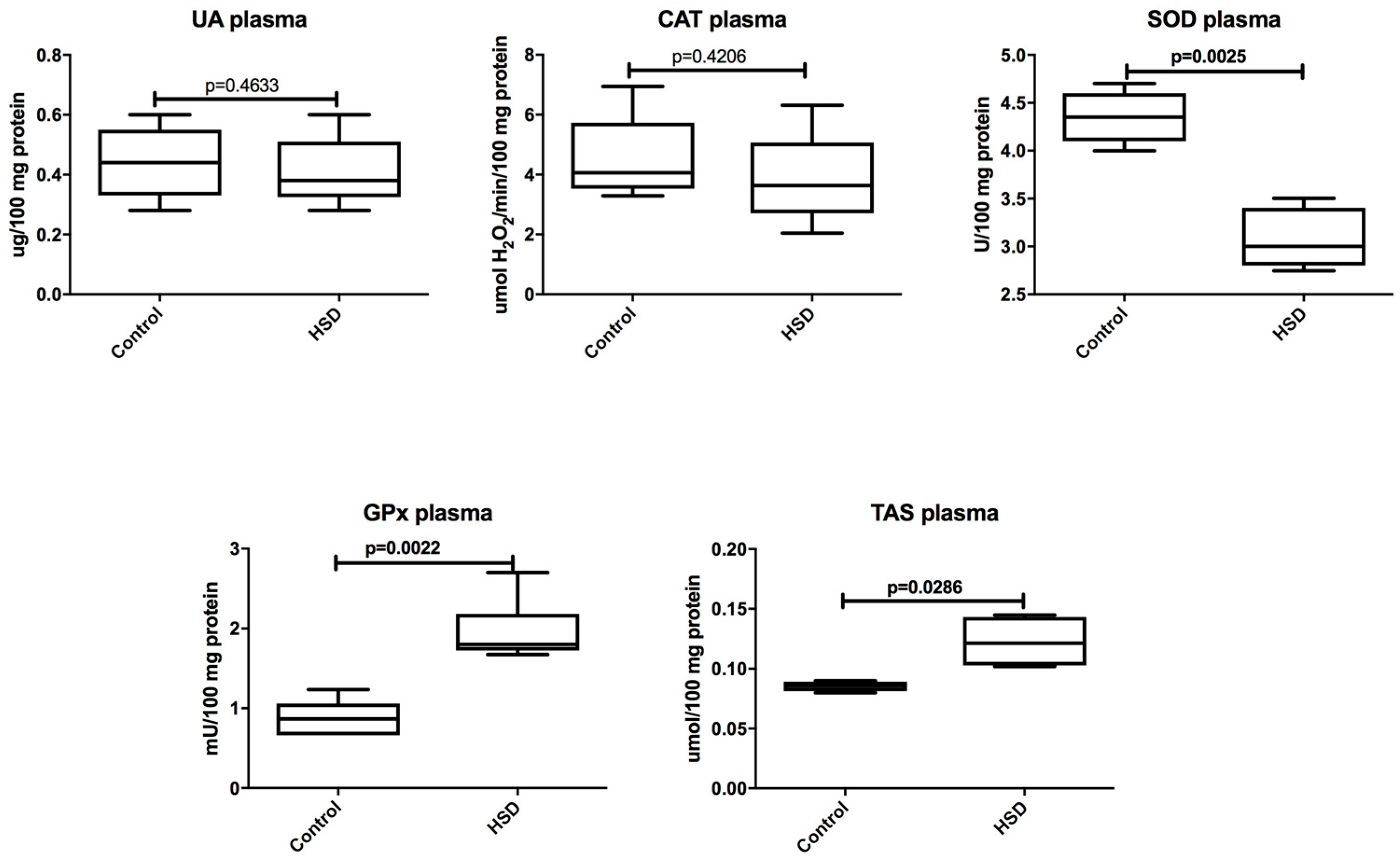
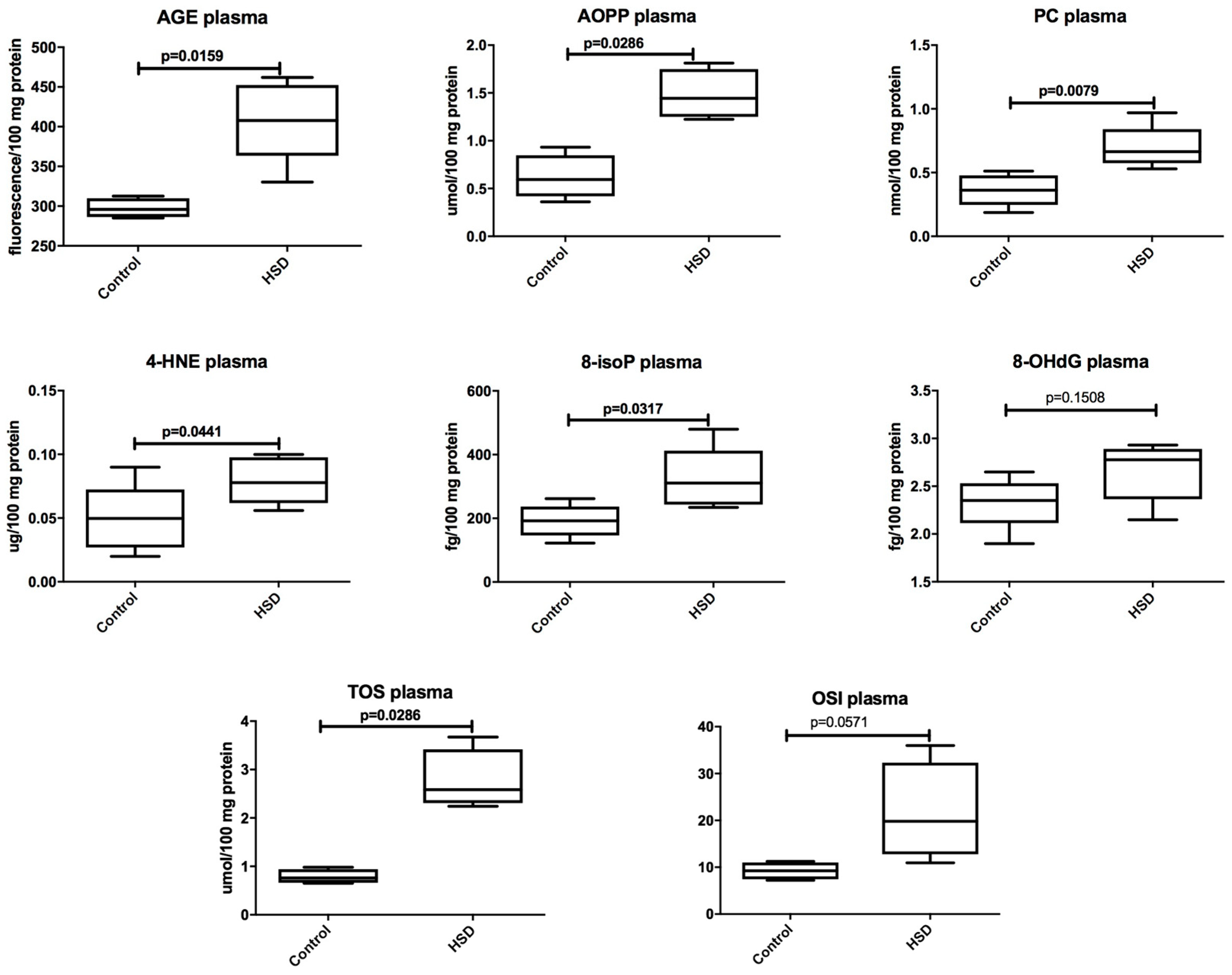
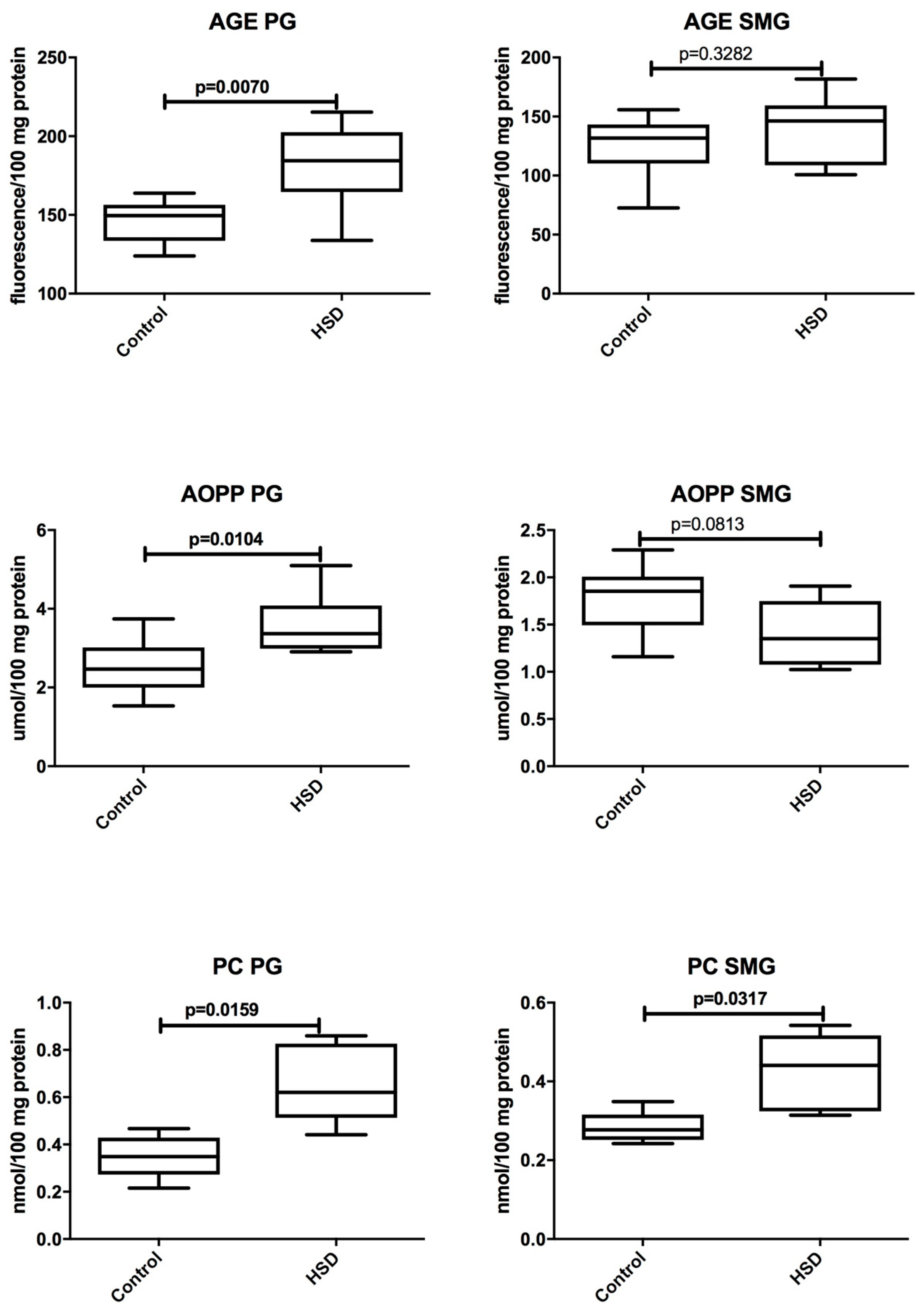
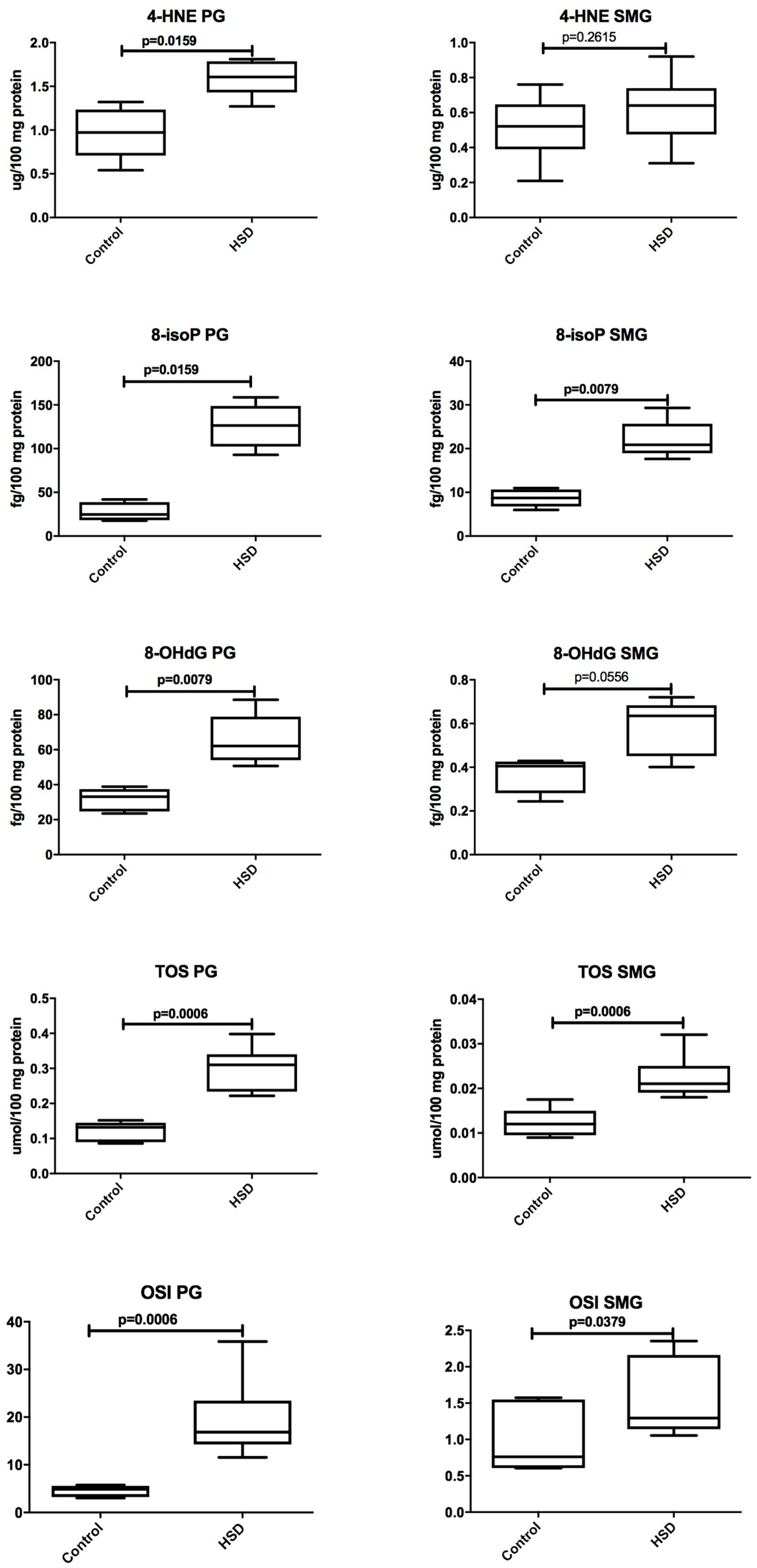
3.2. Plasma Antioxidants and Oxidative Modification Products
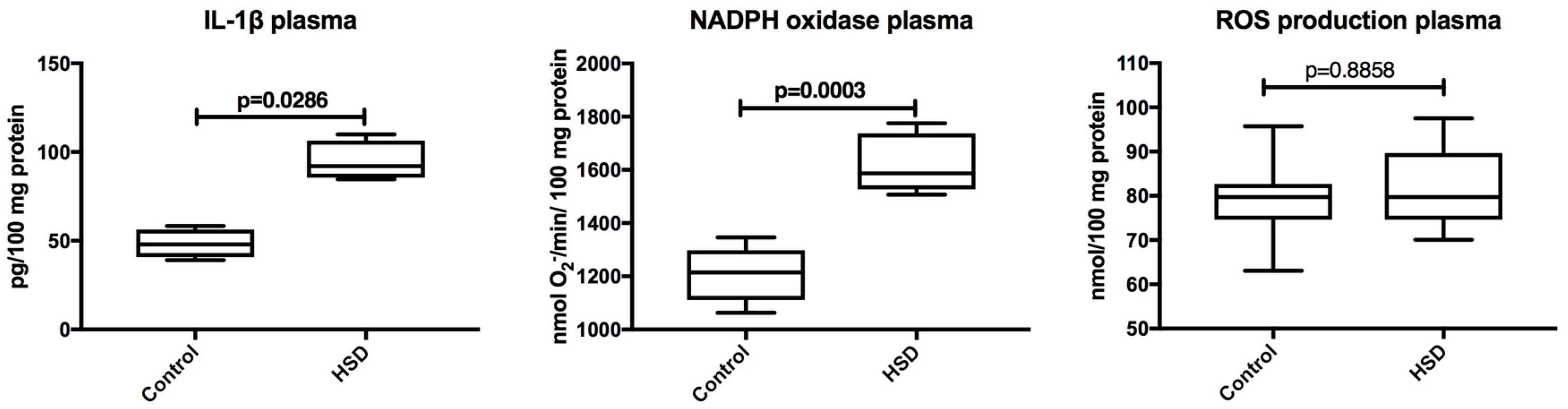
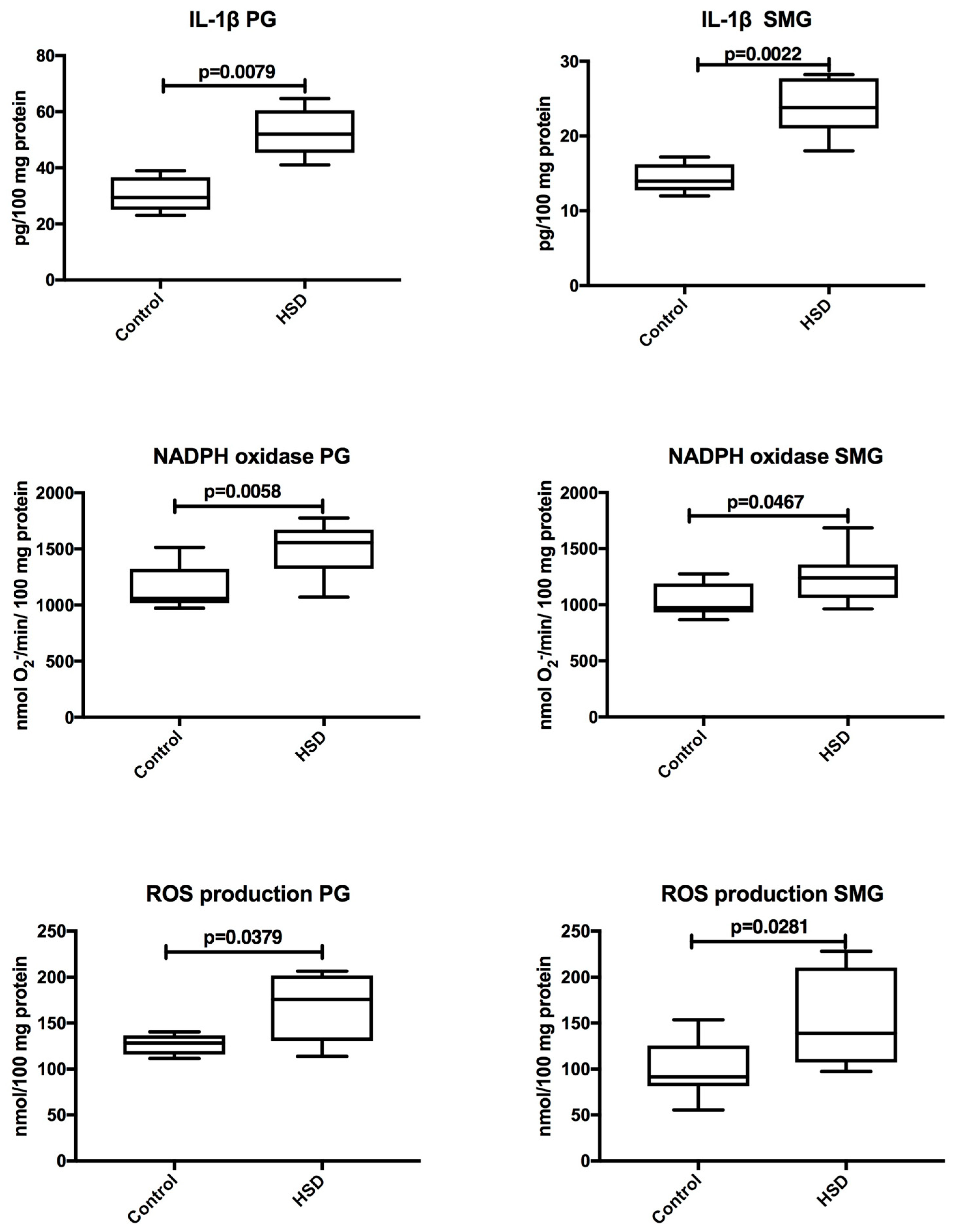
3.3. Plasma Inflammation and ROS Production
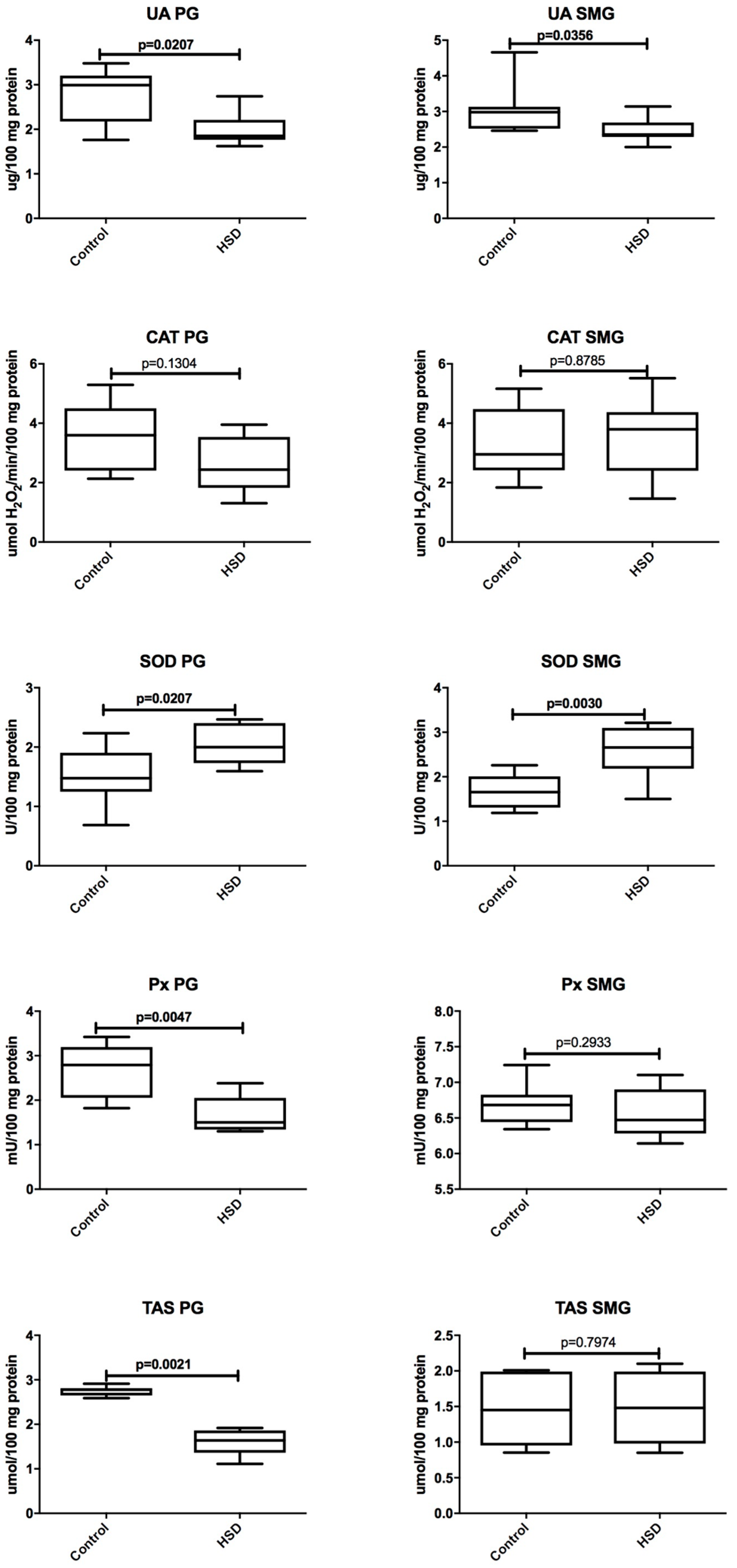
3.4. Salivary Glands Antioxidants and Oxidative Modification Products
3.5. Salivary Glands Inflammation and ROS Production
3.6. Parotid Glands vs. Submandibular Glands
3.7. Correlations
3.8. Histological Analysis
4. Discussion
5. Conclusions
- A high-sucrose diet disturbs the redox balance of the parotid and submandibular salivary glands. However, the parotid glands are more vulnerable to both antioxidant disturbances and oxidative damage.
- Mechanisms involved in the synthesis/secretion of proteins are hindered in both of the salivary glands of HSD rats as compared to the controls.
- A high-sucrose diet reduces the stimulated secretory capacity of salivary glands vs. the control.
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary sugars intake and cardiovascular health: A scientific statement from the american heart association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- WHO. Energy and Protein Requirements. Report of a joint FAO/WHO/UNU Expert Consultation; Series 724; WHO: Geneva, Switzerland, 1985. [Google Scholar]
- WHO. Sugar Intake for Adults and Children; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Blouet, C.; Mariotti, F.; Azzout-Marniche, D.; Mathé, V.; Mikogami, T.; Tomé, D.; Huneau, J.-F. Dietary cysteine alleviates sucrose-induced oxidative stress and insulin resistance. Free Radic. Biol. Med. 2007, 42, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, C.; Durand, A.; Peyrol, S.; Chanseaume, E.; Chauvin, M.-A.; Morio, B.; Vidal, H.; Rieusset, J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Investig. 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busserolles, J.; Zimowska, W.; Rock, E.; Rayssiguier, Y.; Mazur, A. Rats fed a high sucrose diet have altered heart antioxidant enzyme activity and gene expression. Life Sci. 2002, 71, 1303–1312. [Google Scholar] [CrossRef]
- Diniz, Y.S.; Rocha, K.K.H.R.; Souza, G.A.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Novelli Filho, J.L.V.B.; Cicogna, A.C.; Novelli, E.L.B. Effects of n-acetylcysteine on sucrose-rich diet-induced hyperglycaemia, dyslipidemia and oxidative stress in rats. Eur. J. Pharmacol. 2006, 543, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Feillet-Coudray, C.; Sutra, T.; Fouret, G.; Ramos, J.; Wrutniak-Cabello, C.; Cabello, G.; Cristol, J.P.; Coudray, C. Oxidative stress in rats fed a high-fat high-sucrose diet and preventive effect of polyphenols: Involvement of mitochondrial and nad(p)h oxidase systems. Free. Radic. Biol. Med. 2009, 46, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Han, D.; Xu, R.; Wu, H.; Qu, C.; Wang, F.; Wang, X.; Zhao, Y. Glycine protects against high sucrose and high fat-induced non-alcoholic steatohepatitis in rats. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Knaś, M.; Maciejczyk, M.; Waszkiel, D.; Zalewska, A. Oxidative stress and salivary antioxidants. Dent. Med. Probl. 2013, 50, 461–466. [Google Scholar]
- Maciejczyk, M.; Mikołuć, B.; Pietrucha, B.; Heropolitańska-Pliszka, E.; Pac, M.; Motkowski, R.; Car, H. Oxidative stress, mitochondrial abnormalities and antioxidant defense in Ataxia-teleangiectasia, Bloom syndrome and Nijmegen breakage syndrome. Redox Biol. 2017, 11, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Gerdin, E.W.; Angbratt, M.; Aronsson, K.; Eriksson, E.; Johansson, I. Dental caries and body mass index by socio-economic status in swedish children. Community Dent. Oral Epidemiol. 2008, 36, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A.; James, W.P.T. A new understanding of the relationship between sugars, dental caries and fluoride use: Implications for limits on sugars consumption. Public Heal. Nutr. 2014, 17, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Tieche, J.M.; Leonora, J.; Steinman, R.R. High sucrose diet inhibits basal secretion of intradentinal dye penetration-stimulating parotid hormone in pigs. J. App. Physiol. 1994, 76, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Tieche, J.-M.; Leonora, J. Acute secretion of immunoreactive parotid hormone in response to different diets in the pig. Arch. Oral Biol. 1995, 40, 559–565. [Google Scholar] [CrossRef]
- Zalewska, A.; Knaś, M.; Waszkiewicz, N.; Waszkiel, D.; Sierakowski, S.; Zwierz, K. Rheumatoid arthritis patients with xerostomia have reduced production of key salivary constituents. Oral Surg. Oral Med. Oral Pathol. 2013, 115, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Knaś, M.; Kuźmiuk, A.; Waszkiewicz, N.; Niczyporuk, M.; Waszkiel, D.; Zwierz, K. Salivary innate defense system in type 1 diabetes mellitus in children with mixed and permanent dentition. Acta Odontol. Scand. 2013, 71, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Knaś, M.; Gińdzieńska-Sieśkiewicz, E.; Waszkiewicz, N.; Klimiuk, A.; Litwin, K.; Sierakowski, S.; Waszkiel, D. Salivary antioxidants in patients with systemic sclerosis. J. Oral Pathol. Med. 2014, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Farley, C.; Cook, J.A.; Spar, B.D.; Austin, T.M.; Kowalski, T.J. Meal Pattern Analysis Of Diet-Induced Obesity In Susceptible And Resistant Rats. Obes. Res. 2003, 11, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, U.; Maciejczyk, M.; Niklińska, W.; Waszkiel, D.; Żendzian-Piotrowska, M.; Żukowski, P.; Zalewska, A. Chronic high-protein diet induces oxidative stress and alters the salivary gland function in rats. Arch. Oral Biol. 2017, 84, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, U.; Maciejczyk, M.; Miąsko, A.; Matczuk, J.; Knaś, M.; Żukowski, P.; Żendzian-Piotrowska, M.; Borys, J.; Zalewska, A. Oxidative modification in the salivary glands of high fat-diet induced insulin resistant rats. Front. Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Kossakowska, A.; Szulimowska, J.; Klimiuk, A.; Knas, M.; Car, H.; Niklinska, W.; Ladny, J.R.; Chabowski, A.; Zalewska, A. Lysosomal exoglycosidase profile and secretory function in the salivary glands of rats with streptozotocin-induced diabetes. J. Diabetes Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Żebrowska, E.; Zalewska, A.; Chabowski, A. Redox balance, antioxidant defense, and oxidative damage in the hypothalamus and cerebral cortex of rats with high fat diet-induced insulin resistance. Oxid. Med. Cell Longev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Mansson-Rahemtulla, B.; Baldone, D.C.; Pruitt, K.M.; Rahemtulla, F. Specific assays for peroxidases in human saliva. Arch. Oral Biol. 1986, 31, 661–668. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 227–285. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Skrha, J.; Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar] [PubMed]
- Maciejczyk, M.; Szulimowska, J.; Skutnik, A.; Taranta-Janusz, K.; Wasilewska, A.; Wisniewska, N.; Zalewska, A. Salivary Biomarkers of Oxidative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Krętowski, J.; Antonowicz, B.; Ratajczak-Wrona, W.; Jabłońska, E.; Załęski, P.; Waszkiel, D.; Ładny, J.R.; Żukowski, P.; et al. The redox balance in erythrocytes, plasma and periosteum of patients with titanium fixation of the jaw. Front. Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C.; Guo, S.X. Effect of ethanol treatment on indices of cumulative oxidative stress. Eur. J. Pharmacol. 1994, 270, 349–355. [Google Scholar] [CrossRef]
- Haber, C.A.; Lam, T.K.; Yu, Z.; Gupta, N.; Goh, T.; Bogdanovic, E.; Giacca, A.; Fantus, I.G. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: Possible role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E744–E753. [Google Scholar] [CrossRef] [PubMed]
- Stentz, F.B.; Brewer, A.; Wan, J.; Garber, C.; Daniels, B.; Sands, C.; Kitabchi, A.E. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: Randomized control trial. BMJ Open Diabetes Res. Care 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Suwannaphet, W.; Meeprom, A.; Yibchok-Anun, S.; Adisakwattana, S. Preventive effect of grape seed extract against high-fructose diet-induced insulin resistance and oxidative stress in rats. Food Chem. Toxicol. 2010, 48, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Ebertz, C.E.; Bonfleur, M.L.; Bertasso, I.M.; Mendes, M.C.; Lubaczeuski, C.; Araujo, A.C.; Paes, A.M.; Amorim, E.M.P.; Balbo, S.L. Duodenal jejunal bypass attenuates non-alcoholic fatty liver disease in western diet-obese rats. Acta Cir. Bras. 2014, 29, 609–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, K.X.; Wang, C.; Chen, J.H.; Zhu, C.J.; Song, G.Y. Mitofusin-2 ameliorates high-fat diet- induced insulin resistance in liver of rats. World J. Gastroenterol. 2013, 19, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, F.; Shimizu, K.; Muroi, E.; Haseqawa, M.; Takehara, M.; Sato, S. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology 2006, 45, 815–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocco, T.; Di Paola, M.; Papa, S.; Lorusso, M. Arachidonic acid interaction with the mitochondrial electron transport chain promotes reactive oxygen species generation. Free Radic. Biol. Med. 1999, 27, 51–59. [Google Scholar] [CrossRef]
- Levi, B.; Werman, M.J. Long-term fructose consumption accelerates glycation and several age-related variables in male rats. J. Nutr. 1998, 128, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.; Zheng, X.F. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.L. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Gurtu, S.; Sulaiman, S.A.; Ab Wahab, M.S.; Sirajudeen, K.N.; Salleh, M.S. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2010, 80, 74–82. [Google Scholar] [PubMed]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.; Narcy, A.; Rayssiguier, Y.; Mazur, A.; Remesy, C. Lipid metabolism and antioxidant status in sucrose vs. potato-fed rats. J. Am. Coll. Nutr. 2008, 27, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Karin, M. JNK1 and IKKbeta: Molecular links between obesity and metabolic dysfunction. FASEB J. 2010, 24, 2596–2611. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Cerulein pancreatitis: Oxidative stress, inflammation, and apoptosis. Gut Liver 2008, 2, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant defence, oxidative stress and oxidative damage in saliva, plasma and erythrocytes of dementia patients. Can salivary AGE be a marker of dementia? Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Żukowski, P.; Maciejczyk, M.; Matczuk, J.; Kurek, K.; Waszkiel, D.; Żendzian-Piotrowska, M.; Zalewska, A. Effect of N-acetylcysteine on antioxidant defense, oxidative modification, and salivary gland function in a rat model of insulin resistance. Oxid. Med. Cell Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Perez, P.; Kwon, Y.J.; Alliende, C.; Leyton, L.; Aguilera, S.; Molina, C.; Labra, C.; Julio, M.; Leyton, C.; Gonzalez, M.J. Increased acinar damage of salivary glands of patients with Sjogren’s syndrome is paralleled by simultaneous imbalance of matrix metalloproteinase 3/tissue inhibitor of metalloproteinases 1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinases 1 ratios. Arthritis Rheum. 2005, 52, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.C.; Garrett, J.R.; Thulin, A.; Proctor, G.B. Effects of streptozocin-induced diabetes on sympathetic and parasympathetic stimulation of parotid salivary gland function in rats. Diabetes 1989, 38, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Busch, L.; Sterin-Borda, L.; Borda, E. Differences in the regulatory mechanism of amylase release by rat parotid and submandibular glands. Arch. Oral Biol. 2002, 47, 717–722. [Google Scholar] [CrossRef]
- Morris, P.A.; Prout, R.E.S.; Proctor, G.B.; Garrett, J.R.; Anderson, L.C. Lipid analysis of the major salivary glands in streptozotocin-diabetic rats and the effects of insulin treatment. Arch. Oral Biol. 1992, 37, 489–494. [Google Scholar] [CrossRef]
- Aren, G.; Sepet, E.; Ozdemir, D.; Dinccag, N.; Guvener, B.; Firatli, E. Periodontal health, salivary status, and metabolic control in children with type 1 diabetes mellitus. J. Periodontol. 2003, 74, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.E.; Colloca, M.E.; Paez, R.G.; Schallmach, J.N.; Koss, M.A.; Chervonagura, A. Salivary characteristics of diabetic children. Braz. Dent. J. 2003, 14, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siudikiene, J.; Machiulskiene, V.; Nyvad, B.; Tenovuo, J.; Nedzelskiene, I. Dental caries increments and related factors in children with type 1 diabetes mellitus. Caries Res. 2008, 42, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Aitken-Saavedra, J.; Lund, R.G.; Gonzalez, J.; Huenchunao, R.; Perez-Vallespir, I.; Morales-Bozo, I.; Urzua, B.; Tarquinio, S.C.; Maturana-Ramirez, A.; Martos, J.; et al. Diversity, frequency and antifungal resistance of Candida species in patients with type 2 diabetes mellitus. Acta Odontol. Scand. 2018. [Google Scholar] [CrossRef] [PubMed]
- Navea Aguilera, C.; Guijarro de Armas, M.G.; Monereo Megias, S.; Merino Viveros, M.; Toran Ranero, C. The relationship between xerostomia and diabetes mellitus: A little known complication. Endocrinol. Nutr. 2015, 62, 45–46. [Google Scholar] [CrossRef] [PubMed]


| Control | HSD | p | |
|---|---|---|---|
| Body weight (mg) | 355.2 (311.5–415.0) | 407.1 (367.6–449.2) | 0.0046 |
| Food intake (g/day) | 18.97 (12.84–28.22) | 20.73 (12.93–31.03) | 0.7203 |
| Energy intake (g)/per rat/per week | 67.45(63.6–76.6) | 78.8(63.33–85.1) | 0.6300 |
| PG weight (mg) | 0.092 (0.081–0.11) | 0.101 (0.092–0.128) | 0.0447 |
| SMG weight (mg) | 0.305 (0.271–0.342) | 0.31 (0.28–0.373) | 0.0780 |
| Glucose concentration (mg/dL) | 142.5 (100.6–159.4) | 172.0 (145.3–189.2) | 0.0008 |
| Insulin concentration (mU/mL) | 0.178 (0.155–0.197) | 0.2683 (0.192–0.355) | 0.0159 |
| HOMA-IR | 2.704 (1.873–3.251) | 18.272 (15.471–25.136) | <0.0001 |
| PG | SMG | p | |
|---|---|---|---|
| Total protein (mg/mL) | 3842 (3338–4773) | 5118 (3574–6998) | 0.0499 |
| Amylase (μg/100 mg protein) | 3.355 (2.917–4.635) | 2.028 (1.787–2.948) | 0.0159 |
| UA (μg/100 mg protein) | 2.991 (1.761–3.481) | 2.981 (2.461–4.662) | 0.5992 |
| CAT (μmol H2O2/min/100 mg protein) | 3.596 (2.134–5.290) | 2.95 (1.839–5.163) | 0.7209 |
| SOD (U/100 mg protein) | 1.477 (0.6850–2.235) | 1.656 (1.188–2.258) | 0.5737 |
| Px (mU/100 mg protein) | 2.791 (1.821–3.421) | 6.682 (6.342–7.242) | 0.0002 |
| TAS (μmol/100 mg protein) | 2.780 (2.590–2.912) | 1.450 (0.853–2.010) | 0.0021 |
| AGE (fluorescence/100 mg protein) | 149.6 (123.9–163.8) | 131.7 (72.62–155.7) | 0.1049 |
| AOPP (μmol/100 mg protein) | 2.467 (1.532–3.742) | 1.854 (1.159–2.291) | 0.0148 |
| PC (nmol/100 mg protein) | 0.3485 (0.2157–0.4675) | 0.2774 (0.2422–0.3490) | 0.3095 |
| 8-isoP (fg/100 mg protein) | 24.78 (17.64–41.88) | 8.713 (5.974–10.97) | 0.0159 |
| 4-HNE (μg/100 mg protein) | 0.9725 (0.5400–1.320) | 0.521 (0.2100–0.7600) | 0.0173 |
| 8-OHdG (fg/100 mg protein) | 33.14 (23.51–38.87) | 0.4047 (0.2439–0.4287) | 0.0079 |
| TOS (umol/100 mg protein) | 0.1320 (0.086–0.152) | 0.0120 (0.009–0.018) | 0.0006 |
| OSI | 4.871 (3.060–5.736) | 0.7600 (0.603–1.572) | 0.0006 |
| IL-1β (pg/100 mg protein) | 29.41 (23.0–38.96) | 13.95 (11.98–17.2) | 0.0043 |
| NADPH oxidase (nmol O2−/min/100 mg protein) | 1061.0 (972.5–1514.0) | 975.5 (867.6–1275.0) | 0.1605 |
| ROS production (nmol/100 mg protein) | 128.4 (11.6–140.4) | 91.61 (55.47–153.7) | 0.0650 |
| PG | SMG | p | |
|---|---|---|---|
| Total protein (μg/mL) | 3246 (2929–3847) | 3599 (3459–4632) | 0.0207 |
| Amylase (μg/100 mg protein) | 0.4090 (0.2340–0.5423) | 0.3668 (0.2772–1.177) | 0.8413 |
| UA (μg/100 mg protein) | 1.851 (1.620–2.741) | 2.351 (2.000–3.141) | 0.0156 |
| CAT (umol H2O2/min/100 mg protein) | 2.440 (1.306–3.951) | 3.799 (1.462–5.518) | 0.1605 |
| SOD (U/100 mg protein) | 1.999 (1.595–2.467) | 2.657 (1.503–3.210) | 0.0499 |
| Px (mU/100 mg protein) | 1.5 (1.300–2.381) | 6.472 (6.142–7.102) | 0.0002 |
| TAS (μmol/100 mg protein) | 1.640 (1.110–1.920) | 1.480 (0.850–2.100) | 0.9015 |
| AGE (fluorescence/100 mg protein) | 184.4 (133.8–215.3) | 146.2 (100.7–181.7) | 0.0104 |
| AOPP (μmol/100 mg protein) | 3.367 (2.905–5.098) | 1.352 (1.025–1.907) | 0.0007 |
| PC (nmol/100 mg protein) | 0.6203 (0.4416–0.8595) | 0.4408 (0.3141–0.5422) | 0.0317 |
| 8-isoP (fg/100 mg protein) | 126.5 (92.96–158.5) | 20.88 (17.64–29.32) | 0.0079 |
| 4-HNE (μg/100 mg protein) | 1.608 (1.270–1.810) | 0.64 (0.3100–0.9200) | 0.0043 |
| 8-OHdG (fg/100 mg protein) | 62.14 (50.74–88.58) | 0.6352 (0.4009–0.7201) | 0.0079 |
| TOS (μmol/100 mg protein) | 0.3100 (0.222–0.398) | 0.0210 (0.018–0.032) | 0.0006 |
| OSI | 16.850 (11.560–35.860) | 1.295 (1.055–2.353) | 0.0006 |
| IL-1β (pg/100 mg protein) | 52.0 (41.0–64.7) | 23.8 (18.01–28.22) | 0.0043 |
| NADPH oxidase (nmol O2−/min/100 mg protein) | 1556.0 (1071.0–1775.0) | 1248.0 (963.4–1685.0) | 0.0499 |
| ROS production (nmol/100 mg protein) | 175.9 (113.9–206.6) | 139.0 (97.44–228.2) | 0.0410 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejczyk, M.; Matczuk, J.; Żendzian-Piotrowska, M.; Niklińska, W.; Fejfer, K.; Szarmach, I.; Ładny, J.R.; Zieniewska, I.; Zalewska, A. Eight-Week Consumption of High-Sucrose Diet Has a Pro-Oxidant Effect and Alters the Function of the Salivary Glands of Rats. Nutrients 2018, 10, 1530. https://doi.org/10.3390/nu10101530
Maciejczyk M, Matczuk J, Żendzian-Piotrowska M, Niklińska W, Fejfer K, Szarmach I, Ładny JR, Zieniewska I, Zalewska A. Eight-Week Consumption of High-Sucrose Diet Has a Pro-Oxidant Effect and Alters the Function of the Salivary Glands of Rats. Nutrients. 2018; 10(10):1530. https://doi.org/10.3390/nu10101530
Chicago/Turabian StyleMaciejczyk, Mateusz, Jan Matczuk, Małgorzata Żendzian-Piotrowska, Wiesława Niklińska, Katarzyna Fejfer, Izabela Szarmach, Jerzy Robert Ładny, Izabela Zieniewska, and Anna Zalewska. 2018. "Eight-Week Consumption of High-Sucrose Diet Has a Pro-Oxidant Effect and Alters the Function of the Salivary Glands of Rats" Nutrients 10, no. 10: 1530. https://doi.org/10.3390/nu10101530






