Association of Dietary Fatty Acids with Blood Lipids is Modified by Physical Activity in Adolescents: Results from the GINIplus and LISA Birth Cohort Studies
Abstract
:1. Introduction
2. Materials and Methods
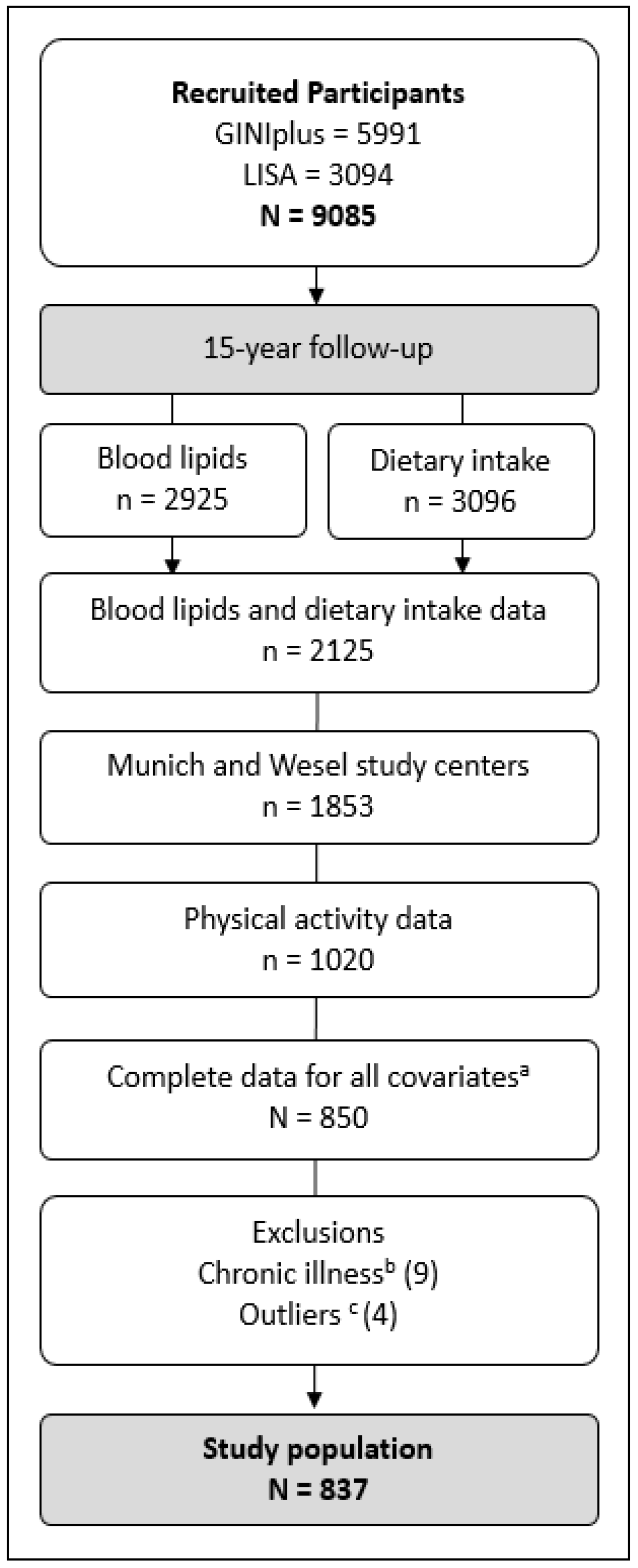
2.1. Participants
2.2. Blood Lipids
2.3. Dietary Intake
2.4. Physical Activity
2.5. Statistical Analyses
3. Results
3.1. Study Population and Basic Characteristics
3.2. Analyses of Associations of Dietary Fatty Acids (When Theoretically Replacing CHO) with Blood Lipids
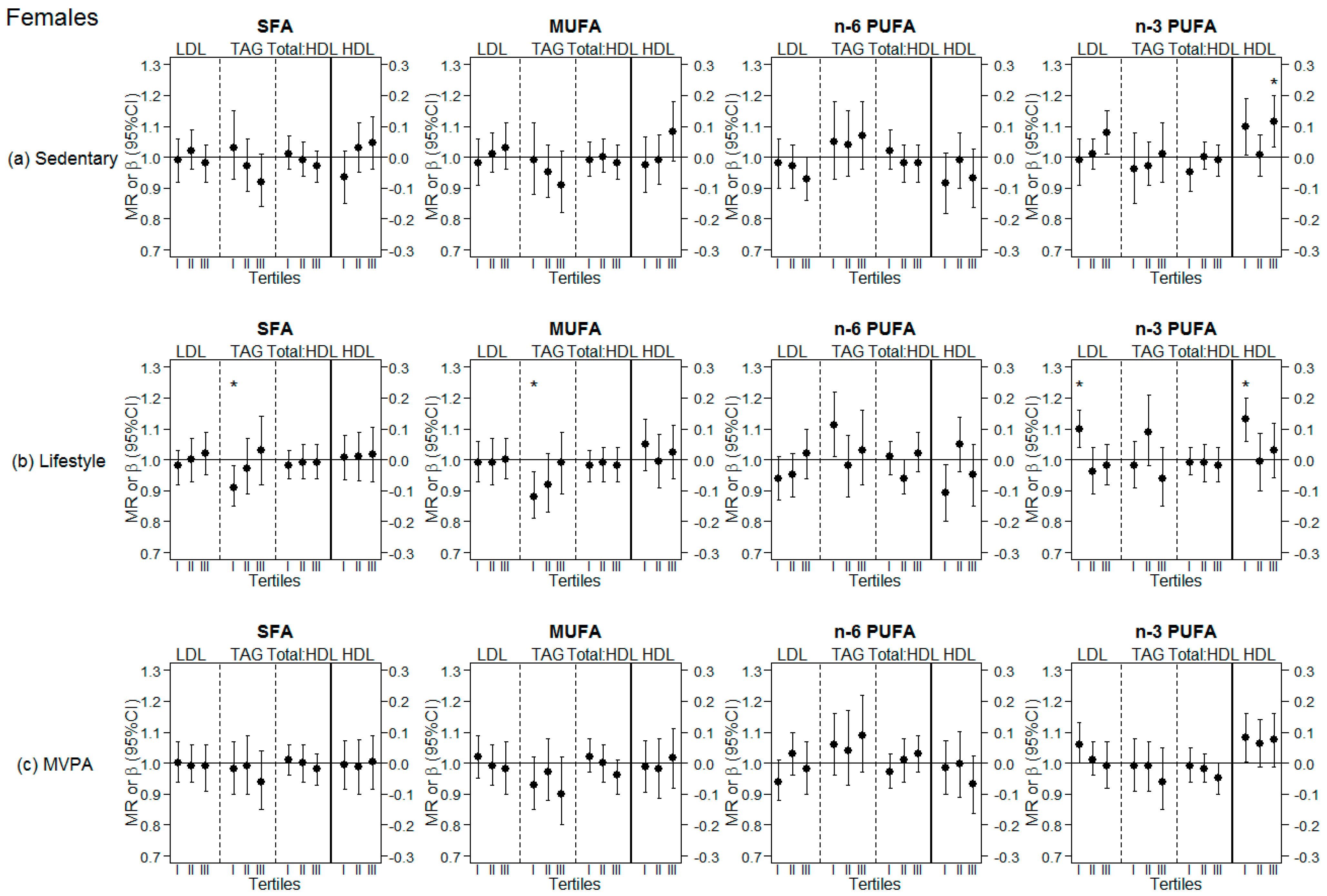
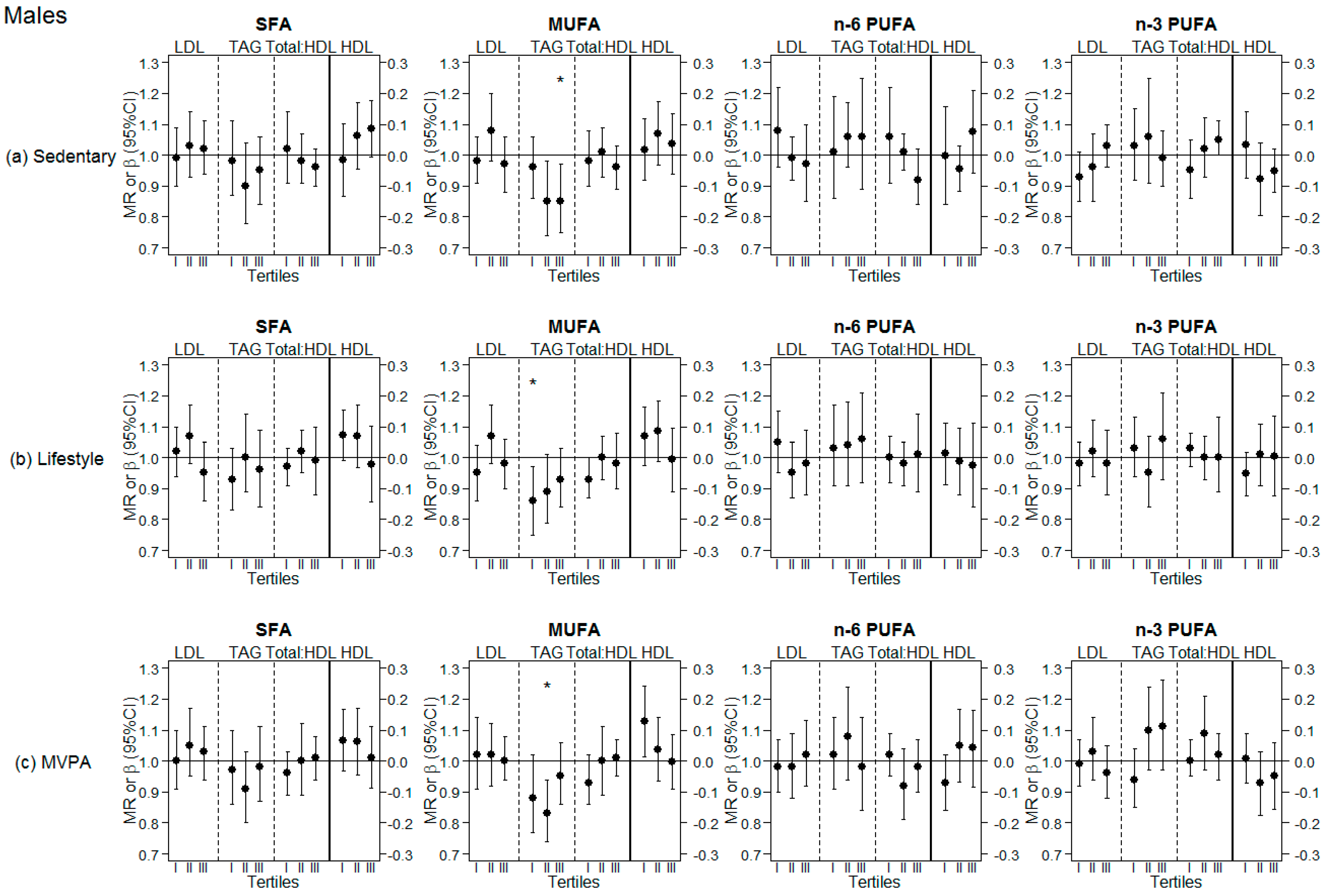
3.3. Analyses Stratified by Tertiles of Different PA Levels (Sedentary, Lifestyle PA, MVPA)
3.4. Sensitivity Analyses Stratified by Fasting Status
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Juhola, J.; Magnussen, C.G.; Viikari, J.S.; Kahonen, M.; Hutri-Kahonen, N.; Jula, A.; Lehtimaki, T.; Akerblom, H.K.; Pietikainen, M.; Laitinen, T.; et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: The cardiovascular risk in young finns study. J. Pediatr. 2011, 159, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, T.A.; von Duvillard, S.P.; Berenson, G.S. Tracking of serum lipids and lipoproteins from childhood to dyslipidemia in adults: The Bogalusa heart study. Int. J. Sports Med. 2002, 23, S39–S43. [Google Scholar] [CrossRef] [PubMed]
- W.H.O. Expert Committee on Prevention in Childhood and Youth of Adult Cardiovascular Diseases. In Prevention in Childhood and Youth of Adult Cardiovascular Diseases: Time for Action/Report of A WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013.
- Baranowski, T. Why combine diet and physical activity in the same international research society? Int. J. Behav. Nutr. Phys. Act. 2004, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (pure): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Mansoor, N.; Vinknes, K.J.; Veierød, M.B.; Retterstøl, K. Effects of low-carbohydrate diets v. Low-fat diets on body weight and cardiovascular risk factors: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 115, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Willett, W.C. Optimal diets for prevention of coronary heart disease. JAMA 2002, 288, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Hegsted, D.M.; Ausman, L.M.; Johnson, J.A.; Dallal, G.E. Dietary fat and serum lipids: An evaluation of the experimental data. Am. J. Clin. Nutr. 1993, 57, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Katan, M.B. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. Vasc. Biol. 1992, 12, 911–919. [Google Scholar] [CrossRef]
- FAO Food and Nutrition Paper. Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr. Pap. 2010, 91, 1–166. [Google Scholar]
- Mente, A.; Dehghan, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Li, W.; Chen, H.; Yi, S.; et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: A cross-sectional analysis from the pure study. Lancet Diabetes Endocrinol. 2017, 5, 774–787. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J. The cardiometabolic consequences of replacing saturated fats with carbohydrates or Ω-6 polyunsaturated fats: Do the dietary guidelines have it wrong? Open Heart 2014, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Lucan, S.C.; O’Keefe, J.H. The evidence for saturated fat and for sugar related to coronary heart disease. Prog. Cardiovasc. Dis. 2016, 58, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Annu. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Anderson, J.J.; Celis-Morales, C.A.; Mackay, D.F.; Iliodromiti, S.; Lyall, D.M.; Sattar, N.; Gill, J.M.R.; Pell, J.P. Adiposity among 132 479 UK Biobank participants; contribution of sugar intake vs other macronutrients. Int. J. Epidemiol. 2017, 46, 492–501. [Google Scholar] [CrossRef]
- Sadler, M.J.; McNulty, H.; Gibson, S. Sugar-fat seesaw: A systematic review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 338–356. [Google Scholar] [CrossRef]
- Rogol, A.D.; Roemmich, J.N.; Clark, P.A. Growth at puberty. J. Adolesc. Health 2002, 31, 192–200. [Google Scholar] [CrossRef]
- Meredith, C.N.; Dwyer, J.T. Nutrition and exercise: Effects on adolescent health. Annu. Rev. Public Health 1991, 12, 309–333. [Google Scholar] [CrossRef]
- Harris, C.; Buyken, A.; Koletzko, S.; von Berg, A.; Berdel, D.; Schikowski, T.; Koletzko, B.; Heinrich, J.; Standl, M. Dietary fatty acids and changes in blood lipids during adolescence: The role of substituting nutrient intakes. Nutrients 2017, 9, 127. [Google Scholar] [CrossRef]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensink, R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Arab, L.; Akbar, J. Biomarkers and the measurement of fatty acids. Public Health Nutr. 2002, 5, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Thyfault, J.P.; Krogh-Madsen, R. Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J. Appl. Physiol. 2011, 111, 1218–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durstine, J.L.; Grandjean, P.W.; Davis, P.G.; Ferguson, M.A.; Alderson, N.L.; DuBose, K.D. Blood lipid and lipoprotein adaptations to exercise. Sports Med. 2001, 31, 1033–1062. [Google Scholar] [CrossRef]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Hardman, A.E. Interaction of physical activity and diet: Implications for lipoprotein metabolism. Public Health Nutr. 1999, 2, 369–376. [Google Scholar] [CrossRef]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia 2012, 55, 2895–2905. [Google Scholar] [CrossRef]
- Heinrich, J.; Bolte, G.; Holscher, B.; Douwes, J.; Lehmann, I.; Fahlbusch, B.; Bischof, W.; Weiss, M.; Borte, M.; Wichmann, H.E. Allergens and endotoxin on mothers’ mattresses and total immunoglobulin e in cord blood of neonates. Eur. Respir. J. 2002, 20, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Von Berg, A.; Kramer, U.; Link, E.; Bollrath, C.; Heinrich, J.; Brockow, I.; Koletzko, S.; Grubl, A.; Filipiak-Pittroff, B.; Wichmann, H.E.; et al. Impact of early feeding on childhood eczema: Development after nutritional intervention compared with the natural course-the giniplus study up to the age of 6 years. Clin. Exp. Allergy 2010, 40, 627–636. [Google Scholar]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, MS, USA, 1998. [Google Scholar]
- Harris, C.; Flexeder, C.; Thiering, E.; Buyken, A.; Berdel, D.; Koletzko, S.; Bauer, C.P.; Bruske, I.; Koletzko, B.; Standl, M. Changes in dietary intake during puberty and their determinants: Results from the giniplus birth cohort study. BMC Public Health 2015, 15, 841. [Google Scholar] [CrossRef]
- Hartmann, B.M.; Bell, S.; Vásquez-Caicedo, A.L. The German Nutrient Database; Federal Research Centre for Nutrition and Food (BfEL): Karlsruhe, Germany, 2005. [Google Scholar]
- Atwater, W.O.; Benedict, F.G. Experiments on the Metabolism of Matter and Energy in the Human Body, 1898–1900; US Office of Experiment Stations Bulletin No. 109; Government Printing Office: Washington, DC, USA, 1902.
- Smith, M.P.; Berdel, D.; Nowak, D.; Heinrich, J.; Schulz, H. Physical activity levels and domains assessed by accelerometry in german adolescents from giniplus and lisaplus. PLoS ONE 2016, 11, e0152217. [Google Scholar] [CrossRef]
- Pfitzner, R.; Gorzelniak, L.; Heinrich, J.; von Berg, A.; Klümper, C.; Bauer, C.P.; Koletzko, S.; Berdel, D.; Horsch, A.; Schulz, H.; et al. Physical activity in german adolescents measured by accelerometry and activity diary: Introducing a comprehensive approach for data management and preliminary results. PLoS ONE 2013, 8, e65192. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.; Pober, D.; Janz, K.F. Calibration of accelerometer output for children. Med. Sci. Sports Exerc. 2005, 37, S523–S530. [Google Scholar] [CrossRef]
- Reed, D.; McGee, D.; Yano, K.; Hankin, J. Diet, blood pressure, and multicollinearity. Hypertension 1985, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Acebo, C. A self-administered rating scale for pubertal development. J. Adolesc. Health 1993, 14, 190–195. [Google Scholar] [CrossRef]
- Standl, M.; Lattka, E.; Stach, B.; Koletzko, S.; Bauer, C.-P.; von Berg, A.; Berdel, D.; Krämer, U.; Schaaf, B.; Röder, S.; et al. FADS1 FADS2 gene cluster, PUFA intake and blood lipids in children: Results from the GINIplus and LISAplus studies. PLoS ONE 2012, 7, e37780. [Google Scholar] [CrossRef] [Green Version]
- Markevych, I.; Standl, M.; Sugiri, D.; Harris, C.; Maier, W.; Berdel, D.; Heinrich, J. Residential greenness and blood lipids in children: A longitudinal analysis in giniplus and lisaplus. Environ. Res. 2016, 151, 168–173. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; version 3.4.4; R Foundation for Statistical Computing: Vienna, Austria, 2018.
- Gidding, S.S.; Dennison, B.A.; Birch, L.L.; Daniels, S.R.; Gillman, M.W.; Lichtenstein, A.H.; Rattay, K.T.; Steinberger, J.; Stettler, N.; Van Horn, L. Dietary recommendations for children and adolescents: A guide for practitioners. Pediatrics 2006, 117, 544–559. [Google Scholar] [PubMed]
- Harris, C.; Demmelmair, H.; von Berg, A.; Lehmann, I.; Flexeder, C.; Koletzko, B.; Heinrich, J.; Standl, M. Associations between fatty acids and low-grade inflammation in children from the LISAplus birth cohort study. Eur. J. Clin. Nutr. 2017, 71, 1303–1311. [Google Scholar] [CrossRef]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American heart association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjaerg-Hansen, A.; Jorgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 2013, 61, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Bays, H. Fish oils in the treatment of dyslipidemia and cardiovascular disease. In The Johns Hopkins Textbook of Dyslipidemia; Lippincott Williams & Wilkins/Wolters Kluwer Health: Philadelphia, PA, USA, 2010; pp. 245–257. [Google Scholar]
- Bays, H.E.; Tighe, A.P.; Sadovsky, R.; Davidson, M.H. Prescription omega-3 fatty acids and their lipid effects: Physiologic mechanisms of action and clinical implications. Expert Rev. Cardiovasc. Ther. 2008, 6, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S. n-3 fatty acids and serum lipoproteins: Human studies. Am. J. Clin. Nutr. 1997, 65, 1645s–1654s. [Google Scholar] [CrossRef] [PubMed]
- Pownall, H.J.; Brauchi, D.; Kilinc, C.; Osmundsen, K.; Pao, Q.; Payton-Ross, C.; Gotto, A.M., Jr.; Ballantyne, C.M. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis 1999, 143, 285–297. [Google Scholar] [CrossRef]
- Paoli, A.; Pacelli, Q.F.; Moro, T.; Marcolin, G.; Neri, M.; Battaglia, G.; Sergi, G.; Bolzetta, F.; Bianco, A. Effects of high-intensity circuit training, low-intensity circuit training and endurance training on blood pressure and lipoproteins in middle-aged overweight men. Lipids Health Dis. 2013, 12, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, F.W.; Laye, M.J.; Lees, S.J.; Rector, R.S.; Thyfault, J.P. Reduced physical activity and risk of chronic disease: The biology behind the consequences. Eur. J. Appl. Physiol. 2008, 102, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Matthews, C.E.; Dunstan, D.W.; Winkler, E.A.; Owen, N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–2006. Eur. Heart J. 2011, 32, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Sedentary lifestyle and risk of obesity and type 2 diabetes. Lipids 2003, 38, 103–108. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Church, T.S.; Craig, C.L.; Bouchard, C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med. Sci. Sports Exerc. 2009, 41, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.; Yates, T.; Biddle, S.J.; Edwardson, C.L.; Khunti, K.; Wilmot, E.G.; Gray, L.J.; Gorely, T.; Nimmo, M.A.; Davies, M.J. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia 2013, 56, 1012–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chastin, S.F.M.; De Craemer, M.; De Cocker, K.; Powell, L.; Van Cauwenberg, J.; Dall, P.; Hamer, M.; Stamatakis, E. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br. J. Sports Med. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bey, L.; Hamilton, M.T. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: A molecular reason to maintain daily low-intensity activity. J. Physiol. 2003, 551, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J.; Hellerstein, M.K. Carbohydrate-induced hypertriacylglycerolemia: Historical perspective and review of biological mechanisms. Am. J. Clin. Nutr. 2000, 71, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Amiel, S.A.; Sherwin, R.S.; Simonson, D.C.; Lauritano, A.A.; Tamborlane, W.V. Impaired insulin action in puberty. N. Engl. J. Med. 1986, 315, 215–219. [Google Scholar] [CrossRef]
- Harris, C.; Buyken, A.; von Berg, A.; Berdel, D.; Lehmann, I.; Hoffmann, B.; Koletzko, S.; Koletzko, B.; Heinrich, J.; Standl, M. Prospective associations of meat consumption during childhood with measures of body composition during adolescence: Results from the giniplus and LISAplus birth cohorts. Nutr. J. 2016, 15, 101. [Google Scholar] [CrossRef]
- Pei, Z.; Flexeder, C.; Fuertes, E.; Standl, M.; Buyken, A.; Berdel, D.; von Berg, A.; Lehmann, I.; Schaaf, B.; Heinrich, J. Food intake and overweight in school-aged children in Germany: Results of the giniplus and lisaplus studies. Ann. Nutr. Metab. 2014, 64, 60–70. [Google Scholar] [CrossRef]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. Vitamin D supplementation, glycemic control, and insulin resistance in prediabetics: A meta-analysis. J. Endocr. Soc. 2018, 2, 687–709. [Google Scholar] [CrossRef]
| Males (N = 365) | Females (N = 472) | p-Value a | |
|---|---|---|---|
| Blood lipids | |||
| LDL cholesterol (mmol/L) | 2.22 (1.8; 2.7) | 2.35 (2.0; 2.8) | 0.008 |
| HDL cholesterol (mmol/L) | 1.40 (0.36) | 1.57 (0.36) | <0.001b |
| TAG (mmol/L) | 1.04 (0.7; 1.4) | 0.98 (0.7; 1.3) | 0.139 |
| Total:HDL | 3.00 (2.6; 3.6) | 2.87 (2.5; 3.3) | 0.002 |
| Fatty acids | |||
| SFA (% EI) | 13.0 (2.9) | 12.7 (3.1) | 0.290 b |
| MUFA (% EI) | 11.2 (2.5) | 10.9 (2.7) | 0.100 b |
| n-6 PUFA (% EI) | 3.9 (3.3; 4.7) | 3.9 (3.3; 4.8) | 0.712 |
| n-3 PUFA (% EI) | 0.6 (0.5; 0.7) | 0.6 (0.5; 0.7) | 0.425 |
| Physical activity | |||
| Sedentary (min/d) | 583 (530; 632) | 599 (563; 644) | <0.001 |
| Lifestyle PA (min/d) | 258 (222; 296) | 241 (209; 275) | <0.001 |
| MVPA (min/d) | 43 (31; 61) | 35 (25; 47) | <0.001 |
| Covariates | |||
| Age (years) | 15.2 (0.3) | 15.2 (0.3) | 0.773 b |
| BMI (kg/m2) | 19.9 (18.5; 22.1) | 20.3 (18.7; 22.1) | 0.135 |
| Parental education (high) | 272 (74.5) | 345 (73.1) | 0.699 c |
| Fasting blood (yes) | 187 (51.2) | 203 (43.0) | 0.022c |
| Daily calories (kcal) | 2397 (668) | 1848 (563) | <0.001b |
| Total carbohydrate (% EI) | 52.6 (6.7) | 53.3 (7.5) | 0.127 b |
| Total fat (% EI) | 31.3 (5.7) | 30.9 (6.4) | 0.350 b |
| Total protein (% EI) | 15 (2.5) | 14.6 (2.9) | 0.047b |
| Study | 0.089 c | ||
| GINI intervention | 117 (32.1) | 174 (36.9) | |
| GINI observation | 135 (37.0) | 183 (38.8) | |
| LISA | 113 (31.0) | 115 (24.4) | |
| Region | 0.001c | ||
| Munich | 260 (71.2) | 283 (60.0) | |
| Wesel | 105 (28.8) | 189 (40.0) | |
| Pubertal stage | <0.001c | ||
| Early | 21 (5.8) | 0 (0.0) | |
| Mid | 134 (36.7) | 20 (4.2) | |
| Late | 207 (56.7) | 377 (79.9) | |
| Post | 3 (0.8) | 75 (15.9) |
| SFA | MUFA | n-6 PUFA | n-3 PUFA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | ||||||||||||
| LDL | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value |
| 1.00 | (0.97, 1.04) | 0.814 | 1.00 | (0.96, 1.04) | 0.902 | 0.97 | (0.93, 1.01) | 0.184 | 1.02 | (0.98, 1.06) | 0.304 | |
| HDL | β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value |
| 0.01 | (−0.04, 0.05) | 0.751 | 0.02 | (−0.03, 0.06) | 0.543 | −0.04 | (−0.09, 0.01) | 0.120 | 0.07 | (0.03; 0.12) | 0.002 | |
| TAG | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value |
| 0.98 | (0.93, 1.04) | 0.544 | 0.94 | (0.89, 1.00) | 0.049 | 1.05 | (0.99, 1.12) | 0.089 | 0.98 | (0.93, 1.03) | 0.419 | |
| Total:HDL | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value |
| 1.00 | (0.97, 1.02) | 0.760 | 0.99 | (0.96, 1.02) | 0.449 | 1.00 | (0.97, 1.03) | 0.902 | 0.980 | (0.95, 1.01) | 0.163 | |
| Males | ||||||||||||
| LDL | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value |
| 1.01 | (0.96, 1.07) | 0.603 | 1.00 | (0.95, 1.05) | 0.902 | 1.00 | (0.94, 1.05) | 0.932 | 1.00 | (0.95, 1.05) | 0.915 | |
| HDL | β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value |
| 0.05 | (−0.01, 0.11) | 0.078 | 0.05 | (−0.00, 0.11) | 0.064 | 0.00 | (−0.06, 0.06) | 0.972 | −0.02 | (−0.08; 0.03) | 0.384 | |
| TAG | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value |
| 0.95 | (0.88, 1.01) | 0.113 | 0.87 | (0.82, 0.94) | <0.001 | 1.04 | (0.97, 1.12) | 0.268 | 1.03 | (0.96, 1.09) | 0.401 | |
| Total:HDL | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value | MR | 95% CI | p-value |
| 0.99 | (0.94, 1.03) | 0.542 | 0.98 | (0.93, 1.02) | 0.326 | 0.99 | (0.94, 1.04) | 0.618 | 1.02 | (0.98, 1.07) | 0.381 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, C.P.; Von Berg, A.; Berdel, D.; Bauer, C.-P.; Schikowski, T.; Koletzko, S.; Heinrich, J.; Schulz, H.; Standl, M. Association of Dietary Fatty Acids with Blood Lipids is Modified by Physical Activity in Adolescents: Results from the GINIplus and LISA Birth Cohort Studies. Nutrients 2018, 10, 1372. https://doi.org/10.3390/nu10101372
Harris CP, Von Berg A, Berdel D, Bauer C-P, Schikowski T, Koletzko S, Heinrich J, Schulz H, Standl M. Association of Dietary Fatty Acids with Blood Lipids is Modified by Physical Activity in Adolescents: Results from the GINIplus and LISA Birth Cohort Studies. Nutrients. 2018; 10(10):1372. https://doi.org/10.3390/nu10101372
Chicago/Turabian StyleHarris, Carla P., Andrea Von Berg, Dietrich Berdel, Carl-Peter Bauer, Tamara Schikowski, Sibylle Koletzko, Joachim Heinrich, Holger Schulz, and Marie Standl. 2018. "Association of Dietary Fatty Acids with Blood Lipids is Modified by Physical Activity in Adolescents: Results from the GINIplus and LISA Birth Cohort Studies" Nutrients 10, no. 10: 1372. https://doi.org/10.3390/nu10101372





